
Hemostatic abnormalities in adult patients with Marfan syndrome
Introduction
Marfan syndrome (MFS) is an autosomal dominant genetic disease with a prevalence of 1–5 per 10,000 (1). In classic MFS, up to 95% of the disease-causing mutations are located in the FBN1 gene on chromosome 15q21. The typical characteristic of MFS is the ectatic or aneurysmatic deformation of the aortic root. Other cardiovascular manifestations include aneurysm formation in more distally located aortic segments and the pulmonary artery (2,3), as well as disorders of the heart valves (3).
The typical aortic root ectasia or aneurysm associated with patients with MFS may induce hemostatic deviations. In contrast to the laminar blood flow with homogeneous shearing forces as observed in the normal aorta, aortic dilatation induces changes in blood flow and rheological parameters (4). Moreover, other clinical manifestations of MFS, such as aortic valve insufficiency or severe thoracic scoliosis, may lead to regurgitation, and flow turbulence (5,6) and increased shear stress (7), respectively. These rheological disturbances affect hemostasis by interfering with platelet activation or behaviour, the transport of clotting factors, and cell-to-cell interactions (8-10). Moreover, Heyde syndrome, a combination of an acquired aortic valve stenosis and angiodysplasias of the gastrointestinal tract, demonstrates a similar relationship between rheology and hemostasis in a clinical context. In this disease, the altered shear forces affect the von Willebrand factor (VWF) and thus the hemostasis (11,12). However, after surgical correction of such an aortic valve stenosis, the tendency toward increased bleeding, which is generally associated with Heyde syndrome, is significantly reduced (12).
Increased markers for platelet and thrombin activation, fibrinolysis, as well as a correlation of the parameters to the size of the aortic aneurysm have already been described in thoracic aortic aneurysms of different etiology and in abdominal aortic aneurysms (4,13,14).
Thus, a correlation between rheology and hemostasis can be assumed and this correlation may also be present in MFS patients with aortic pathologies. Due to the increased diameter of the aortic root, the blood flow patterns in MFS are altered and thus it can be argued that the aortic dilatation has the ability to influence hemostasis. Changes in hemostasis, probably also playing a causal role in the development and progression of aneurysms, may complicate cardiovascular and other surgical procedures in MFS patients.
To assess the feasibility of a correlation between rheology and hemostasis, laboratory parameters from MFS patients were compared with data from healthy, age- and sex-matched controls and further correlated with the size of the aortic root diameter in the MFS patients.
Methods
Study design and study subjects
For this cross-sectional case-control study, 78 patients with proven MFS were recruited between December 2012 and July 2017 from a tertiary care centre for adults with congenital heart disease. The control group consisted of 50 healthy age- and sex-matched persons recruited from March to June 2017 at various health care locations. Inclusion requirements were a clinically and molecular genetic based MFS diagnosis, according to the 2010 revised Ghent-criteria, and ≥18 years of age. Exclusion criteria for both patient and control groups were the use of anticoagulant as well as acetylsalicylic acid, the occurrence of inflammatory, coagulation and malignant diseases, lack of cognitive competency and refusal to consent. An additional exclusion criterion for the control group was the occurrence of cardiovascular diseases.
Ethical standards
All participants gave informed consent. The study complies with the requirements of the Declaration of Helsinki and was approved by the local ethical committee (number 5313/12).
Clinical data and imaging
Clinical data, cardiovascular imaging with magnetic resonance imaging (MRI) and echocardiography, as well as laboratory parameters were collected for each participant. The clinical data of the MFS patients were recorded retrospectively using patient files, while the clinical data of the controls were collected using partially standardized questionnaires. Both the MFS patients and control group underwent echocardiographic examinations (device type: Philips Epic 7G and GE Vivid E9 4D), either on or near the date on which blood was drawn. Additionally, the cardiac MRI results from the MFS patients were also evaluated (device type: 1.5 Tesla scanner, Avanto, Siemens Healthcare). If the patients had already undergone cardiac surgery, echocardiographic and cardiac MRI findings from a time point prior to surgery were analyzed. In each case we studied the diameter of the aortic root in diastole, always regarding the largest diameter in the three-dimensional measurement and measuring inner edge to inner edge. Z-scores were calculated based on patient’s body surface area (15). Furthermore, the cardiac valves were examined for the presence of insufficiency, prolapse or stenosis and the aorta for the presence of dissection.
Blood collection and laboratory assays
Venous blood was collected from each study participant and directly analyzed. The platelet count was measured by resistance impedance measurement, the platelet function by the Multiplate® and the PFA-100® systems. The Multiplate® test is usually used to monitor and differentiate an anticoagulation therapy. In this test, the platelet aggregation is measured after stimulating different receptors on platelets with different reagents (ADP, arachidonic acid and TRAP-6 as internal control). The PFA-100® measures the closure time after stimulating the platelets with collagen, shear stress and either ADP or epinephrine. In this test all values above 300 s are given as 300 s by the laboratory. D-dimers and VWF antigens and activity were determined by particle-enhanced immune-turbidimetry. Factor VIII activity, PTT and fibrinogen were measured by clotting assays at the Siemens BCS XP instrument. Prothrombin fragment 1+2 were determined by enzyme-linked immunosorbent assays (ELISA).
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics for Mac 2016, version 24.0 (Armonk, NY: IBM Corp.). All data were represented by mean and standard deviation, as well as median, minimum and maximum. Nominal data were described by relative and absolute frequencies. For the comparison of variables between two or more groups the Mann-Whitney U-test, the t-test or the Chi-square test were used depending on the characteristics of the groups and the variables of the t-test. The correlation between two continuous, non-normally distributed data were calculated with the Spearman correlation; the relationship between two continuous, normally distributed variables with the Pearson correlation. The correlation is purely descriptive and uncorrected for multiple testing. Statistical testing was performed at the 2-tailed alpha level of 0.05.
Results
Study subjects
Of the 78 primary MFS patients surveyed, 27 were excluded for various reasons. These included the use of anticoagulants in 15 cases, sequence analysis detected MFS variants of uncertain significance in eight cases, missing data in the main target variables in three cases, and age <18 years in one case.
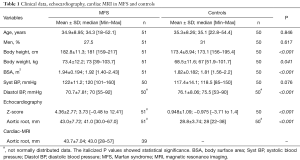
Clinical, echocardiographic and cardiac MRI data are summarized in Table 1. There were no significant differences in age (P=0.846) and gender (P=0.617) between the MFS patients and the control group. Height (P<0.001), weight (P=0.041), and body surface area (P<0.001) at the time of echocardiography imaging differed significantly between both groups. Sixteen of the 51 MFS patients underwent cardiac surgery.
Full table
Imaging
MFS patients showed significantly higher Z-scores (P<0.001) and aortic root diameters (P<0.001) (Table 1). The mitral valve (86.3%, n=44) in MFS patients was most commonly found to be pathological (in 56.9% insufficiency and prolapse, in 15.7% only a insufficiency, in 13.7% only a prolapse), followed by the tricuspid valve (78.4%, n=40), the aortic valve (43.1%, n=22), and finally the pulmonary valve (27.5%, n=14). Only two of the MFS patients had no abnormality of any of the four valves. The valves of the control group showed very few abnormalities (in total 1% of all valves, n=2) including one aortic valve insufficiency and one mitral valve prolapse. Two MFS patients had a chronic aortic dissection type Stanford B. No aortic dissection type Stanford A was found in any of the patients.
A total of 19 cardiovascular operations for MFS-related cardiovascular manifestations were performed in 16 (31%) of the 51 MFS patients. In each of these operations at least one transfusion was necessary.
Laboratory measurements
Markers of hemostasis
Platelet count and function
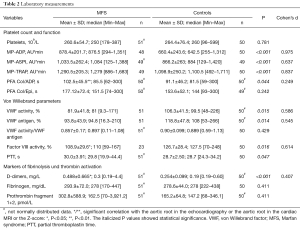
No difference in the platelet count was observed between MFS patients and controls, nor did the values correlate with aortic diameter (Table 2).
Full table
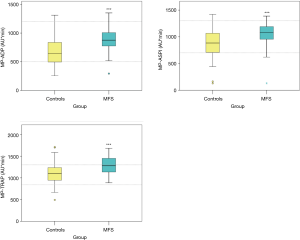
The MFS patients showed significantly higher values in all three Multiplate® tests compared to the control group (P<0.001) (Figure 1, Table 2). None of these values showed a correlation to the aortic diameter.
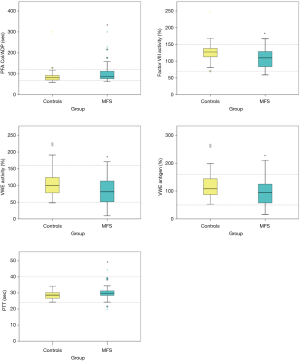
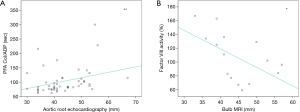
The PFA Col/ADP test was significantly longer in the MFS patients compared to the controls (P=0.044) (Figure 2), whereas PFA Col/Epi did not differ between the MFS patients and the control group (Table 2). PFA Col/ADP also correlated positively with the size of the aortic root in the echocardiography (r=0.43, P<0.01) and the related Z-score (r=0.45, P<0.01) (Figure 3).
VWF
VWF activity (P=0.015) and antigen (P=0.014) were significantly reduced in MFS patients compared to controls (Figure 2). The VWF activity/VWF antigen ratio did not differ significantly between the MFS patients and the control group (Table 2). No significant correlation could be found with the size of the aortic root either.
Factor VIII activity
Factor VIII activity was significantly decreased in MFS patients compared to controls (P=0.016) (Figure 2, Table 2). Factor VIII activity correlated significantly negatively with the diameter of the aortic root in cardiac MRI (r=−0.55, P<0.05) (Figure 3). There was no correlation to the diameter of the aortic root and the Z-score in echocardiography.
Partial thromboplastin time
PTT was significantly higher in MFS patients compared to controls (P=0.047) (Figure 2, Table 2). A correlation to the diameter of the aorta was not found.
D-dimers
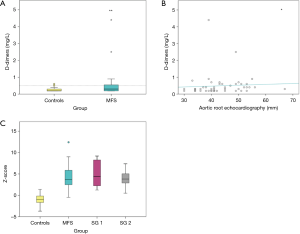
The D-dimers were significantly elevated in MFS patients when compared to the control group (P<0.001) (Table 2) and also correlated positively with the diameter of the aortic root (r=0.28, P<0.05) and the Z-scores in the echocardiography (r=0.29, P<0.05) (Figure 4).
Fibrinogen
Fibrinogen levels were similar in MFS patients and the control population (Table 2).
Prothrombin fragments 1 and 2
Prothrombin fragments 1 and 2 showed no significant differences between the two groups (Table 2).
Subgroup analysis
Subgroup 1: VWF
Eight MFS patients (subgroup 1) showed VWF antigen levels and VWF activity levels below the respective minimum values of the control group. For these eight MFS patients, the VWF activity/VWF antigen ratio was significantly smaller (P<0.001) compared to the control group and the aortic root showed values above the mean and the median of the control group. In seven of these patients, the echocardiographic Z-score was above the maximum value of the control group (Figure 4). Seven of these eight patients had prolonged values in the PFA Col/Epi test and five in the PFA Col/ADP test. No specific correlation could be found with factor VIII activity.
Subgroup 2: D-dimers
Subgroup 2 was formed from eight MFS patients with D-dimer values above the maximum value of the control group. In seven patients of this subgroup, the aortic root and the associated Z-score were larger than the respective maximum value of the controls (Figure 4). Six patients from the subgroup also had elevated levels of prothrombin fragment 1+2.
Discussion
This study demonstrates the presence of hemostatic alterations in a large group of patients with proven MFS in relation of the size of the aortic root and in comparison with data from a group of healthy, sex- and age-matched controls. To the best of our knowledge there is only one case control study exploring this issue in MFS patients (4) and in addition several single case reports (4,16-18).
The main finding of the study is the possible presence of an acquired von Willebrand syndrome type 2A in patients with MfS This syndrome is characterized by a qualitative and quantitative defect of large VWF multimers followed by a dysfunctional interaction with platelets (19,20). It may be induced by increased shear stress caused by the ectatic aortic root in MFS patients (9) which gives rise to a conformational change of the VWF antigens and results in an increased susceptibility to proteases (ADAMTS13) (10,11,21-24).
Further evidence supporting an acquired von Willebrand syndrome type 2A was found in the laboratory findings (25,26). These included an abnormal PFA-100® test, significantly VWF antigen and activity (the VWF activity was more frequent and its mean more decreased than that of the VWF antigen), normal to moderately decreased factor VIII activity and normal platelet counts. PFA Col/ADP also correlated positively with the aortic root size as determined by echocardiography and the related Z-scores. Through the analysis of a selected patient subgroup (subgroup 1) these specific patterns became more apparent.
Various other studies have shown similar laboratory deviations in patients with aortic valve stenosis (12,27), dysfunctional aortic or mitral valve replacement, severe aortic or mitral valve regurgitation (28,29) and in patients with acquired von Willebrand syndrome type 2A (30). It was also reported that the altered laboratory parameters normalized after the repair of an aortic valve stenosis (30-32).
In order to confirm our theory, further investigations will be necessary. A standardized, universally accepted diagnostic criteria for acquired von Willebrand disease is still lacking (24) and there are different opinions about the sensitivity of the laboratory tests (33-36). Another point which may add greatly to this field will be the development of further laboratory tests to differentiate between different subtypes of the von Willebrand syndromes. Analyzing the laboratory parameters before and after surgery in one patient could determine a possible regression of hemostatic changes after the correction of aortic aneurysm or pathological cardiac valves. This would be important to prevent acquired coagulation changes, such as the mentioned von Willebrand syndrome type 2A, by early correction of pathological heart valves or aortic aneurysms. Lastly, the majority of the MFS patients had pathological heart valves, which might also have an effect on shear stress and thereby on hemostasis. In future studies it would be interesting to examine the correlation between hemostasis and parameters like regurgitant volume or pressure half time in MFS patients.
Subgroup 2 of MFS patients with enlarged aortic root and Z-score had increased fibrinolysis and thrombin activation with elevated levels of D-dimer and prothrombin fragment 1+2. Other studies on patients with ectatic or aneurysmatic thoracic and abdominal aortic segments have also revealed increased fibrinolysis and thrombin activation (4,13,37-40). Indeed, studies on the thoracic aortic aneurysms typical of MFS are rare: Touat et al. [2008] studied 52 Marfan patients and found increased platelet and thrombin activation in patients with aortic diameter over 45 mm. In patients with thoracic aortic aneurysm significantly elevated levels of D-dimers and a correlation to the aneurysmal size were found (37,41). In contrast to abdominal aortic aneurysms, this location is not characterized by the formation of mural thrombi contributing to coagulation (4), thus implying that other mechanisms must be responsible for this occurrence.
The patients represented in subgroup 1 and subgroup 2 did not overlap, indicating that both previous discussed theories may exist independently in MfS Both situations were positively correlated with the size of the aortic root. Identifying and comparing the exact genotypes of the two subgroups would be an interesting topic for future studies. However, it remains unclear whether the altered hemostasis is a consequence of the ectatic aortic root or whether it plays a causal role in the development and progression of an enlarged aortic root. If the altered hemostasis leads to an enlarged aortic root, it may be due to microthrombosis in the vessel wall inducing a necrosis.
Other inherited diseases leading to thoracic aortic aneurysm, like Ehlers-Danlos syndrome, were also associated with platelet dysfunction, measured, e.g., by PFA-100® tests. In contrast, von Willebrand disease or factor VIII deficiency could be excluded in Ehlers-Danlos patients (42,43). There are no studies available on hemostasis in Loeys-Dietz syndrome as the third important genetic disease with aortic aneurysm. Patients with bicuspid aortic valve and thoracic aortic dilatation showed increased fibrinolysis and thrombin activation (4). Bilen et al. [2012] found increased platelet activation measured by mean platelet volume and a correlation to aortic wall stiffness in patients with bicuspid aortic valve (44).
Limitations
This study has various limitations: the data collection was partly done in a retrospective manner. The recruitment of a part of the control population in the Outpatients’ Clinic for Prevention, Rehabilitation and Sports Medicine, may have led to a selection bias, as patients at this clinic are often more physically active than normal controls. Furthermore, the controls did not undergo cardiac MRI screening. However, there was no clear need for this, as echocardiography did not reveal an aortic root ectasia or aneurysm in any case. A biological, pre-analytical and analytical variability must be considered, especially when analysing manually measured coagulation parameters.
In addition other contributing factors for the findings must be considered: in this study we focused on the correlation between hemostatic parameters and aortic dilatation, but also the pathological cardiac valves play a decisive role in the development of shear forces and thus influence the hemostasis. Second, in MFS patients altered wall shear stress was found in absence of changes in aortic morphology (45), which may indicate an intrinsic coagulation disorder. Third, the effects of the occurred surgical interventions and patient’s blood group were not considered. As endothelial cells synthesize von VWF, endothelial dysfunction found in MFS patients might cause reduced VWF levels (46-48). PTT did not show relevant abnormalities, so a haemophilia as another cause for the findings could be excluded. Lastly, multiple linear regression analyses using hemostasis parameters as dependent variables and gender, weight, height, body surface area, age and blood pressure as independent variables did not show statistically significant and relevant findings (data not shown).
Conclusions
The current study reveals relevant hemostatic deviations in MFS patients, which could be attributed to either an acquired von Willebrand syndrome type 2A or a nonspecific coagulation activation with increased fibrinolysis and thrombin activation. The hemostatic deviations are associated with increased diameters of the aortic root, possibly explicable by the altered blood flow patterns present in these patients.
These novel findings may help to reduce peri- and postoperative bleeding complications and improve common therapeutic and preventive measures for MFS patients. However, the clinical relevance of the findings needs further definition. Until then the hemostatic parameters should be carefully monitored when use of anticoagulants or surgical interventions are necessary.
Acknowledgments
This work was supported by the “German Cardiology Society” (“Deutsche Herzstiftung”); “Herzkind e.V.”; and health insurance company “AOK-Bayern” for the unrestricted promotion of ACHD research. A special thanks also goes to Vida Ungerer for her English language corrections.
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study complies with the requirements of the Declaration of Helsinki and was approved by the local ethical committee (number 5313/12). All participants gave informed consent.
References
- Jondeau G. 2010. Marfan Syndrome. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=558
- Kaemmerer H, Oechslin E, Seidel H, et al. Marfan syndrome: what internists and pediatric or adult cardiologists need to know. Expert Rev. Cardiovasc Ther 2005;3:891-909. [Crossref] [PubMed]
- Rutz T, Seidel H, Hauser M, et al. Das Marfan-Syndrom als komplexe kardiovaskuläre Erkrankung. Die Medizinische Welt - aus der Wissenschaft in die Praxis 2013;64:97-101.
- Touat Z, Lepage L, Ollivier V, et al. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol 2008;28:940-6. [Crossref] [PubMed]
- Bogren HG, Mohiaddin RH, Yang GZ, et al. Magnetic resonance velocity vector mapping of blood flow in thoracic aortic aneurysms and grafts. J Thorac Cardiovasc Surg 1995;110:704-14. [Crossref] [PubMed]
- Geiger J, Markl M, Herzer L, et al. Aortic flow patterns in patients with Marfan syndrome assessed by flow-sensitive four-dimensional MRI. J Magn Reson Imaging 2012;35:594-600. [Crossref] [PubMed]
- Bellini C, Korneva A, Zilberberg L, et al. Differential ascending and descending aortic mechanics parallel aneurysmal propensity in a mouse model of Marfan syndrome. J Biomech 2016;49:2383-9. [Crossref] [PubMed]
- Brown CH, Leverett LB, Lewis CW, et al. Morphological, biochemical, and functional changes in human platelets subjected to shear stress. J Lab Clin Med 1975;86:462-71. [PubMed]
- Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol 2006;26:1729-37. [Crossref] [PubMed]
- Gogia S, Neelamegham S. Role of fluid shear stress in regulating VWF structure, function and related blood disorders. Biorheology 2015;52:319-35. [Crossref] [PubMed]
- Horiuchi H. A hemostatic disorder caused by high shear stress: acquired von Willebrand syndrome. Rinsho Ketsueki 2018;59:2233-7. [PubMed]
- Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med 2003;349:343-9. [Crossref] [PubMed]
- Sidloff DA, Stather PW, Choke E, et al. A systematic review and meta-analysis of the association between markers of hemostasis and abdominal aortic aneurysm presence and size. J Vasc Surg 2014;59:528-35.e4. [Crossref] [PubMed]
- Yuan SM, Shi YH, Wang JJ, et al. Elevated plasma D-dimer and hypersensitive C-reactive protein levels may indicate aortic disorders. Rev Bras Cir Cardiovasc 2011;26:573-81. [Crossref] [PubMed]
- Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥ 15 years of age. Am J Cardiol 2012;110:1189-94. [Crossref] [PubMed]
- Humphries JE, Stouffer GA, Kelly TE, et al. Hypercoagulability in a patient with Marfan syndrome. J Med Genet 1991;28:349-51. [Crossref] [PubMed]
- Mamiya S, Endo Y, Miura AB, et al. Disseminated intravascular coagulation accompanying thoracic and abdominal aortic aneurysm; report of three cases. Jpn J Med 1988;27:91-5. [Crossref] [PubMed]
- Alarcón-Segovia D, Fierro FJ, Villalobos JD, et al. Bilateral renal vein thrombosis and nephrotic syndrome in a patient with the Marfan syndrome. Dis Chest 1968;54:153-6. [Crossref] [PubMed]
- Fressinaud E. 2009. Von-Willebrand-Syndrom Typ 2. Available online: http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=de&Expert=166081
- Universitätsklinikum Ulm NN. 2017. von Willebrand-Faktor. Available online: https://www.uniklinik-ulm.de/fileadmin/default/09_Sonstige/Klinische-Chemie/Seiteninhalte/Seiteninhalte_V/von_Willebrand_Faktor.pdf
- Dent JA, Berkowitz SD, Ware J, et al. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc Natl Acad Sci U S A 1990;87:6306-10. [Crossref] [PubMed]
- Siedlecki CA, Lestini BJ, Kottke-Marchant KK, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood 1996;88:2939-50. [Crossref] [PubMed]
- Tsai HM, Sussman II, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood 1994;83:2171-9. [Crossref] [PubMed]
- Petricevic M, Knezevic J, Samoukovic G, et al. Diagnosis and Management of Acquired von Willebrand Disease in Heart Disease: A Review of the Literature. Thorac Cardiovasc Surg 2018. [Crossref] [PubMed]
- Stone ME, Mazzeffi M, Derham J, et al. Current management of von Willebrand disease and von Willebrand syndrome. Curr Opin Anaesthesiol 2014;27:353-8. [Crossref] [PubMed]
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- Iijima M, Itoh N, Murase R, et al. A surgical case of aortic stenosis with recurrent gastrointestinal bleeding: Heyde syndrome. Int J Surg Case Rep 2018;53:281-4. [Crossref] [PubMed]
- Blackshear JL, Wysokinska EM, Safford RE, et al. Shear stress-associated acquired von Willebrand syndrome in patients with mitral regurgitation. J Thromb Haemost 2014;12:1966-74. [Crossref] [PubMed]
- Blackshear JL, McRee CW, Safford RE, et al. von Willebrand Factor abnormalities and Heyde Syndrome in dysfunctional heart valve prostheses. JAMA Cardiol 2016;1:198-204. [Crossref] [PubMed]
- Frank RD, Lanzmich R, Haager PK, et al. Severe aortic valve stenosis. Clin Appl Thromb Hemost 2017;23:229-34. [Crossref] [PubMed]
- Panzer S, Badr Eslam R, Schneller A, et al. Loss of high-molecular-weight von Willebrand factor multimers mainly affects platelet aggregation in patients with aortic stenosis. Thromb Haemost 2010;103:408-14. [Crossref] [PubMed]
- Marggraf O, Schneppenheim S, Daubmann A, et al. Correction of acquired von Willebrand syndrome by transcatheter aortic valve implantation. J Invasive Cardiol 2014;26:654-8. [PubMed]
- Tiede A, Priesack J, Werwitzke S, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J Thromb Haemost 2008;6:569-76. [Crossref] [PubMed]
- Fressinaud E, Veyradier A, Truchaud F, et al. Screening for von Willebrand disease with a new analyzer using high shear stress: a study of 60 cases. Blood 1998;91:1325-31. [Crossref] [PubMed]
- Cattaneo M, Federici AB, Lecchi A, et al. Evaluation of the PFA-100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost 1999;82:35-9. [Crossref] [PubMed]
- Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost 2008;34:709-33. [Crossref] [PubMed]
- Ihara A, Kawamoto T, Matsumoto K, et al. Relationship between hemostatic markers and circulating biochemical markers of collagen metabolism in patients with aortic aneurysm. Pathophysiol Haemost Thromb 2003;33:221-4. [Crossref] [PubMed]
- Vele E, Kurtcehajic A, Zerem E, et al. Plasma D-dimer as a predictor of the progression of abdominal aortic aneurysm. J Thromb Haemost 2016;14:2298-303. [Crossref] [PubMed]
- Wallinder J, Bergqvist D, Henriksson AE. Haemostatic markers in patients with abdominal aortic aneurysm and the impact of aneurysm size. Thromb Res 2009;124:423-6. [Crossref] [PubMed]
- Kapetanios DM, Karkos CD, Papazoglou KO. Changes in circulating markers of coagulation and fibrinolysis after endovascular repair of abdominal aortic aneurysms. Int Angiol 2018;37:440-50. [Crossref]
- Nomura F, Ihara A, Yoshitatsu M, et al. Relationship between coagulation cascade, cytokine, adhesion molecule and aortic aneurysm. Eur J Cardiothorac Surg 2003;23:1034-8; discussion 1038-9. [Crossref] [PubMed]
- Artoni A, Bassotti A, Abbattista M, et al. Hemostatic abnormalities in patients with Ehlers-Danlos syndrome. J Thromb Haemost 2018;16:2425-31. [Crossref] [PubMed]
- Busch A, Hoffjan S, Bergmann F, et al. Vascular type Ehlers-Danlos syndrome is associated with platelet dysfunction and low vitamin D serum concentration. Orphanet J Rare Dis 2016;11:111. [Crossref] [PubMed]
- Bilen E, Tanboga IH, Kurt M, et al. Mean platelet volume is increased in patients with bicuspid aortic valve. Clin Appl Thromb Hemost 2012;18:351-5. [Crossref] [PubMed]
- Geiger J, Arnold R, Herzer L, et al. Aortic wall shear stress in Marfan syndrome. Magn Reson Med 2013;70:1137-44. [Crossref] [PubMed]
- Lomelí O, Perez-Torres I, Marquez R, et al. The Evaluation of Flow-Mediated Vasodilation in the Brachial Artery Correlates With Endothelial Dysfunction Evaluated by Nitric Oxide Synthase Metabolites in Marfan Syndrome Patients. Front Physiol 2018;9:965. [Crossref] [PubMed]
- Sellers SL, Milad N, Chan R, et al. Inhibition of Marfan Syndrome Aortic Root Dilation by Losartan: Role of Angiotensin II Receptor Type 1-Independent Activation of Endothelial Function. Am J Pathol 2018;188:574-85. [Crossref] [PubMed]
- Soto ME, Soria-Castro E, Guarner-Lans V, et al. Preliminary analysis of the association of TRPV1 to the formation of Marfan syndrome aneurysms. Histol Histopathol 2019. [Epub ahead of print]. [PubMed]


