
Patients over 70 years of age with moderate ischemic mitral regurgitation undergoing surgical revascularization plus mitral valve repair: insights from a single-center study of propensity-matched data
Introduction
Ischemic mitral regurgitation (IMR) is a subset of secondary mitral regurgitation (MR) caused by coronary artery disease, that may be acute (e.g., papillary muscle ischemia or rupture) or chronic, in which adverse remodeling occurs after myocardial infarction (1,2). The development of IMR is associated with an increased risk of mortality and heart failure, and this risk increases with the severity of regurgitation (3). Surgical approaches of moderate IMR include coronary artery bypass grafting (CABG) alone and mitral valve procedure at the time of CABG. Previous studies have reported that CABG alone is adequate for patients with moderate IMR (2-4); nevertheless, CABG alone cannot be sufficient to eliminate MR (5). Although recurrent MR does occur, mitral valve repair at the time of CABG is an effective therapy for moderate IMR because it not only eliminates MR immediately after surgery but also improves cardiac function and reduces MR at follow-up (4-7).
With the acceleration of population aging, the advancement of surgical techniques, and the improvements in perioperative management, the current CABG population has seen increased preponderances of aging and post-infarction complications (5,8,9). Elderly patients, compared with the young, have a higher burden of surgical risk factors with reduced functional capacity and increased comorbidities conditions, and may have worse clinical outcomes (10-12). So, a question arises: whether elderly patients with moderate IMR receive poor clinical outcomes after mitral valve procedure at the time of CABG? However, few studies so far have focused on the comparison of clinical outcomes between elderly patients and non-elderly ones with moderate chronic IMR undergoing CABG plus mitral valve procedure.
In this study, 324 eligible patients with moderate chronic IMR who underwent CABG plus mitral valve repair in a single center were included and were entered into an elderly group or a control group according to patients’ age (over 70 years or not). After propensity-score matching (1:1), in-hospital and follow-up clinical outcomes were analyzed to evaluate the impacts of age-based grouping (elderly patients vs. non-elderly ones) on clinical outcomes of CABG plus mitral valve repair in the treatment of moderate chronic IMR.
Methods
Grade of IMR
Standard transthoracic echocardiography was performed within 3 days before surgery to determine the etiology and severity of MR. MR grade was defined as mild (small central jet area <20% LA on Doppler, and vena contracta <3.0 mm), moderate [effective regurgitant orifice area (EROA) <20 mm2, regurgitant volume <30 mL, and regurgitant fraction <50%], or severe (EROA ≥20 mm2, regurgitant volume ≥30 mL, and regurgitant fraction ≥50%) (13). MR was graded independently by 2 level-3 readers (discrepancies were resolved by a third reader as needed) as suggested by current guidelines with an integrated approach using all available parameters.
Study protocol
This study protocol was approved by the ethics committee of Zhongshan Hospital Fudan University (No. B2018-118) and was consistent with the Declaration of Helsinki.
Consecutive documented patients with moderate chronic IMR who underwent CABG plus mitral valve repair during the study period (from January 2008 to December 2017) were assessed for study eligibility by checking the following criteria: (I) prior myocardial infarction by electrocardiogram or regional wall motion abnormalities by echocardiography; (II) structurally normal mitral valve; and (III) sinus rhythm. Exclusion criteria were moderate or severe aortic stenosis or insufficiency, concomitant tricuspid annuloplasty, mitral valve organic pathology (prolapse, rheumatic, endocarditis, leaflet perforation, or extensive annular calcification), valve prosthesis, Marfan syndrome, and unstable clinical conditions.
Based on patients’ age, all eligible patients were entered into an elderly group (patients over 70 years of age) or a control group (patients with age of 70 or less). Baseline and surgical characteristics were investigated and analyzed. Patients were regularly followed up at 3 and 6 months following surgery, and thereafter at 6-month intervals. The in-hospital and follow-up clinical outcomes were compared after propensity score matching (1:1). In-hospital outcomes were surgical mortality and major postoperative morbidity (14,15). Major postoperative morbidity included myocardial infarction associated with CABG (15), intra-aortic balloon pump (IABP) support, redo for bleeding, blood transfusion, pulmonary complications (including postoperative pneumonia and respiratory failure) (16,17), stroke (14), renal failure requiring hemodialysis, and deep sternal wound infection (15). Additionally, follow-up outcomes included all-cause death and recurrent MR. All-cause death rather than cardiac death was chosen because it was the most robust and unbiased index which exempted us from misreading the cause of death with the subjective and sometimes inaccurate medical records. Recurrent MR was defined as the occurrence of moderate or more MR at follow-up determined by transthoracic echocardiography.
Surgical procedures
All surgical procedures were performed through a midline sternotomy. The internal mammary artery was harvested in a skeletonized or a pedicled fashion, and the left internal mammary artery grafting to the left anterior descending artery has been the first choice. Saphenous vein and radial artery were harvested with an open technique, and sequential or separate aortocoronary bypass grafting was performed in the remaining coronary arteries. The details of the on-pump CABG procedure were consistent with those of previous studies (18). The quality of anastomosis was assessed after grafting with a transit-time flow probe (Medistim Butterfly Flowmeter, Oslo, Norway) during the operation.
The mitral valve was exposed through a vertical transseptal approach along the right border of the foramen ovale, leaving the left atrial roof untouched. Ring size was determined after careful measurement of the height of the anterior leaflet, and then downsizing. Rings were inserted using deep U-shaped simple horizontal sutures using Ethibond 2-0 (Ethicon, Inc., Somerville, NJ) or Ti-Cron 2-0 (Syneture, Norwalk, CT). Leaflet repair using the edge-to-edge technique or mitral leaflet augmentation was available. A repair of the sub-valvular apparatus including secondary chordal cutting and/or papillary muscle approximation was sometimes performed as well. The quality of mitral repair was determined by intraoperative transesophageal echocardiography immediately after discontinuation of cardiopulmonary bypass.
Statistics analysis
Perioperative data were obtained from our institutional database and were reviewed using a standard data collection form. Follow-up data were obtained by a clinic visit or telephone. Data collection was performed by trained staff (two people). The trained staff, however, were not informed of the purpose of this study. Normally distributed continuous variables were expressed as the mean ± standard deviation and non-normally distributed continuous variables were expressed as median and interquartile range (IQR). Categorical variables were expressed as frequency distributions and single percentages.
To control for measured potential confounders in the data set, a propensity score (PS) was generated for each patient from a multivariable logistic regression model that was based on 17 baseline characteristics as independent variables with age-based grouping (patients over 70 years vs. patients with age of 70 or less) as a binary dependent variable. The 17 independent variables consisted of 16 variables referring to risk model of EuroSCORE system (19) as listed in Table 1 (including demographics, concomitant diseases and preoperative cardiac status except Euroscore) and the EROA (a key quantitative evaluation indicator of severity of MR). The discrimination power and calibration of the PS model were tested with the c-statistic and the Hosmer-Lemeshow goodness-of-fit. Two pairs of matched patients were obtained using the greedy-matching algorithm to implement nearest-neighbor 1:1, with a caliper width of 0.2 of standard deviation of the logit of the PS. The quality of the match was assessed by comparing selected pretreatment variables in matched patients by use of the standardized mean difference, by which an absolute standardized difference of >10% was suggested to represent meaningful covariate imbalance.
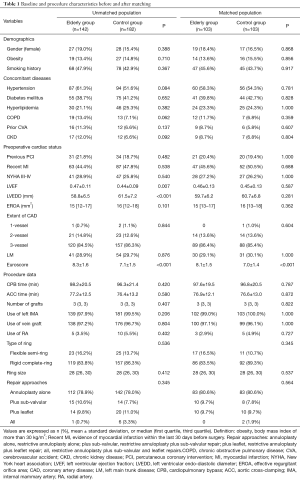
Full table
Baseline differences between groups were assessed before matching using the Mann-Whitney U test or the Student’s t-test for continuous variables and χ2 test or Fisher’s exact test for categoric variables. Group comparisons on the same data after matching were performed with the Wilcoxon rank-sum test or paired t-test for continuous data and the McNemar’s test for categorical data. For the analysis of in-hospital outcomes in the matched population, the McNemar’s test with odds ratios and 95% confidence interval (CI) was used for categorical outcomes. The impacts of grouping (the matched elderly group vs. the matched control group) as independent risk factors on in-hospital outcomes were evaluated using conditional mixed-effects logistic regression analysis. Overall survival and recurrent MR-free survival were estimated using the Kaplan-Meier method with the stratified log-rank test to compare the equality of the survival curves in the PS-matched population. The Cox regression model stratified on the matched pairs was used to estimate the PS-adjusted hazard ratio (HR) and 95% CI of midterm mortality between the two matched groups. A two-sided P value less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS statistical package version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Study population
A total of 338 patients who met the inclusion criteria were reviewed. Fourteen patients were excluded due to moderate or more aortic stenosis or insufficiency (4 patients), concomitant tricuspid annuloplasty (2 patients), non-ischemic mitral valve prolapse (4 patients), and endocarditis or leaflet perforation (4 patients), which left 324 eligible patients for data analysis. Among 324 included patients, 142 patients with age over 70 years were entered into the elderly group, and the remaining 182 patients with age of 70 or less were entered into the control group.
Baseline characteristics are shown in Table 1. The elderly group, compared with the control group, had a higher left ventricular ejection fraction but a smaller left ventricular endo-diastolic diameter (0.47±0.11 vs. 0.44±0.09, P=0.007, and 58.8±6.5 vs. 61.5±7.2 mm, P<0.001, respectively). No significant differences between groups were observed in other baseline characteristics. Compared with the control group, the elderly group had a higher additive Euroscore (8.3±1.6 vs. 7.1±1.5, P<0.001).
Surgical characteristics are presented in Table 1. No significant differences between groups were found with regard to cardiopulmonary bypass time and aortic cross-clamping time. Both the two groups received similar number of grafts (P=0.407). A total of 276 patients (83.8% for the elderly group vs. 86.3% for the control group, P=0.536) underwent the annular repair with downsized rigid complete-ring (Edwards Physio “O” type ring). The median size of ring was 28 mm. Forty-one patients underwent leaflet repair, 36 patients sub-valvular repair, and 254 patients restrictive annuloplasty alone. No significant difference between groups was observed with regard to the use of repair techniques (P=0.345).
Propensity score-matched cohort
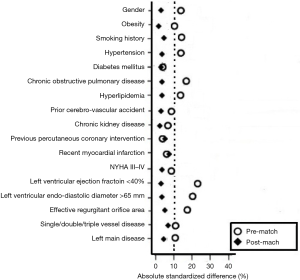
Bivariate analyses were conducted to examine differences in baseline characteristics between patients in the elderly group (n=142) and those in the control group (n=182). Propensity scores were then calculated using multivariate logistic regression model based on the predefined 17 variables. The Hosmer-Lemeshow goodness for the model was 4.72 (P=0.786). Also, the model achieved good discrimination power with the receiver operating curve resulting in the area under the curve of 0.74 (95% CI, 0.64–0.81, P=0.012). Finally, 103 pairs of patients were successfully established in a 1:1 ratio. As shown in Figure 1, the two groups had larger common support domains. As shown in Figure 2, all the absolute standardized differences after matching were <10%, indicating an adequate balance.

The two matched groups were comparable for baseline variables (as shown in Table 1). Note that the matched elderly group, compared with the matched control group, had a higher additive Euroscore (8.1±1.5 vs. 7.0±1.4, P<0.001). Additionally, no significant differences were found between the two matched groups with regard to surgical characteristics.
In-hospital outcomes in the matched population
No significant difference between the two matched groups was found in surgical mortality (5.8% vs. 3.9%, P=0.754). The incidences of major postoperative morbidity are listed in Table 2. The two matched groups received similar incidences of major postoperative morbidity.
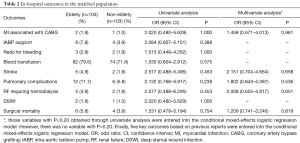
Full table
The impacts of grouping (the matched elderly group vs. the matched control group) on surgical mortality and major postoperative morbidity are also shown in Table 2. Grouping was not an independent risk factor for surgical mortality or major postoperative morbidity (including MI associated with CABG, stroke, pulmonary complications, renal failure requiring hemodialysis, and surgical mortality) via conditional mixed-effects logistic regression analysis.
Midterm outcomes in the matched population
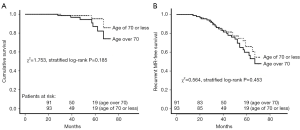
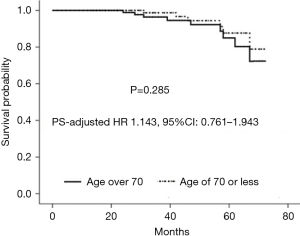
A total of 184 patients received regular follow-up visit with the median duration of 38 months (IQR, 27–56 months). A total of 10 deaths (7 in the elderly group and 3 in the control group) and 41 cases of recurrent MR (21 in the elderly group and 20 in the control group) occurred during follow-up. Only one patient in the matched elderly group experienced repeat mitral valve procedure (mitral valve replacement) due to severe MR at 24 months after initial surgery, whereas 2 patients in the matched control group underwent repeat mitral valve procedure (one patient underwent repeat mitral valve replacement due to mitral valve stenosis at 31 months after initial surgery, and the other received repeat mitral valve replacement due to moderate residual MR and infective endocarditis at 46 months after initial surgery). As shown in Figure 3A, the two matched groups received a similar cumulative survival (χ2=1.753, stratified log-rank P=0.185). There was no significant difference between the two matched groups regarding recurrent MR-free survival (χ2=0.564, stratified log-rank P=0.453) (Figure 3B). Additionally, follow-up death among the matched population was modeled using Cox regression analysis (Figure 4). The result showed that that age over 70 years as compared to age of 70 or less was not associated with midterm mortality (PS-adjusted HR 1.143, 95% CI: 0.761–1.943, P=0.285).
Discussion
It is now a common clinical scenario to see octogenarians undergoing surgical revascularization. Even a healthy elderly is very different physiologically from a young as they have age-related thickening and stiffening of blood vessel walls leading to less elasticity, reduced functional reserve of other organ systems and poor effort tolerance, and may have worse clinical outcomes (20,21). This is probably the first study to compare clinical outcomes of CABG plus mitral valve repair in the treatment of moderate chronic IMR between elderly patients and non-elderly ones. Based on a single-center study of propensity score-matched data, this study demonstrated that elderly patients with moderate IMR undergoing mitral valve repair at the time of CABG received similar in-hospital and midterm outcomes as did non-elderly ones.
An important finding of this study is that elderly patients with moderate chronic IMR who underwent CABG plus mitral valve repair received similar in-hospital outcomes as did non-elderly ones. In this study, after adjusting for differences in baseline characteristics by propensity score matching, no significant differences between the two groups were found with regard to each individual in-hospital adverse event. Also, grouping was not an independent risk factor for surgical mortality or major postoperative morbidity via conditional mixed-effects logistic regression analysis. So, this study suggested that elderly patients with moderate chronic IMR who underwent CABG plus mitral valve repair received favorable in-hospital outcomes. A previous study (20), which evaluated early outcomes of 55 elderly patients with moderate IMR who underwent CABG plus mitral valve procedure, have reported that elderly patients received poor early outcomes (including 30-day mortality of 20% and incidences of renal dysfunction and new onset stroke of 34.55% and 23.64%, respectively). This evidence (poor early outcomes) differed from the results of this study. The reason of this difference may have been the study population, regarding that patients in this study had a higher left ventricle ejection fraction (0.47±0.15 vs. 0.37±0.13) and a lower ratio of NYHA III–IV (28.9% vs. 43.6%) than those in that previous study. Higher left ventricle ejection fraction and better preoperative cardiac function may be contributed to favorable in-hospital outcomes. It was also important to note that the previous study was actually a comparison of off-pump CABG alone versus combined CABG and mitral repair, and was a retrospective cohort study. As a result, there were multiple confounding factors that may have influenced the decision to include mitral repair, and these factors may have influenced the mortality and morbidity rate. In addition, favorable in-hospital outcomes in this study may be related to the improvements of surgical proficiency and perioperative management, which are directly related to high surgery volume. With the improvements of surgical proficiency and perioperative management, postoperative complications were on the decrease. In our center, where adult valve procedure and CABG procedure have now become common practices over the years with the annual procedure volumes of over 2,500 cases and 800 cases, respectively.
Another important finding is that elderly patients received similar midterm outcomes of CABG plus mitral valve repair in the treatment of moderate chronic IMR as did non-elderly ones. In this matched population, elderly patients and non-elderly ones received similar cumulative survival and recurrent MR-free survival. Also, age over 70 years as compared to age of 70 or less was not associated with midterm mortality via the Cox proportional hazard regression. So, this study suggested that elderly patients with moderate chronic IMR who underwent CABG plus mitral valve repair received favorable midterm outcomes. Unfortunately, the research about the comparison of follow-up outcomes of CABG plus mitral valve repair in the treatment of moderate chronic IMR between elderly patients and non-elderly ones has been rarely reported.
Patients in the matched elderly group compared with the matched control group had a significantly higher Euroscore, which was only related to age.
This single-center study based on propensity score-matched data demonstrated that patients over 70 years of age received similar in-hospital and midterm outcomes of CABG plus mitral valve repair in the treatment of moderate chronic IMR as did patients with age of 70 or less. However, further studies were warranted to substantiate and validate the evidence. Additionally, favorable in-hospital and midterm outcomes may support CABG plus mitral valve repair as an alternative approach for treating elderly patients with moderate chronic IMR. However, it did not translate into CABG plus mitral valve repair as a preferred strategy for treatment of elderly patients with moderate chronic IMR relative to CABG alone or CABG plus mitral valve replacement. The strategy of CABG plus mitral valve repair in the treatment of elderly patients with moderate chronic IMR may be a relatively favorable choice in the case of symptoms of cardiac insufficiency and/or concomitant left ventricle enlargement.
There were several limitations to this study. First, it was only a single-center observational study involving a small sample size, which may influence the generalizability of its results. Although using propensity score matching, confounds and selection biases among the two groups cannot be eliminated. A final determination would need a prospective, multi-center study involving a larger sample size. Second, this study showed that adverse event rates were very low in both groups, which may be related to a small sample size. However, only 324 patients with moderate chronic IMR who underwent mitral valve repair at the time of CABG out of 6,488 consecutive patients undergoing CABG with or without concomitant open-heart surgery in our center were included in this study. Third, due to a retrospective observational study, the time course of changes in the left ventricular geometry was not dynamically monitored during follow-up. Also, assessment of quality of life was not conducted at follow-up. Finally, the duration of follow-up was relatively short.
In conclusion, patients over 70 years of age with moderate chronic IMR undergoing combined CABG and mitral valve repair may receive favorable clinical outcomes.
Acknowledgments
Funding: The authors thank for the support from the National Natural Science Foundation of China (Project Number: 81770341).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of Zhongshan Hospital Fudan University (No. B2018-118). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [Crossref] [PubMed]
- Piérard LA, Carabello BA. Ischaemic mitral regurgitation: pathophysiology, outcomes and the conundrum of treatment. Eur Heart J 2010;31:2996-3005. [Crossref] [PubMed]
- Virk SA, Tian DH, Sriravindrarajah A, et al. Mitral valve surgery and coronary artery bypass grafting for moderate-to-severe ischemic mitral regurgitation: Meta-analysis of clinical and echocardiographic outcomes. J Thorac Cardiovasc Surg 2017;154:127-36. [Crossref] [PubMed]
- Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012;126:2502-10. [Crossref] [PubMed]
- Salmasi MY, Harky A, Chowdhury MF, et al. Should the mitral valve be repaired for moderate ischemic mitral regurgitation at the time of revascularization surgery? J Card Surg 2018;33:374-84. [Crossref] [PubMed]
- Timek TA, Hooker RL, Collingwood R, et al. Five-year real world outcomes of GeoForm ring implantation in patients with ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2014;148:1951-6. [Crossref] [PubMed]
- Sun X, Huang J, Shi M, et al. Predictors of moderate ischemic mitral regurgitation improvement after off-pump coronary artery bypass. J Thorac Cardiovasc Surg 2015;149:1606-12. [Crossref] [PubMed]
- Sandoval Y, Sorajja P, Harris KM. Contemporary management of ischemic mitral regurgitation: a review. Am J Med 2018;131:887-95. [Crossref] [PubMed]
- Malhotra AK, Evans AS, Weiner MM, et al. Ischemic mitral regurgitation: a paradigm shift in surgical management? J Cardiothorac Vasc Anesth 2018;32:580-5. [Crossref] [PubMed]
- Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 1997;112:676-92. [Crossref] [PubMed]
- Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816-22. [Crossref] [PubMed]
- Yap CH, Yan BP, Akowuah E, et al. Does prior percutaneous coronary intervention adversely affect early and mid-term survival after coronary artery surgery? JACC Cardiovasc Interv 2009;2:758-64. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Carmona P, Paredes F, Mateo E, et al. Is off-pump technique a safer procedure for coronary revascularization? A propensity score analysis of 20 years of experience. Interact Cardiovasc Thorac Surg 2016;22:612-8. [Crossref] [PubMed]
- Ji Q, Shi Y, Xia L, et al. Revascularization of left coronary system using a skeletonized left internal mammary artery - sequential vs. separate grafting. Circ J 2017;82:102-9. [Crossref] [PubMed]
- Sachdev G, Napolitano LM. Postoperative pulmonary complications: pneumonia and acute respiratory failure. Surg Clin North Am 2012;92:321-44. ix. [Crossref] [PubMed]
- Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest 2011;140:1207-15. [Crossref] [PubMed]
- Ji Q, Mei Y, Wang X, et al. On-pump versus off-pump coronary artery bypass surgery in high-risk patients. Int Heart J 2014;55:484-8. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Malhotra A, Ananthanarayanan C, Wadhawa V, et al. OPCABG for moderate CIMR in elderly patients: a superior option? Braz J Cardiovasc Surg 2018;33:15-22. [PubMed]
- Karavidas A, Lazaros G, Tsiachris D, et al. Aging and the cardiovascular system. Hellenic J Cardiol 2010;51:421-7. [PubMed]


