
Age-dependent clinical and echocardiographic manifestations of aortic stenosis in an unselected, non-biased cohort
Introduction
Aortic stenosis (AS) is the most common acquired aortic valve disease in western countries, with a prevalence of 1–2% (1). Both the prevalence and the incidence increase up to 12% with age (2). Clinical symptoms diverge broadly: heart failure, angina, syncope, and sudden death are common; however, some patients remain asymptomatic (3). Curative therapy options include replacing the aortic valve, either with conventional surgical aortic valve replacement (SAVR), which requires a thoracotomy, or with transcatheter aortic valve replacement (TAVR). In some cases, balloon angioplasty is an option for treating AS. Treatment decision pathways are predominantly established based on age: SAVR is recommended for younger patients, and TAVR is an option for older patients that are at high risk (4,5).
However, the management of AS is a controversial issue. The first TAVR was performed in 2002. Since then, the method has improved with the production of smaller devices and the introduction of a sheath, which was less harmful (6). Currently, an increasing number of patients are being treated with TAVR. In Germany, 15 964 patients received TAVR during 2011–2013 (7).
Surprisingly, very few studies have scrutinized whether the echocardiographic clinical manifestations and comorbidities of AS might depend on patient age. Available data are largely from tertiary heart centers and carry a significant referral bias. Unbiased data are lacking, and it remains unclear whether the severity of aortic valve disease and the presence of comorbidities might be correlated with patient age.
In this retrospective, observational cohort study (8), we analyzed the clinical and echocardiographic manifestations of AS according to age. This study included 321 adults with AS that were admitted to Reinbek hospital.
Methods
We performed a retrospective, systematic analysis of all patient records admitted to our hospital from 1/1/2014 to 1/6/2018. To identify patients with AS, we defined AS with echocardiographic parameters, according current guidelines (9). All data were derived from a primary care hospital (Reinbek Hospital). All patients were investigated in this hospital.
We collected detailed data on the medical history and physical examination for all patients. In all patients with AS, we performed and evaluated a complete set of echocardiographic measurements. We used the aortic valve area (AVA) to characterize the disease stage. We defined the stages, based on the aortic orifice, as follows: mild AS: 1.5–2 cm2; moderate AS: 1.0–1.5 cm2, and severe AS: <1.0 cm2. All clinical and echocardiographic data were extracted from electronic data records (IMED One©, Deutsche Telekom Clinical Solutions GmbH, Köln, Germany) in pseudonymized manner. We classified the patients into 4 age categories, according to earlier recommendations: <65, 65–74, 75–84, and >85 years (4).
All echocardiographic investigations were performed by experienced investigators. The GE Vivid E9© Ultrasound machine was used with GE EchoPac© software tools for analyzing raw data (both products from General Electronics Healthcare).
Statistics
We described quantitative data as the mean ± standard deviation, and qualitative data as the number and proportion (%), unless otherwise specified. We compared continuous data with one-way Analysis of Variance (ANOVA) and the post-hoc Tukey Test for comparing multiple variables. We compared categorical data with Pearson's chi squared test and contingency tables. The Pearson correlation analysis and scatter plots were calculated to determine relationships between continuous variables.
We considered P values P<0.05 as an indicator of significance between patient groups. We performed all statistical analyses with SPSS software (SPSS for Windows, Release 25.0, SPSS Inc., 1993 to 2007, Chicago, Illinois, USA).
The authors of this manuscript certify that they have complied with the principles of ethical publishing. The study and analyses were performed according to the guidelines of the local Ethics Committee (Ärztekammer Schleswig-Holstein). All information in the data bank was encoded in a pseudonymized manner.
Results
We identified 321 patients with AS in our records. Of these patients, 2 had bicuspid valves and 15 had massive calcifications. Consequently, it was not possible to classify the aortic valve type as uni-, bi-, or tricuspid types.
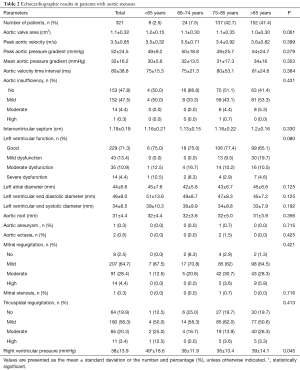
Of all 321 patients, 74.1% had symptoms of angina and dyspnea, with no differences between age categories (Table 1). The mean New York Heart Association (NYHA) class was 1.5. Only 11.5% of patients experienced syncope. Coronary heart disease and atrial fibrillation were the most common comorbidities. We found no differences between age categories regarding the incidences of coronary heart disease (CAD), myocardial infarction, peripheral artery disease, cerebral insults, or atrial fibrillation. The prevalence of cardiovascular risk factors was the same in all groups (Table 1).
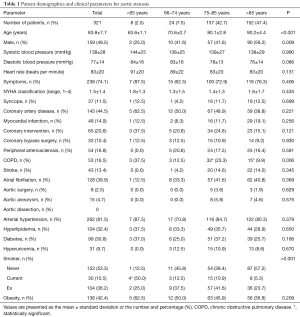
Full table
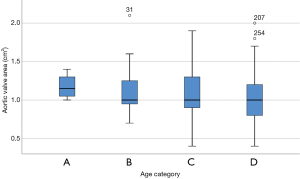
The AVAs were 1.2±0.15 cm2 in patients under 65 years, 1.1±0.3 cm2 in patients 65–74 years, 1.1±0.35 cm2 in patients 75–84 years, and 1.0±0.3 cm2 in patients 85 years and older (P=0.061; Figure 1).
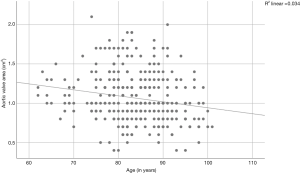
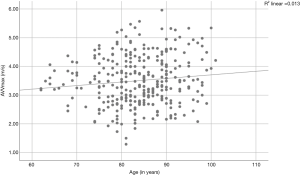
A Pearson product-moment correlation coefficient was computed to assess the relationships between AVA, aortic velocity, pressure gradients, and age. We found a correlation between the AVA and age (r=−0.185, P=0.001; Figure 2) and between aortic velocity and age (r=0.114, P=0.042; Figure 3). Furthermore, there was a correlation between peak pressure and age (r=0.134, P=0.017), but there was no correlation between mean pressure and age (r=0.108, P=0.054).
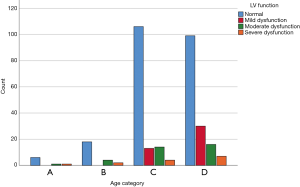
Patient echocardiographic results are presented in Table 2. No differences were found in the peak aortic velocity or aortic pressure gradients among the different age categories. Other echocardiographic criteria, like left ventricular function, were similar in all age categories (Figure 4). Interestingly, right ventricular pressure (RVP) was elevated in the youngest age category (age <65 years); in that category, the mean pressure was 49±18.6 mmHg, compared to 38±11.9 mmHg among patients 65–74 years, 36±13.4 mmHg among patients 75–84 years, and 39±14.1 mmHg in patients 85 years and older. We did not detect any other echocardiographic differences between these age groups.
Full table
Discussion
The main findings of our study were: (I) with increasing age, the AVA declined and the aortic velocity increased; (II) the symptoms of aortic valve dysfunction were common across all age groups, and (III) the incidences of cardiovascular comorbidities and risk factors were similar in all age groups.
Severity of AS and age
Our data were consistent with the traditional ideas that severe AS is a disease of older individuals, and that the severity of AS increases with age. Interestingly, other cardiac disorders or risk factors did not correlate with severe AS. Older patients had smaller AVAs, higher aortic velocities, and greater pressure gradients. The decision of whether to treat with conservative observation or a palliative, surgical, or interventional procedure often depends on the patient’s age (5). Most TAVR trials included patients older than 75 or 80 years. However, recent trials have shown that patient characteristics and outcomes were independent of age. Interestingly, older patients had a better outcome than younger patients in the PARTNER Trial (10). Of note, in the present study, we included only 8 patients under 65 years old.
Clinical manifestation of AS
In our cohort, the patients had the same clinical presentation and symptoms, independent of age. Of all 321 patients included, 74% had symptoms. The NYHA classifications were low in all categories. The syncope frequency was about 11%. However, in contrast to earlier observations, our frequency of symptomatic AS was high. In a recent meta-analysis, 50% of patients with AS were reported to be asymptomatic (11). In many patients, it is difficult to define symptoms—particularly in patients that are older or with multimorbidities. Nevertheless, the indication and timing for interventional or surgical valve replacement is based on clinical symptoms (5). Symptomatic patients are referred for interventional or surgical treatment earlier than asymptomatic patients; in the latter group, a “watchful waiting” strategy is possible (5).
A recent trial (FRAILTY-AVR-Trial) investigated different frailty scores for patients before TAVR or SAVR. They showed that frailty was correlated with outcome after the procedure (12). In that study, the prevalence of frailty varied broadly in the cohort, depending on the scoring method used: frailty was diagnosed in 12% to 56% among patients that received SAVR and from 35% to 74% among patients that received TAVR (12). Further investigations are needed to unravel the complex clinical characteristics of patients with AS.
Cardiovascular comorbidities
The incidence of cardiovascular comorbidities and risk factors was high in all age categories. In our cohort, at the time of the AS diagnosis, 143 patients (44.5%) had CAD; 48 patients (14.9%) had prior myocardial infarctions; 65 patients (20.6%) had percutaneous coronary interventions; and 33 patients (10.4%) had undergone coronary artery bypass surgery. Interestingly, the incidence of CAD was the same in all age categories; i.e., it did not significantly increase with age. This observation was novel, because in previous studies, CAD severity was correlated with age.
Cardiovascular comorbidities have a crucial impact on AS. Among patients with AS, those with concomitant CAD have a worse outcome than those without CAD (13). Many patients with AS had CAD and/or myocardial infarctions, and they had previously received coronary interventions or aorto-coronary bypass surgery (13).
Clinical variables that influence mortality
The prognosis of patients with AS depends on several clinical factors. A previous study evaluated 241 patients with severe AS and preserved left ventricular (LV) ejection fraction that did not undergo valve replacement. They showed that older age, a history of hypertension, and LV diastolic dysfunction were independent predictors of mortality (14). Interestingly, in our analysis, these factors were equally distributed throughout our predefined age categories. Nevertheless, these predictors of death should be considered, when deciding on the mode and timing of therapy. We confirmed that LV function and arterial hypertension were not correlated with age.
A large analysis of patients with AS that were treated with aortic valve surgery showed that patients with isolated AS, with none or with only a few comorbidities, had better postprocedural and long-term outcomes than patients with AS and CAD (13). A complete evaluation of clinical characteristics is essential in the treatment decision process. For example, metabolic syndrome was associated with accelerated progression and calcification in AS (15). In our analysis, we did not find any differences in clinical characteristics, particularly metabolic syndrome, between our predefined age groups.
Study limitations
We could not completely exclude biases in our study, due to the inclusion of patients that received both outpatient and in-hospital treatments. Patients that were treated in the hospital had a different clinical presentation and probably had more severe symptoms. Additionally, we did not perform a systematic analysis or screening of a healthy population. Finally, although symptomatic complaints are perceived very subjectively and variably, by both patients and physicians, we did not perform “objective” stress tests or pro-BNP tests to ascertain the level of symptomatic AS.
Conclusions
Based on our results, we conclude that age is a weak parameter for making decisions about the optimal AS therapy. AVAs in AS decreases moderately with age. Age does not impact any clinical or echocardiographic parameters. Cardiovascular diseases and symptomatic AS are found in all age categories.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was performed according to the guidelines of the local ethics committee (Ärztekammer Schleswig Holstein) as a retrospective, pseudonymized research and is listed as a research project at the University Hospital Hamburg-Eppendorf. All patients have given written consent accepting the data processing and analysis before beginning of the treatment.
References
- d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515-22. [Crossref] [PubMed]
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002-12. [Crossref] [PubMed]
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66. [Crossref] [PubMed]
- Vandvik PO, Otto CM, Siemieniuk RA, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 2016;354:i5085. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Cribier A, Durand E, Eltchaninoff H. TAVR, 15 years down: shooting for the moon, reaching the stars. J Am Coll Cardiol 2017;70:56-9. [Crossref] [PubMed]
- Walther T, Hamm CW, Schuler G, et al. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol 2015;65:2173-80. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [Crossref] [PubMed]
- Adegunsoye A, Mundkur M, Nanda NC, et al. Echocardiographic evaluation of calcific aortic stenosis in the older adult. Echocardiography 2011;28:117-29. [Crossref] [PubMed]
- Don CW, Johnson A, Burke C, et al. Tavr age paradox: the oldest patients have better than expected outcomes in the partner study. J Am Coll Cardiol 2016. [Crossref]
- Genereux P, Stone GW, O'Gara PT, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2016;67:2263-88. [Crossref] [PubMed]
- Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the frailty-avr study. J Am Coll Cardiol 2017;70:689-700. [Crossref] [PubMed]
- Beach JM, Mihaljevic T, Svensson LG, et al. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol 2013;61:837-48. [Crossref] [PubMed]
- Barasch E, Petillo F, Pollack S, et al. Clinical and echocardiographic correlates of mortality in medically treated patients with severe isolated aortic stenosis and normal left ventricular ejection fraction. Circ J 2014;78:232-9. [Crossref] [PubMed]
- Capoulade R, Clavel MA, Dumesnil JG, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol 2012;60:216-23. [Crossref] [PubMed]


