
Advanced mapping strategies for ablation therapy in adults with congenital heart disease
Introduction
The population of patients with congenital heart disease (CHD) is continuously increasing as today about 90% of patients reach adulthood due to improved therapeutic options (1). Approximately half of these patients are expected to experience arrhythmias because of the underlying congenital heart defect itself or as a sequela of interventional or surgical treatment (1,2). Catheter ablation in this cohort is an important therapeutic option attributable to frequently experienced drug refractoriness and possible hemodynamic deterioration (3,4). Three-dimensional (3D) electroanatomic mapping systems are commonly used in these patients as their role in producing favorable outcome in CHD-related catheter ablation has been demonstrated (5-7). Currently, further technological advances, such as ultra-high density mapping (HDM) including multipolar catheters with small electrode size and spacing, are providing further solutions for the various challenges encountered during electrophysiological studies in adult patients with CHD (8-12). However, data reporting on acute ablation outcome in patients with CHD using these novel technologies are sparse (8,12). Therefore, the aim of the present study was to investigate the contribution of HDM in conjunction with novel automated annotation algorithms to the treatment of cardiac arrhythmias in patients with moderate to great CHD complexity.
Methods
Study design and patient selection
The study was approved by the local ethics committee of the University of Hamburg (No. WF-79/16) and informed consent was taken from all patients.
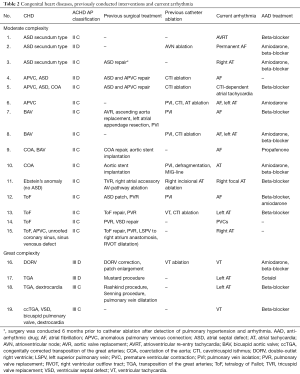
We investigated 19 consecutive adult patients with a history of treated or untreated CHD and an indication for catheter ablation due to symptomatic arrhythmia using the Rhythmia™ mapping system in conjunction with automated annotation algorithms incorporated in the novel Lumipoint™ software (Boston Scientific Corporation, Marlborough, MA, USA). Only patients with CHD of moderate to great complexity were included into the study, according to the ACHD AP classification including anatomical and physiological criteria defined by the current guidelines of the management of adults with CHD (3). Anatomical abnormalities such as anomalous pulmonary venous connection, congenital aortic valve disease, Ebstein anomaly, ventricular septum defect, supravalvar aortic stenosis or unrepaired secundum atrial septum defect were considered as CHD of moderate complexity (3). Patients presenting with transposition of the great arteries (TGA), cyanotic CHD, Fontan procedure, interrupted aortic arch, mitral/pulmonary atresia, truncus arteriosus, a single/double-outlet ventricle or other abnormalities of atrioventricular and ventriculoarterial connection were classified as CHD of great complexity (3). Moreover, physiological variables including arrhythmia severity, exercise capacity and end-organ dysfunction were considered for classification (physiological stage A–D), as many have prognostic value in patients with CHD. Patients were classified based on the “highest” relevant anatomic or physiological feature (3).
Electrophysiological study and periprocedural imaging
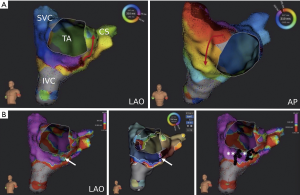
Prior to ablation, transthoracic and/or transesophageal echocardiography were performed for evaluation of global cardiac function and exclusion of intracardial thrombi. Additional imaging was performed and imported into the HDM system prior to the procedure for better evaluation of complex anatomy if reasonable (Figure 1). Patients were under conscious sedation by intravenous propofol and fentanyl administration throughout the procedure (13). Hemodynamic monitoring was conducted by continuous assessment of saturation, non-invasive or invasive blood pressure as well as surface and intracardiac ECG recording. All tracings were recorded and stored on a digital amplifier/recorder system (LabSystem PRO®, Bard Electrophysiology Inc., Lowell, MA, USA). The general catheter setting consisted of the following: (I) a steerable 6F decapolar diagnostic catheter (Inquiry™, 5 mm spacing; St. Jude Medical, Saint Paul, MN, USA) positioned in the coronary sinus and serving as the reference of the Rhythmia™ 3-D electroanatomical mapping system (Boston Scientific Corporation, Marlborough, MA, USA); as for ventricular procedures, an additional 5F quadripolar catheter (IBI Inquiry™; St. Jude Medical) was placed in the right ventricular apex; (II) an expandable, open irrigated 64-pole basket mapping catheter (IntellaMap Orion™, Boston Scientific) comprising of 8 splines with 8 electrodes (electrode spacing 2.5 mm, electrode surface area 0.4 mm2) for atrial as well as ventricular procedures as described elsewhere (14,15); (III) an open-irrigated tip, bidirectional mapping and ablation catheter (IntellaNav MiFi™ OI, Boston Scientific). For pulmonary vein isolation (PVI), catheters were introduced into the left atrium by double transseptal access using a fixed curve long sheath (SL0, 8-F; St. Jude Medical, for ablation catheter) and a long steerable sheath (Zurpaz™, medium curl, 8.5-F, Boston Scientific, for mini basket catheter). Intravenous heparin administration and continuous activated clotting time monitoring were conducted after first access of the left atrium or after introduction of the basket catheter into any chamber of the heart to maintain an activated clotting time >300 seconds.
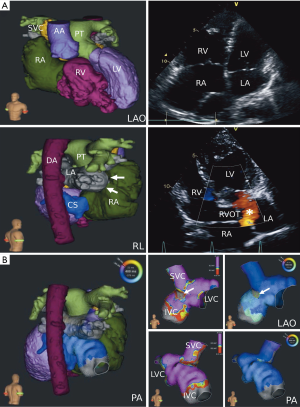
Ultra-high density 3-D mapping
HDM of the cardiac chambers was performed with the basket catheter acquiring data during sinus rhythm, during pacing from the coronary sinus and/or during ongoing arrhythmia. If patients were in sinus rhythm at the beginning of the procedure, arrhythmias were induced by burst pacing or programmed stimulation. Electrogram annotation was performed automatically by the mapping system using the following criteria for beat acceptance: (I) cycle length stability: ±10 ms; (II) stability of relative timing of reference electrograms: ±5 ms; (III) respiration gating: automatic measurement and filtering of motion above maximum inspiration movement by change of impedance of the ECG-electrodes; (IV) electrode location stability (catheter stability): 2–3 mm. A detailed description of the systems electrogram annotation has been published before (16). Maps were considered complete when the entire chamber anatomy was reconstructed with the best achievable electrode-tissue contact. Complete activation maps of macroreentrant supraventricular tachycardia or ventricular tachycardia (VT) were defined as mapping of ≥90% of the cycle length. If necessary, additional mapping was performed at the operator’s discretion using the ablation catheter.
Catheter ablation
Radiofrequency current was applied with a maximum power of 30–40 W and an irrigation rate of 17–30 mL/min for up to 120 seconds not exceeding an upper temperature limit of 48 °C. The energy level was reduced to 20–25 W at the posterior left atrium wall. After restoration of sinus rhythm, pacing maneuvers and pharmacological arrhythmia provocation were repeatedly performed to test for non-inducibility of arrhythmias until atrial refractoriness or 200 ms cycle length were reached. A bidirectional block was confirmed whenever linear lesions were generated and additional activation mapping was performed if necessary (17). When indicated, PVI or cavotricuspid isthmus (CTI) ablation was added to the procedure as described previously (18,19). Subsequently, endpoints of previously performed ablations were verified and completed if necessary.
Offline HDM analysis
Maps were analyzed offline after the procedures using a non-commercial version of the Rhythmia™ software. Lumipoint™ algorithms were applied. These included the ‘activation search’ feature (highlighting regions of the map which contain electrograms that show activity in the time-of-interest), the ‘complex activation’ feature (highlighting regions of the map that both activate within the time-of-interest period and exhibit multiple components of activation), the ‘Skyline graph’ feature (reflecting the size of the depolarizing region throughout the mapping window), the ‘split activation’ feature (highlighting areas of the map that exhibit discontinuous activation), as well as the ‘trend tool’ (a visualization concept aiming to identify gaps in lines). Lumipoint™ confidence was applied aiming to estimate the likelihood or probability of an electrogram having a genuine biological activation at a particular point in time.
Follow-up
Patients underwent outpatient clinical visits 3 to 6 months after ablation. Pacemaker and implantable cardioverter-defibrillator were followed up through remote monitoring. 12-channel-electrocardiograms (ECGs) as well as 24-hour Holter ECGs were conducted in all patients without implantable devices. Any recurrence of arrhythmia >30 seconds during Holter ECG or registration of atrial high-rate episodes in patients with implantable devices were regarded as a recurrence (20). Additional outpatient clinical visits were conducted whenever symptomatic episodes occurred suggestive of arrhythmia recurrence or in case of a deteriorated clinical condition of the patient.
Statistical analysis
All continuous variables were tested for normal distribution using the Shapiro-Wilk test. Parametric data are expressed as mean ± standard error of the mean, whereas results of non-parametric data are provided as medians with interquartile ranges (IQR). If applicable, minimum and maximum values are also indicated. All analyses were performed using Graphpad Prism 8® (Graphpad Inc., La Jolla, CA, USA) and Microsoft Excel. For calculation of differences of continuous data between the CHD group of moderate and great complexity, the Mann-Whitney U test was used. A P value <0.05 was considered statistically significant.
Results
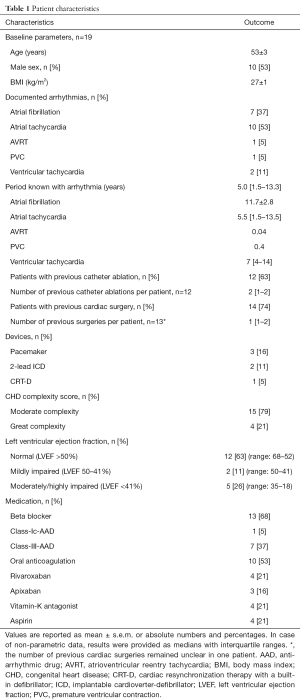
Patient characteristics
We studied data from 19 consecutive adult patients with CHD (53±3 years; 10 male; n=14 with previous cardiac surgery/n=12 with catheter ablation procedures) and an indication for catheter ablation of cardiac arrhythmias. Detailed patient characteristics are presented in Table 1. CHD conditions consisted of patients with moderate complexity in 15 (79%) and great complexity in 4 patients (21%). A detailed overview including underlying CHD and current ACHD AP classification, previous interventions and detected arrhythmia is presented in Table 2. Atrial tachycardia (AT) was diagnosed in 10 patients (53%) and atrial fibrillation (AF) in 7 patients (37%). Atrioventricular reentrant tachycardia was detected in 1 patient (5%). Ventricular arrhythmias were observed in 3 patients (16%) [frequent premature ventricular contractions: n=1; ventricular tachycardia (VT): n=2]. Reduced left ventricular ejection fraction was detected in 5 patients (26%), of which 3 presented with CHD of great complexity.
Full table
Full table
Procedural characteristics
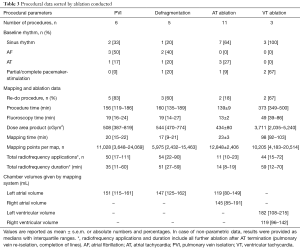
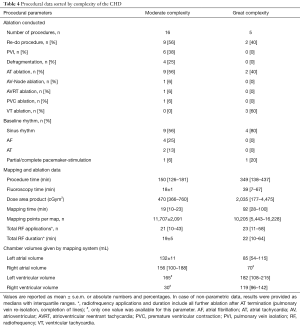
Detailed procedural characteristics sorted by ablation conducted and CHD complexity are shown in Tables 3 and 4. In total, 21 procedures were conducted with a mean procedural time of 189±23 minutes, mean fluoroscopy time of 23±4 minutes and a mean area dose product of 992±279 cGym2. Eleven out of 21 procedures (52%) displayed a re-do catheter ablation (AF: n=5; AT: n=3; VT: n=2; atrioventricular node ablation: n=1). Moreover, one patient with a history of CTI ablation presented for first-time AF ablation (Table 2, No. 4).
Full table
Full table
At the beginning of 15 procedures, sinus rhythm or intermittent/permanent pacemaker stimulation were present (67%; supraventricular n=11; ventricular n=4) with subsequent arrhythmia induction during these procedures in all but 2 patients who underwent atrial fibrillation ablation. Clinical arrhythmia was present at the beginning of the electrophysiological study in 6 procedures (29%). Non-inducibility of stable AT was accomplished in 7 out of 11 procedures. In ventricular tachycardia ablation, non-inducibility was reached in 1 out of 3 procedures, respectively. No arrhythmia induction attempt was performed after ablation in one procedure of AT and VT ablation each due to poor hemodynamic toleration and advanced procedure duration, whereas ablation of the critical isthmus based on activation and voltage map was successful with consecutive arrhythmia termination.
Acute procedural success was reached in all but one patient. In 1 patient with an Ebstein’s anomaly (Table 2, No. 11), severely enlarged right atrium (volume: 257 mL) and moderate tricuspid insufficiency, postprocedural monitoring on the intensive care unit was initiated after beginning cardiac decompensation. This patient fully recovered rapidly without prolonged hospitalization. Another patient experienced retroperitoneal and scrotal hemorrhage after VT ablation and received successful conservative treatment. No other periprocedural complications occurred.
3-D electroanatomical high-density mapping and ablation
In total, 56 complete electroanatomical high-density maps with a median number of 11,073 (IQR, 5,217–16,593) mapping points per map were generated. Per procedure 1–4 critical areas were defined which were treated with 21 (IQR, 11–45) radiofrequency energy applications within a median ablation time of 16 (IQR, 12–45) minutes.
Atrial tachycardia ablation
In 11 procedures with AT, 19 activation maps were completed during arrhythmia with a range of 1–3 reentry circuits per procedure. Baseline AT cycle length in these patients was 301±22 ms (total range, 210–470 ms). AT mechanisms displayed as following: Localized reentry was detected in 3 (27%) and macroreentry in 7 AT procedures (64%) (Figure 2), whereas focal AT was present in 1 procedure (9%) (Figure 3). Critical AT arrhythmia substrate was detected in the left atrium in 6 (55%) and in the right atrium in 5 procedures (45%). Critical sites were distributed among the left atrium (roof: n=2; left pulmonary veins: n=1; mitral anulus: n=1; ridge: n=1; systemic venous antrum: n=1), whereas the majority of sites were located at the free wall (n=3; 75%) in the right atrium. Ablation resulted in acute successful arrhythmia termination in 10 out of these initial procedures. In one patient with transposition of the great arteries and previous Mustard procedure (Table 2, No. 17), no critical arrhythmic site of the clinically predominant tachycardia was detected in the left atrium while transbaffle puncture (suspected right atrial origin) was initially disapproved by this patient. After recurrence of arrhythmia during a follow up of 97 days, the patient approved inferior transbaffle puncture. Ablation of 3 ATs was conducted in the antero-lateral systemic as well as pulmonary atrium with successful acute termination and freedom from any arrhythmia during a 3 month follow-up period.
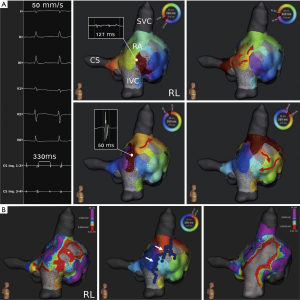
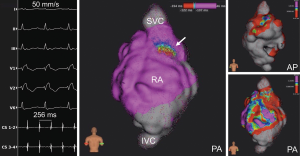
Atrial fibrillation ablation
AF ablation was performed in 7 procedures (33%), where partial or complete reconnection of pulmonary veins after previous PVI was detected in 6 patients [left pulmonary veins: n=3 (50%); right pulmonary veins: n=5 (83%)]. Ablation of complex fractionated atrial electrograms (CFAE) was conducted in 5 procedures (CFAE ablation only due to isolated pulmonary veins: n=1; CFAE + re-do PVI: n=4) and a re-do PVI only in 2 procedures. A total of 20 CFAE sites were registered (3.3±1.1 per procedure) equally distributed among the left atrium (roof: n=4; anterior wall: n=3; septal wall: n=3; lateral wall: n=1; inferior wall: n=2; posterior wall: n=2; ridge/left atrial appendage: n=4 coronary sinus: n=1). Catheter ablation resulted in AF termination in 6 patients, with 1 patient receiving subsequent amiodarone application and electrocardioversion for sinus rhythm restitution.
Ablation of ventricular tachycardia and premature ventricular contractions
A total number of 3 VT procedures were conducted in 2 patients (Table 2, No. 16 and No. 19; Figure 4A). Overall, 7 morphologies were registered with a median VT cycle length of 380 (IQR, 345–400) ms and consecutive completion of 7 maps (right ventricle: n=1; left ventricle: n=6). Ablation resulted in successful clinical VT termination in both patients. In one patient with surgically treated tetralogy of Fallot (Table 2, No. 14), frequent premature ventricular contractions were addressed in 2 localizations (left ventricular apex, ventricular outflow tract).
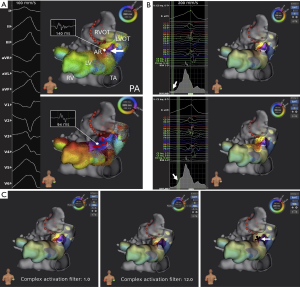
Postprocedural signal processing
Postprocedural signal processing using Lumipoint™ allowed automated annotation of fractionated signals within specific windows of interest supporting arrhythmia mechanism characterization and identification of critical isthmus sites. Independent of activation time annotation, the ‘activation search’ feature identified the isthmus where the automatically annotated map did not clearly indicate it (Figure 4B). The ‘complex activation search’ feature identified a subset of late potentials with fractionated signals during substrate mapping in all patients undergoing VT ablation (Figure 4C). Overall, Lumipoint™ successfully displayed critical isthmus sites in 10 out of 15 procedures (67%) and 20 out of 27 completed AT, atrioventricular reentrant tachycardia and VT maps (74%).
Postprocedural signal processing could not be applied due to subsequent partial (n=1) or complete (n=2) arrhythmia mapping using the ablation catheter in 3 out of 15 procedures (20%). Moreover, critical isthmus detection using Lumipoint™ was not possible in 2 out of 15 procedures (13%) with microreentrant AT and intermittent AF. In both procedures, postprocedural signal processing using Lumipoint™ was not applicable due to repeatedly occurring rapid degeneration of AT into AF, which consecutively lead to incomplete generation of activation maps during the clinically relevant AT. Nevertheless, ablation guided by middiastolic low amplitude fractionated signals resulted in acute success in both procedures.
Using the ‘split activation’ and ‘trend tool’ feature, gap confirmation in a previously set CTI ablation line was successful in a patient with recurrent CTI-dependent macroreentrant AT (Table 2, No. 5; Figure 5). Two right-atrial accessory pathways were also displayed by activation mapping including the Lumipoint™ software and successfully treated in a patient with atrioventricular reentrant tachycardia (Table 2, No. 1).
Follow-up
Acute procedural success was achieved in all but one patient. One patient was lost during follow-up due to a considerable distance to the patient’s residence. During a median follow-up time of 140 (IQR, 91–190) days, recurrence of AF/AT/VT after a single HDM-guided procedure occurred in 4, 4, and 2 patients, respectively. Two out of these patients with AF/AT showed different arrhythmias (compared with the initial HDM-guided procedure) at follow-up. When comparing patients with CHD of moderate and great complexity, no statistically significant difference regarding arrhythmia recurrence was registered between both groups [n=6 (40%) vs. n=4 (100%); P=0.09].
For treatment of recurrent arrhythmia, 4 patients received an antiarrhythmic drug treatment only [beta-blocker: n=4 (100%); flecainide: n=1 (25%)]. An additional ablation procedure was conducted in 6 patients (AF: n=2; AT: n=3; VT: n=1) within a median time of 176 (IQR, 73–313) days between both procedures. Five out of these 6 patients showed freedom of arrhythmia after a second HDM-guided ablation procedure, with one patient showing recurrence of AF. No long-term complications were observed in all patients.
Discussion
The present findings suggest that HDM of cardiac arrhythmias can provide detailed insights into arrhythmia substrate and mechanisms. Acquired maps reflected underlying arrhythmogenic substrates with high spatial resolution and allowed target-orientated activation mapping of complex focal as well as macro- and microreentrant arrhythmias. Additionally, novel automated annotation algorithms supported rapid identification of regions critical for arrhythmia preservation. This might facilitate catheter ablation in patients with moderate to great CHD.
The role of catheter ablation in CHD
Supraventricular as well as ventricular tachycardias are a leading cause of hospitalization and predictor of mortality in patients with CHD (21,22). Especially in patients with CHD and pulmonary arterial hypertension, occurrence of supraventricular arrhythmia may be regarded as a progression of hemodynamic deterioration (23). Antiarrhythmic drug management is often limited in CHD, as it is mostly adapted from clinical studies conducted in patients with acquired heart diseases. No randomized controlled trials are available for specific guidance of antiarrhythmic drug treatment due to the heterogeneity and continued evolution of CHD in adults (1). Beta-blockers are probably useful, while sotalol and amiodarone should be avoided—at least as first-intention treatment—because of the risk of proarrhythmia and potential long-term side effects (24-26). In line with recent international consensus, amiodarone may be considered for AF and AT recurrence prevention in patients with CHD and ventricular dysfunction, hypertrophy of systemic ventricle, or coronary artery disease, in whom catheter ablation fails or is otherwise no option. Class I antiarrhythmic drugs may be harmful in case of a diseased ventricle (26,27). Therefore, catheter ablation guided by electro-anatomical 3D-mapping is regarded as the first-line therapy and is preferred to long-term pharmacological treatment in CHD related arrhythmias whenever no reversible hemodynamic or other factors can be addressed (1).
Clinical implications of HDM in patients with CHD
Corrected anatomy after pediatric cardiac surgery, abnormal anatomical localization or disturbances within the conduction system and iatrogenic scarring can create complex arrhythmogenic substrate (28) while multiple arrhythmia mechanisms are frequently present in patients with CHD (26).
Conventional point-by-point 3D electro-anatomical mapping has been developed more than two decades ago and has been found to produce favorable outcome in CHD-related catheter ablation (6). However, the collection of even a few hundred points is time-consuming and still often need additional manual annotation. Moreover, the electrode size, spacing and design within most presently used single-tip catheters (in conjunction with classical signal to noise ratios within classical 3-D mapping algorithms) only show limited electrogram resolution and can be additionally limited by far-field signals (29).
Noteworthy, within the last 5 years HDM has been found to open up new avenues in the treatment of cardiac arrhythmias in a wide range of patients (30). HDM deepened our understanding of cardiac electrophysiology and arrhythmogenesis by rapid acquisition of thousands of activation points independent of activation time. Small and closely spaced electrodes enable characterization of arrhythmogenic substrate with high spatiotemporal resolution. The low noise level (0.01 mV) allows registration of electrograms with very low amplitude, which for instance can improve identification of reconnection gaps in pulmonary veins (31) or concealed low voltage propagation within the pulmonary vein antra (32) in patients with AF. Moreover, HDM can enhance the precise display of complex arrhythmia mechanisms in atrial as well as ventricular tachycardia (8,33). It becomes now evident that multiple isthmus, double-loop and localized reentries are more frequently present than previously thought (34,35). This is of importance as HDM can indicate conduction and substrate variability where long post-pacing intervals during entrainment alone can be misleading (19,30). HDM furthermore supports fast arrhythmogenic substrate characterization in patients with either hemodynamically not tolerated, non-inducible or short lasting episodes of VT (9,15), as pacemapping of VT circuits can be aggravated in case of multiple entrances and exits (8).
And entrainment mapping alone can overestimate isthmus dimensions in some cases (36).
As a result, several HDM guided ablation strategies aiming to avoid extensive radiofrequency current delivery and minimize the risk of a stiff left atrial syndrome have been proposed (37). These recent developments have also been found to be useful in the treatment of patients with CHD, but have only been reported in a limited number of patients (12,38). Not surprisingly, the here presented data also show that arrhythmia-free survival following HDM-guided ablation is not guaranteed. Therefore, further studies are needed to investigate long-term efficacy.
Safety
HDM has been found to result in similar safety in comparison to classical point-by-point mapping in various settings (9,18). The recently published TRUE HD prospective multicenter study (39) as well as smaller studies (40) confirmed acute safety, effectiveness and feasibility of HDM for catheter ablation of a wide spectrum of arrhythmias. Device-related serious adverse events were reported to be 0.57–1.25% (39,40) and might be even lower as supported by our experience from more than 500 procedures. Our findings regarding radiation exposure and safety outcome were comparable to previously published studies having used conventional point-by-point mapping in adult patients with different CHD-related arrhythmias (41,42).
Lumipoint™ algorithm
The Lumipoint™ automated annotation algorithm may enhance interpretation, detection and ablation of complex arrhythmogenic substrate using the following features: First, the ‘activation search’ feature highlights areas with simultaneous depolarization in the activation map independent of activation time annotation. As annotation of single activation signals may be insufficient at sites with complex multiple fractionation of signals (43), demarcation of multiple simultaneously activated regions can improve the characterization of complex arrhythmogenic substrate and potential arrhythmia-maintaining regions. Moreover, farfield signals can be automatically annotated by the algorithm, consecutively improving ablation guidance (44). Second, the area of fewest overall depolarized tissue can be marked by using the skyline feature (‘skyline valley’) within a window of interest, which displays the critical isthmus of AT or VT circuits (44,45). As standardized approaches for system adjustments of the different Lumipoint™ features are still to be developed, further studies are needed for optimized utilization of the Lumipoint™ algorithm.
Limitations
The present study has some limitations that need to be addressed. Most importantly, this is a single-center experience investigating the usefulness of novel automated annotation algorithms in a relatively small number of patients with CHD in a retrospective manner. Moreover, the current study does not compare the efficacy of HDM with conventional methods of mapping and ablation. Due to strict exclusion criteria about one-third of maps could not be used for postprocedural signal processing using Lumipoint in this patient series. This was mainly driven by incomplete activation mapping because of usage of the ablation catheter instead of the IntellaMap Orion™ catheter.
Conclusions
The findings of the present study suggest that HDM provides detailed insights into CHD-related arrhythmia substrate and mechanisms. In conjunction with novel automated annotation algorithms this might facilitate tailored catheter ablation in patients with moderate to great CHD complexity. Further studies are needed to assess long-term efficacy of HDM as a treatment modality of arrhythmias in CHD.
Acknowledgments
We thank Ms. Lydia Merbold for excellent technical assistance during ultra-high density mapping using the Rhythmia™ system and Lumipoint™ software application during ablation procedures and offline analysis.
Footnote
Conflicts of Interest: C Meyer: speaker for Boston Scientific and Abbott; consultant for Biosense Webster. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local ethics committee of the University of Hamburg (No. WF-79/16) and informed consent was taken from all patients.
References
- Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital Heart Disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719-53. [Crossref] [PubMed]
- Meyer C, Martinek M, Winter S, et al. Arrhythmias in patients with surgically corrected tetralogy of Fallot. Herzschrittmacherther Elektrophysiol 2010;21:189-95. [Crossref] [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease. Circulation 2019;139:e637-97. [PubMed]
- Maury P, Sacher F, Rollin A, et al. Ventricular arrhythmias and sudden death in tetralogy of Fallot. Arch Cardiovasc Dis 2017;110:354-62. [Crossref] [PubMed]
- Triedman JK, Alexander ME, Love BA, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol 2002;39:1827-35. [Crossref] [PubMed]
- Delacretaz E, Ganz LI, Soejima K, et al. Multiple atrial macro–re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol 2001;37:1665-76. [Crossref] [PubMed]
- Ueda A, Suman-Horduna I, Mantziari L, et al. Contemporary Outcomes of Supraventricular Tachycardia Ablation in Congenital Heart Disease: A Single-Center Experience in 116 Patients. Circ Arrhythm Electrophysiol 2013;6:606-13. [Crossref] [PubMed]
- Martin R, Maury P, Bisceglia C, et al. Characteristics of Scar-Related Ventricular Tachycardia Circuits Using Ultra-High-Density Mapping. Circ Arrhythm Electrophysiol 2018;11:e006569. [Crossref] [PubMed]
- Nührich JM, Kaiser L, Akbulak RÖ, et al. Substrate characterization and catheter ablation in patients with scar-related ventricular tachycardia using ultra high-density 3-D mapping. J Cardiovasc Electrophysiol 2017;28:1058-67. [Crossref] [PubMed]
- Gunawardene M, Munkler P, Eickholt C, et al. A novel assessment of local impedance during catheter ablation: initial experience in humans comparing local and generator measurements. Europace 2019;21:i34-42. [Crossref] [PubMed]
- Kaiser L, Jularic M, Akbulak RÖ, et al. Catheter ablation of hemodynamically unstable ventricular tachycardia in ischemic cardiomyopathy using high-resolution mapping. Clin Case Rep 2017;5:389-93. [Crossref] [PubMed]
- Xue Y, Liu Y, Liao H, et al. Evaluation of Electrophysiological Mechanisms of Post-Surgical Atrial Tachycardias Using an Automated Ultra-High-Density Mapping System. JACC Clin Electrophysiol 2018;4:1460-70. [Crossref] [PubMed]
- Salukhe TV, Willems S, Drewitz I, et al. Propofol sedation administered by cardiologists without assisted ventilation for long cardiac interventions: an assessment of 1000 consecutive patients undergoing atrial fibrillation ablation. Europace 2012;14:325-30. [Crossref] [PubMed]
- Anter E, Tschabrunn CM, Contreras-Valdes FM, et al. Pulmonary vein isolation using the Rhythmia mapping system: Verification of intracardiac signals using the Orion mini-basket catheter. Heart Rhythm 2015;12:1927-34. [Crossref] [PubMed]
- Sultan A, Bellmann B, Luker J, et al. The use of a high-resolution mapping system may facilitate standard clinical practice in VE and VT ablation. J Interv Card Electrophysiol 2019. [Crossref] [PubMed]
- Schaeffer B, Hoffmann BA, Meyer C, et al. Characterization, Mapping, and Ablation of Complex Atrial Tachycardia: Initial Experience With a Novel Method of Ultra High-Density 3D Mapping. J Cardiovasc Electrophysiol 2016;27:1139-50. [Crossref] [PubMed]
- Shah D, Haissaguerre M, Takahashi A, et al. Differential pacing for distinguishing block from persistent conduction through an ablation line. Circulation 2000;102:1517-22. [Crossref] [PubMed]
- Rottner L, Metzner A, Ouyang F, et al. Direct Comparison of Point-by-point and Rapid Ultra High-Resolution Electroanatomical Mapping in Patients Scheduled for Ablation of Atrial Fibrillation. J Cardiovasc Electrophysiol 2017;28:289-97. [Crossref] [PubMed]
- Pathik B, Lee G, Sacher F, et al. New Insights Into an Old Arrhythmia: High-Resolution Mapping Demonstrates Conduction and Substrate Variability in Right Atrial Macro-Re-Entrant Tachycardia. JACC Clin Electrophysiol 2017;3:971-86. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Koyak Z, Harris L, De Groot JR, et al. Sudden Cardiac Death in Adult Congenital Heart Disease. Circulation 2012;126:1944-54. [Crossref] [PubMed]
- Verheugt CL, Uiterwaal CSPM, Van Der Velde ET, et al. The emerging burden of hospital admissions of adults with congenital heart disease. Heart 2010;96:872-8. [Crossref] [PubMed]
- Drakopoulou M, Nashat H, Kempny A, et al. Arrhythmias in adult patients with congenital heart disease and pulmonary arterial hypertension. Heart 2018;104:1963-9. [Crossref] [PubMed]
- Thorne SA, Barnes I, Cullinan P, et al. Amiodarone-Associated Thyroid Dysfunction: Risk Factors in Adults With Congenital Heart Disease. Circulation 1999;100:149-54. [Crossref] [PubMed]
- Moore BM, Cordina RL, McGuire MA, et al. Efficacy and adverse effects of sotalol in adults with congenital heart disease. Int J Cardiol 2019;274:74-9. [Crossref] [PubMed]
- Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease. Heart Rhythm 2014;11:e102-e65. [Crossref] [PubMed]
- Fish FA, Gillette PC, Benson DW. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. J Am Coll Cardiol 1991;18:356-65. [Crossref] [PubMed]
- Ernst S, Saenen J, Rydman R, et al. Utility of noninvasive arrhythmia mapping in patients with adult congenital heart disease. Card Electrophysiol Clin 2015;7:117-23. [Crossref] [PubMed]
- Acosta J, Penela D, Andreu D, et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace 2018;20:512-9. [Crossref] [PubMed]
- Meyer C. High-density mapping-based ablation strategies of cardiac rhythm disorders: the RHYTHMIA experience at new horizons. Europace 2019;21:iii7-10. [Crossref] [PubMed]
- García-Bolao I, Ballesteros G, Ramos P, et al. Identification of pulmonary vein reconnection gaps with high-density mapping in redo atrial fibrillation ablation procedures. Europace 2018;20:f351-8. [Crossref] [PubMed]
- Segerson NM, Lynch B, Mozes J, et al. High-density mapping and ablation of concealed low-voltage activity within pulmonary vein antra results in improved freedom from atrial fibrillation compared to pulmonary vein isolation alone. Heart Rhythm 2018;15:1158-64. [Crossref] [PubMed]
- Mantziari L, Butcher C, Shi R, et al. Characterization of the Mechanism and Substrate of Atrial Tachycardia Using Ultra-High-Density Mapping in Adults With Congenital Heart Disease: Impact on Clinical Outcomes. J Am Heart Assoc 2019;8:e010535. [Crossref] [PubMed]
- Takigawa M, Derval N, Maury P, et al. Comprehensive Multicenter Study of the Common Isthmus in Post-Atrial Fibrillation Ablation Multiple-Loop Atrial Tachycardia. Circ Arrhythm Electrophysiol 2018;11:e006019. [Crossref] [PubMed]
- Takigawa M, Derval N, Frontera A, et al. Revisiting anatomic macroreentrant tachycardia after atrial fibrillation ablation using ultrahigh-resolution mapping: Implications for ablation. Heart Rhythm 2018;15:326-33. [Crossref] [PubMed]
- Anter E, Tschabrunn CM, Buxton AE, et al. High-Resolution Mapping of Postinfarction Reentrant Ventricular Tachycardia. Circulation 2016;134:314-27. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Ernst S, Cazzoli I, Guarguagli S. An initial experience of high-density mapping-guided ablation in a cohort of patients with adult congenital heart disease. Europace 2019;21:i43-53. [Crossref] [PubMed]
- Hindricks G, Weiner S, McElderry T, et al. Acute safety, effectiveness, and real-world clinical usage of ultra-high density mapping for ablation of cardiac arrhythmias: results of the TRUE HD study. Europace 2019.655-61. [Crossref] [PubMed]
- Lackermair K, Kellner S, Kellnar A, et al. Initial single centre experience with the novel Rhythmia(c) high density mapping system in an all comer collective of 400 electrophysiological patients. Int J Cardiol 2018;272:168-74. [Crossref] [PubMed]
- Liang JJ, Frankel DS, Parikh V, et al. Safety and outcomes of catheter ablation for atrial fibrillation in adults with congenital heart disease: A multicenter registry study. Heart Rhythm 2019;16:846-52. [Crossref] [PubMed]
- Anguera I, Dallaglio P, Macías R, et al. Long-Term Outcome After Ablation of Right Atrial Tachyarrhythmias After the Surgical Repair of Congenital and Acquired Heart Disease. Am J Cardiol 2015;115:1705-13. [Crossref] [PubMed]
- Irie T, Yu R, Bradfield JS, et al. Relationship Between Sinus Rhythm Late Activation Zones and Critical Sites for Scar-Related Ventricular Tachycardia: Systematic Analysis of Isochronal Late Activation Mapping. Circ Arrhythm Electrophysiol 2015;8:390-9. [Crossref] [PubMed]
- Martin CA, Takigawa M, Martin R, et al. Use of Novel Electrogram "Lumipoint" Algorithm to Detect Critical Isthmus and Abnormal Potentials for Ablation in Ventricular Tachycardia. JACC Clin Electrophysiol 2019;5:470-9. [Crossref] [PubMed]
- Takigawa M, Martin CA, Derval N, et al. Insights from atrial surface activation throughout atrial tachycardia cycle length: A new mapping tool. Heart Rhythm 2019. [Crossref] [PubMed]


