
Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque
Introduction
Atherosclerotic disease is a leading cause of morbidity and mortality. In carotid atherosclerotic disease, high-risk plaques may rupture and this can lead to neurological sequelae, such as transient ischemic attacks (TIA) and cerebral ischemic infarction (1). Further, when treating symptomatic carotid stenosis, there is an inherent risk of downstream microembolization from manipulating at-risk carotid plaques. High-risk plaques are characterized as “vulnerable” and predispose patients to an elevated risk of cardiovascular events (2).
Duplex ultrasound imaging is well established to assess the degree of carotid stenosis. However, there is clear evidence, that not only the degree of luminal narrowing but also plaque morphology and plaque composition assessed by contrast-enhanced ultrasound (CEUS) play an important role to characterize such vulnerable carotid plaques (3). This review article discusses the use of CEUS for the detection and characterization of carotid vulnerable plaques.
CEUS—basic principles and application
As with every imaging modality, physicians performing CEUS need to familiarize themselves with the technique’s basic principles, in order to be able to accurately interpret findings, to promptly detect artifacts and to successfully address those, for the benefit of the patient. Being an advanced ultrasonographic technique, CEUS makes use of the basic principles of ultrasonography (US) including the emission and reception of an ultrasound beam after its interaction with human tissue. In addition, CEUS is characterized by some additional features, including the ultrasonographic contrast agent (UCA), the hardware needed for the emission of specialized ultrasound beam sequences and the software necessary for post-processing and analysis. In this section, these aspects will be briefly discussed with regards to the carotid application of CEUS.
The UCA
Many UCAs have been made commercially available over the last years, each one with slight differences in composition, interaction with US beam, and variations in the approved indications in different countries. Nevertheless, the structure of all UCAs is ubiquitous and includes a microbubble consisting of an internal gas encapsulated by an external shell made of either phospholipids or albumin. Nowadays, SonoVue® (Bracco) is the UCA most frequently used for vascular applications, being approved for macro-vascular applications including cerebral arteries, extracranial carotid or other peripheral arteries and the portal vein in Europe (4).
SonoVue® (Bracco) is a microbubble of sulfur hexafluoride (SF6) contained inside a monolayer shell of phospholipids. Given that this shell is hydrophilic on the outer surface and hydrophobic on the inner, it is stable and keeps the gas successfully contained within the microbubble. On the other hand, the phospholipid shell is flexible enough to allow changes in size and shape; a phenomenon termed “oscillation” which is crucial for the generation of CEUS signal (5). SonoVue® microbubbles have a mean diameter of 2.5 µm, which is a crucial feature for clinical practice not only because it allows the microbubbles to travel through the entire vascular bed of the human body, reaching the smallest capillary and traversing the pulmonary circulation, but also because it prevents the microbubbles from crossing the endothelium and hence exiting the vascular lumen. As a result, microbubbles are strictly intravascular contrast agents, which is a property of paramount importance, particularly in vascular applications of CEUS (5). Similar to CT and MRI contrast agents, UCAs are administered intravenously but unlike the former, these are never excreted by the kidneys, as the phospholipid capsule is metabolized by the liver and the internal gas is exhaled by the respiratory tract. This feature is valuable as UCAs can be safely used in patients with renal failure, a patient group commonly affected by vascular pathology requiring imaging. The excellent safety profile of UCAs is also complemented by the lack of interaction with the thyroid gland and the very low percentage of allergic reactions (6-8).
CEUS: hardware aspects
The Mechanical Index, commonly known as MI, is one of the most important parameters in US, indicating the insonation power of the ultrasound beam used at any moment during scanning and the “pressure” applied to the tissues scanned. In terms of mathematics, it is defined as the ratio of peak negative pressure with the square root of US frequency. For conventional ultrasonographic techniques such as gray-scale or colour Doppler technique, the MI is typically over 1.6. For CEUS though, a much lower MI should be used for two reasons. First, microbubbles tend to react in a different way to increasing values of MI. Namely, when exposed to a very low MI US beam, microbubbles will oscillate in a linear pattern, meaning that they will expand at the same rate that they will contract. This pattern of response will reflect the same exact frequency with the one initially emitted by the probe. If higher but still low values of MI (ranging from 0.08 to 0.1 for vascular clinical applications) are applied, microbubbles will expand to a higher degree than they contract (a pattern termed non-linear oscillation), thus not only reflecting the baseline frequency but also generating harmonic frequencies. Of course, this reaction cannot last for more than a few minutes, by which time the microbubbles rupture. The same will happen if high MI values (those used for conventional techniques) are applied to microbubbles. The second reason for which a low MI should be used for CEUS is that static tissues (not microbubbles) will produce linear signals when exposed to a low-MI beam but will also produce harmonic frequencies similar to those of microbubbles if a high-MI pulse is emitted. As a consequence, the harmonic frequencies emitted by static tissues will be confounded with those originating from the UCA, making it difficult to exclusively visualize UCA (9-11).
Historically, the first generation of CEUS techniques was based on colour or power Doppler technique and made use of a high-MI intermittent US beam which caused disruption of the microbubbles and hence production of strong signal intensity and improvement of signal-to-noise ratio. Nevertheless, this form of CEUS suffered from all the inherent Doppler artifacts such as overwriting artifact and lacked the real-time nature of currently available techniques (12). Having previously described the principles governing the microbubbles—US interaction, it becomes evident that currently available US machines can use the different frequencies generated by UCA and static tissue to differentiate them. In the pulse-inversion technique, which is currently the most widely used mode of CEUS, the US probe emits two pulses identical in amplitude and frequency but with a difference of 180° in phase. As a result, when those pulses are linearly reflected by static tissues, the second pulse cancels the first, being its inverted copy. However, when these pulses hit microbubbles, harmonic frequencies are produced and reflected towards the transducer. Therefore, hardware is able to selectively visualize microbubbles by suppressing the signal from static tissues at the same time (6).
Two of CEUS’ advantages over CT and MRI are the ability for prolonged scanning of the contrast agent and the real-time scanning pattern characterized by high spatial and temporal resolution. The enhancement pattern of structures with microbubbles is typically recorded in cine loops in everyday clinical practice. Nevertheless, the reperfusion technique is available in the setting of low-MI CEUS. In this technique, a high MI pulse is instantaneously emitted, disrupting every microbubble in the imaging field. In this way, the physician can re-observe the arrival of microbubbles or even quantify them using specialized software, making this technique a useful tool in CEUS such as, identifying or quantifying carotid plaque neovascularization or confirming an area of extravasation (4).
CEUS: software aspects
Once acquired by the transducer, the signal produced by the microbubbles can be visualized on the US machine screen, usually using the dual-screen technique, where the contrast specific image is shown next to a low-MI grayscale image, helping the physicians orient themselves through the scanning field. Currently available devices allow for real-time visualization of microbubbles for more than 4 minutes, thus enabling qualitative evaluation of enhancement. Nevertheless, quantitative analysis of CEUS signal is now feasible using both software integrated in US scanners and commercially available software packages. In this type of analysis, regions of interest can be drawn over parts of the CEUS image and the signal intensity can be plotted against time, leading to the time-intensity curves (TIC) (4,13).
Temporal maximum intensity projection (MIP) is a useful tool for vascular imaging, available in most US scanners. In this mode, a high-MI pulse is initially used to disrupt microbubbles and completely erase the signal in the field-of-view. Subsequently, low-MI continuous scanning is performed, and every frame is aggregated to the previous one, thus creating a complex image containing signal from every microbubble imaged over a period of time. In essence, the scanner acts as an “open shutter camera” and creates detailed images of macro- or micro-vascular anatomy (14).
CEUS: protocol
Ideally, CEUS should be performed after the completion of the conventional US study and the area of interest has been identified. In this way, the scanning location for the CEUS examination can be determined and a focused scan can be performed. Of course, both carotid arteries can be scanned using a single dose of UCA, thanks to the prolonged enhancement time offered by current UCAs. A typical CEUS protocol for carotid CEUS examination is outlined in Table 1.

Full table
CEUS for the detection of plaque surface irregularities and ulceration
CEUS applications in the carotid system provides a significant amount of information both on a macro- and micro-vascular level. Namely, CEUS can accurately delineate carotid plaque irregularities (macro-vascular/luminal level) and can detect intraplaque neovascularization (micro-vascular/intraplaque level) (16). Carotid plaque surface irregularities and ulceration represent an issue of great clinical significance, as many studies have shown significant clinical correlation with the occurrence of neurologic symptoms, embolic signals on transcranial Doppler and stroke (17-19).
Describing carotid plaque surface morphology should be an integral part of every imaging modality used for carotid atherosclerosis evaluation, as also recommended by the American Society of Neuroradiology (ASNR) (20). When it comes to US, this can be done in a straightforward way by assessing the plaque surface morphology. Nonetheless, it should be kept in mind that conventional US techniques such as colour Doppler or power Doppler may occasionally be limited by lower sensitivity to slow blood flow (as in the case of a severely stenotic atherosclerotic lesion or intraplaque neovessels) and inherent technical artifacts such as Doppler angle dependency, inappropriate gain setting and overwriting artifact. Setting a colour gain too high, will lead to the visualization of colour flow signals outside of the vascular lumen or hiding a plaque, while setting it too low will result in inadequate signal filling of the vascular lumen and thus inadequate assessment of the plaque surface morphology (6,17,21). The introduction of UCA has offered a solution to these inherent limitations of US, allowing for more accurate delineation of vascular luminal border and better visualization of plaque surface.
In terms of plaque surface morphology, a carotid atherosclerotic plaque can be subjectively classified as smooth, irregular or ulcerated. The term smooth should be used for those plaques that have a straight and regular surface, with no appreciable irregularities. Irregular plaques are those whose surface is characterized by fluctuations ranging between 0.3 and 0.9 mm, but no overt detectable ulceration. Ulceration represents the highest form of plaque surface irregularity and is defined as a focal cavity measuring at least 1 or 2 mm in depth according to different studies. It should be noted that definitions of ulceration vary depending on the modality used or histology. In the latter instance, which is the gold standard, ulceration is defined as the disruption of the endothelial lining measuring at least 1,000 µm in width, hence leading to the exposure of plaque’s necrotic core to the circulation (8,17,20,22-26). For CEUS, a widely accepted criterion for the diagnosis of ulceration is the projection of microbubbles column within an atherosclerotic plaque measuring at least 1×1 mm. Of note, this criterion has a high sensitivity for carotid plaque ulceration in multiple studies (8,27). For conventional US, the diagnosis of ulceration is less straightforward, with the first criteria introduced back in 1997 defining an ulceration as a cavity which measures ≥2 mm in depth and length, has a well-demarcated basal wall on B-mode and exhibits flow reversal on colour Doppler technique (28). Compared with the previous guidelines, newer criteria are superior by discarding the size criterion and defining ulceration as a cavity situated on the plaque surface and exhibiting surface echogenicity lower than that of the adjacent endothelium (29). The latter criterion of echogenicity reflects the histological disruption of endothelium and is expected to become more relevant thanks to the ongoing improvement of US technology. It is also expected that the combination of high-resolution gray-scale imaging with CEUS in the setting of multi-parametric US (MPUS) would significantly improve the technique’s accuracy for the diagnosis of ulceration.
In terms of diagnostic accuracy, many studies assessed conventional US diagnostic accuracy for ulceration showing conflicting results, with sensitivity values ranging from 23% to 85% when the newer criteria were used (17,29). CEUS has been evaluated and has been directly compared to conventional colour Doppler techniques for the diagnosis of ulceration, demonstrating improved diagnostic accuracy (Figure 1). In a study with symptomatic patients only, colour Doppler technique was only 29% sensitive whereas CEUS was 88% sensitive, with multi-detector computed tomography angiography (MDCTA) as the reference method (8). In a different study with both symptomatic and asymptomatic patients, colour Doppler technique was 41.2% sensitive and CEUS was 94.1% sensitive, while both techniques were 97.95% specific. CEUS also showed better concordance with MDCTA reference imaging (27). In a different research setting, CEUS has been used to assess subclinical atherosclerosis and ulceration in patients with diabetes mellitus, detecting pathological plaque in 8% of carotid segments examined (30). Thanks to its excellent spatial and temporal resolution, CEUS can visualize a swirling movement of microbubbles within ulcerations, a movement pattern indicative of the arterio-arterial embolization (31). This phenomenon can be observed in 18% of ulcerations (27). This swirling movement had previously been shown in an experimental setting using models of vessels (32) and can also be observed on colour Doppler technique in the form of the “yin-yang sign” (33). One limitation of CEUS is that it is a two-dimensional technique which may occasionally be limited in the evaluation of complex three-dimensional (3D) structures such as atherosclerotic blood vessels. This limitation could be overcome by the introduction of 3D CEUS techniques which proved feasible in the evaluation of even complicated cases of atherosclerosis, including ulcerated or heavily calcified plaques. Novel 3D CEUS achieved better agreement with angiography than colour Doppler technique, in quantifying atherosclerosis (34). Albeit of these promising results, it is expected that 3D CEUS might not be widely available for clinical practice in the near future. When interpreting CEUS images, it is important not to misinterpret echogenic parts of the plaque (calcifications) projecting to the contrast-specific part of a dual-display image as ulceration. Comparison with the low-MI grayscale image will readily address this issue (35).
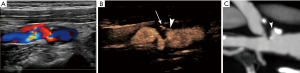
Quantification analysis is one of the current and most essential trends in many aspects of radiology, in an attempt to reduce subjectivity and thus to improve inter-observer agreement. Given the prevalence and clinical significance of carotid plaque irregularities, there have been some attempts to quantitatively analyze and correlate plaque irregularities with neurologic symptomatology. Although the entity of “Bending Energy” failed to discriminate symptomatic from asymptomatic plaques (36), a more recent approach suggested by Kanber et al. provided promising results. Using an intuitive approach, this team used a quantitative index deriving from the summation of angular deviation of the plaque surface from a straight line, divided by the length of the plaque surface. The resulting index was termed the surface irregularity index (SII) and was calculated using a semi-automatic software based on B-mode images. The SII was found to be an independent risk factor in predicting ipsilateral hemispheric symptoms, since it was significantly higher in symptomatic plaques, while it was not associated with the degree of stenosis (37). In a later study, SII was combined with plaque grayscale median (GSM) and stenosis to produce a multi-parametric vulnerability index which was significantly higher in symptomatic plaques, and more importantly outperformed stenosis alone for the prediction of symptomatic plaques (38). These studies showed that quantification of carotid plaque irregularities is feasible and is capable of discriminating symptomatic from asymptomatic plaques, using B-mode and colour Doppler techniques for the delineation of plaque surface.
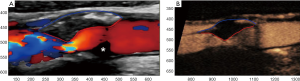
The concept of SII was further confirmed using different manual software based on colour Doppler and CEUS images (Figure 2). In a study directly comparing subjective characterization of plaque morphology with SII, the former did not significantly correlate with the occurrence of stroke, whereas the quantitative index was again significantly higher in symptomatic plaques using both colour Doppler and CEUS (39). In a study assessing the previously suggested multi-parametric index, CEUS allowed for a slightly higher diagnostic accuracy in the detection of symptomatic plaques, although no statistical significance was achieved (40). In a study comparing colour Doppler and CEUS with the histology, Hamada et al. concluded that CEUS was significantly superior to the former for the detection of histologic plaque rupture after performing a quantitative analysis. Moreover, ROC analysis showed that CEUS was 91.3% sensitive for this diagnosis, using the cut-off value of 1.4, 1.3 and 1.88 mm for orifice, depth and width measurement respectively (41). Based on these findings, quantification of carotid plaque irregularities using both Doppler techniques and CEUS appears feasible and a promising technique for the detection of vulnerable carotid plaques. Further studies are needed using different approaches or even 3D ultrasonographic techniques.
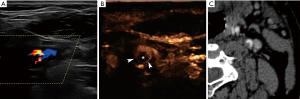
Given its ability to better delineate plaque surface, CEUS is also expected to better detect intraluminal thrombus as a complication of carotid plaque rupture (Figure 3). This has been previously shown for the detection of intra-cardiac thrombus and intraluminal thrombus in abdominal aortic aneurysm (42). Intraluminal thrombus in the carotid artery is expected to be circumferentially outlined by microbubbles or blood flow signals on Doppler techniques on an axial plane, producing the previously reported “donut sign” for computed tomography angiography (CTA). In long-axis images, the thrombus will be seen partially attached to a plaque (43).
CEUS for the detection of intraplaque neovascularization
Multiple risk factors contribute to vulnerable plaque formation, including a large lipid core, thin fibrous cap as well as inflammatory cell infiltration of the plaque (44). In particular, aberrant vasa vasorum and intraplaque neovascularization are implicated in the pathogenesis of vulnerable plaques. The underlying atherosclerotic process can lead to local hypoxia and vessel wall injury, which induce inward vasa vasorum formation further leading to intraplaque neovascularization. When functional, vasa vasorum are essentially a collection of small vessels originating from the adventitia which supplies the vascular wall. However, in atherosclerotic disease, aberrant vasa vasorum neovascularization, along with local hypoxia, can lead to immature intraplaque neovascularization (45). Such vessels are characterized by increased vascular density. These vessels oftentimes lack the critical pericytes to provide vascular integrity. The result is a collection of abundant, leaky and fragile vessels that are prone to intraplaque hemorrhage, which further destabilizes atherosclerotic plaques and may eventually lead to rupture of the plaque with distal embolization.
In the early 2000s, a number of histological studies have confirmed that the presence of intraplaque neovascularization was a consistent feature in clinically significant vascular disease. McCarthy et al. have shown that patients with symptomatic carotid disease had significantly more intraplaque neovessels (P<0.00001). Further, intraplaque hemorrhage and rupture were associated with an increased number of intraplaque neovessels in this study (P<0.017, P=0.001, respectively) (46). On the other hand, Dunmore et al. have demonstrated that the neovessels in symptomatic patients with carotid disease were predominantly immature with a lack of smooth muscle wall (47).
Therefore, intraplaque neovascularization has become an important target for non-invasive assessment of plaque vulnerability. CEUS is uniquely positioned with respect to imaging of intraplaque neovascularization. The contrast microbubbles behave similarly to red blood cells and remain strictly intravascular (48). Therefore, intraplaque signals are almost exclusively reflective of the microvasculature. In addition, intraplaque enhancement can represent intraplaque hemorrhage, a downstream effect of immature, leaky vasa vasorum and neovessels in vulnerable plaques.
The intraplaque enhancement phenomenon was first described by Professor Feinstein in delineating the plaque border in carotid stenosis (49). The authors described visualizing discrete mobile microbubbles as spot enhancement through the intraplaque vasculature. A pattern of adventitial vasa vasorum extending towards the core of the plaque was observed on CEUS. The authors speculated that the signal intensity might correlate with the degree of neovessel density. Further, it was thought that CEUS enhancement was perhaps indicative of plaque vulnerability. Staub et al. have shown that a higher degree of post-contrast plaque enhancement was associated with more imaging-evident vulnerable plaques, measured by a higher degree of stenosis and lesion thickness (P=0.003) (50). Subsequent studies have shown a strong degree of correlation between CEUS enhancement and histologic vascular density of carotid plaques, both qualitatively and quantitatively.
Quantification of intraplaque neovascularization on CEUS
Most studies used a semi-quantitative visual based approach with different scoring systems to grade intraplaque neovascularization (51). Commonly, intraplaque neovascularization was scored by a visual based 3-point grading system. Grade 1 was defined as no appearance of moving bubbles in the plaque or microbubbles confined only to the adjacent adventitial layer (no visible microbubbles) (50). Grade 2 were defined as moderate visible appearance of moving bubbles in the plaque at the adventitial side or plaque shoulder (limited to moderate microbubbles), and grade 3 as extensive intraplaque neovascularization, with clear visible appearance of bubbles moving to the plaque core (extensive appearance of microbubbles within the plaque) (Figure 4).
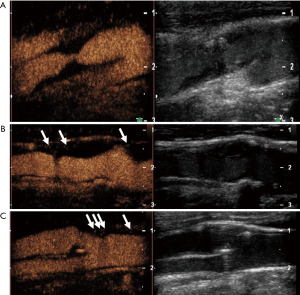
For the assessment of intraplaque neovascularization other studies used a quantitative analysis method by measuring maximal intraplaque video-intensity after bolus application of the contrast agent within the selected region of interest (TIC) (52). Furthermore, quantitative software analysis of intraplaque neovascularization on CEUS used MIP to quantify intraplaque neovascularization (51). More sophisticated software with specific quantification algorithm have been used for automated quantification of intraplaque microvessels (53-55) (Figure 5).
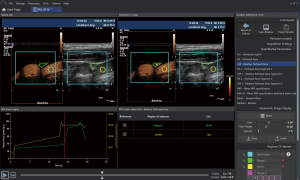
Intraplaque enhancement on CEUS compared with histology
Shah et al. provided the first qualitative measure of CEUS enhancement and its histologic correlation (56). The degree of neovascularization was visually graded on four levels. Grade 0 was defined as no appearance of intraplaque enhancement. Grade 3 was defined as those with pulsating arterial vessel. Grade 1 was then defined as limited enhancement and grade 2 as moderate enhancement laying between grade 1 and 3. Histologic analysis was carried out by measuring the level of vascular markers such as CD31, CD34 and hemosiderin. It was found that there was a significant association between the degree of CEUS neovascularization and histologic vascular density (r2=0.68, P=0.002). Coli et al. conducted the first semi-quantitative study on CEUS histology correlation. Enhancement patterns were graded into two categories: grade 1 was defined as no intraplaque bubbles or bubbles confined to adventitia; and grade 2 was defined as bubbles reaching plaque core or enhancement throughout the plaque (57). Histological analysis was done by counting the number of vessels in the magnified image. The study showed a significantly higher number of vasa vasorum in grade 2 plaques in comparison to grade 1 plaques (3.24 vs. 1.82 mm2, P=0.005). Then in 2011, Hoogi et al. conducted the first quantitative study comparing CEUS imaging to histologic analysis (53). Imaging quantification was done by segmenting all intraplaque enhancement on CEUS throughout one cardiac cycle. The accumulated area enhancement was then divided by the plaque volume. Histologic analysis was performed by measuring the ratio of neovessel area to the total area of the plaque. The authors found that the ratio of histological vessel-to-plaque ratio was well correlated with the degree of contrast enhancement on CEUS (r2=0.79, P<0.01).
Since then, multiple studies have shown comparable correlations between CEUS enhancement and histological vascular density utilizing either qualitative or quantitative CEUS analysis (51). Li et al. have demonstrated that both qualitative and quantitative measurements of plaque enhancement were strongly associated with histological neovascularization (P=0.002 and P<0.001, respectively) (58). A 2016 meta-analysis has shown both qualitative and quantitative analysis were highly sensitive and specific to diagnose intraplaque neovascularization (sensitivity: 0.80 vs. 0.77; specificity: 0.83 vs. 0.68) (59).
Further, studies have revealed some preliminary evidence that the strong correlation between CEUS enhancement and vascular density can be applied to intraplaque hemorrhage as well. Vavuranakis et al. conducted a quantitative study comparing CEUS enhancement to histological vascular marker level (60). Interestingly, although there was a significant correlation between the neovessel density and CEUS enhancement in classic V plaques (fibroatherotic) (P=0.031), such relationship was not observed in class VI plaques (ulcerated or hemorrhagic) (P=0.129). Furthermore, histological analysis has shown a significant increase in vessel density of class VI plaques compared to class V (P=0.021). In addition, no significant difference was observed in post-contrast enhancement between class V and VI plaques (84 vs. 88, P=0.848). Therefore, the findings were indicative of vessel-independent CEUS enhancement in unstable carotid plaques, such as in the case of intraplaque hemorrhage. Schmidt et al. then compared CEUS enhancement to immunochemistry analysis of neovessel density in patients with unstable plaques (61). Similar to prior studies, there was a significant association between the number of intraplaque vessels and the degree of CEUS neovascularization (P=0.006). Moreover, authors have described that areas of prolonged and strong enhancement were typically associated with acute intraplaque hemorrhage as well (P not provided) (61). Therefore, CEUS enhancement may indicate not only an increased vascular density but also a loss of vascular integrity (intraplaque hemorrhage), which are both important features of vulnerable plaques.
Previous studies have shown a significant correlation between CEUS enhancement and histologic neovascularization and possible intraplaque hemorrhage, which are markers for vulnerable plaques. More recently, some authors opted to directly examine the relationship between CEUS enhancement and immunohistological grading of vulnerable plaques. On histology, such plaques are defined by high-risk features, such as the presence of large number of inflammatory cells, thin fibrous capsules and a large lipid core. Amamoto et al. studied CEUS enhancement and the histological grade of plaque vulnerability (62). Post-endarterectomy plaques were analyzed and classified per American Heart Association (AHA) guidelines. Less vulnerable plaques were those without evidence of rupture. More vulnerable plaques included those with status post rupture as well as healed plaques with vascular occlusion. The authors found that CEUS enhancement was closely correlated with the histologic grade of plaque vulnerability (P=0.001). Similarly, Giannoni et al. have shown that a pattern of diffuse plaque enhancement was more commonly seen in patients with symptomatic carotid atherosclerotic disease (P<0.0001) (63). Furthermore, Iezzi et al. compared CEUS to immunohistological diagnosis of plaque vulnerability, defined as those with a large lipid core, heavy staining for macrophages, and minor staining for smooth muscle cell (64). The authors have found that CEUS had 94% sensitivity and 87% positive predictive value in diagnosing histological vulnerable plaque. Therefore, CEUS is not only capable of assessing intraplaque neovascularization, but is also able to directly predict histological plaque vulnerability.
Intraplaque enhancement on CEUS compared with biomarkers
Serum biomarkers
Evidence has shown that intraplaque neovascularization is associated with vulnerable plaques. One of the notable downstream effects of neovascularization is increased intraplaque inflammation. The immature vessels are prone to hemorrhage, which induces inflammatory cell chemotaxis. The localized inflammation will further enhance plaque neovascularization and will lead to the secretion of proteases (65). The vicious cycle results in the expansion and rupture of vulnerable plaques. Indeed, histologic evidence has shown that intraplaque neovascularization was closely associated with localized inflammation. Fleiner et al. have found that the number of intraplaque macrophages and vasa vasorum density were significantly higher in symptomatic patients (P<0.05, P=0.008) (66). Similarly, Hoogi et al. have shown that there was a high degree of correlation between inflammatory cells per square millimeter and the ratio of neovessel area to total plaque area (r2=0.7034, P<0.01) (53). Therefore, given the synergistic relationship between inflammation and neovascularization in vulnerable plaques, it is worthwhile to examine the correlation between serum inflammatory biomarkers and CEUS enhancement, a surrogate imaging biomarker of intraplaque neovascularization. Studies have offered evidence of a strong association between CEUS and serum inflammatory markers.
In this context one of the first studied biomarkers is C-reactive protein (CRP) or high-sensitivity CRP (hs-CRP). CRP is a non-specific inflammatory marker which can be elevated in a variety of inflammatory states. With regard to carotid atherosclerotic disease CRP is an acute phase reactant that accumulates in the macrophage-rich area of the plaque (67). Therefore, a high CRP or hs-CRP level indicates a higher level of intraplaque inflammation and potentially predicts plaque vulnerability. Alvarez Garcia et al. have found that unstable plaques were associated with a higher median hs-CRP value compared to stable plaques (27.1 vs. 4.1 mg/L. P<0.001) (68). Similarly, Kablak-Ziembicka et al. have found that hs-CRP level was significantly higher in those patients with a cardiovascular event than those without (5.36±5.1 vs. 3.74±3.5, P=0.004) (69).
From an imaging perspective, preliminary evidence has shown a significant correlation between hs-CRP level and CEUS enhancement. Chang et al. performed CEUS exams and measured serum hs-CRP level in 48 patients (70). Authors have found a significant positive correlation between CRP level and CEUS enhancement (r=0.69, P<0.01). Similarly, Xu et al. conducted a retrospective cohort study on 146 patients (71). This study found a significant correlation between hs-CRP level and cerebral infarction (P<0.001). More importantly, there was also a significant positive correlation between hs-CRP level and CEUS enhancement intensity (r=0.574, P<0.001).
Another serum inflammatory biomarker is matrix metalloproteinase (MMP), which is a protease secreted by inflammatory cells. It is an important factor in capsular erosion and may contribute to plaque rupture (72). Histologic studies have shown a significant correlation between MMP and plaque vulnerability. Molloy et al. showed co-localization of MMP proteases with macrophages. In addition, symptomatic patients and patients with unstable plaques demonstrated an increased concentration of MMP-8 (P=0.0036 and 0.0002, respectively) (73).
From an imaging perspective, studies have shown preliminary evidence of significant correlations between CEUS enhancement and MMP level. Owen et al. studied the late-phase CEUS enhancement in the assessment of plaque stability. In standard CEUS, the immediate post-contrast enhancement of intraplaque neovascularization is assessed. In comparison, late-phase CEUS utilizes the phenomenon where contrast microbubbles are ingested by monocytes. Intracellular microbubbles can remain acoustically stable for up to 30 minutes (74). In this study, late-phase CEUS signal intensity was used to compare with vasa vasorum density and MMP levels. The result showed significantly increased late-phase CEUS intensity in those with higher MMP-1 and MMP-3 level (P=0.043 and 0.024, respectively) (75). Further, Kim et al. examined the relationship between the degree of neovascularization and levels of serum biomarkers. They measured serum levels of hs-CRP, MMP-2 and MMP-9. The degree of neovascularization on CEUS was categorized into two general categories per Coli et al.’s definition. It was found that both MMP-2 and MMP-9 were significantly elevated in patients with grade 2 enhancement pattern (P=0.003 and 0.001, respectively). Interestingly, no correlation was found between hs-CRP level and CEUS enhancement in this study (76).
Other than hs-CRP and MMP, additional serum inflammatory biomarkers include the level of circulating leukocytes. In atherosclerotic disease, certain circulating leukocytes are recruited into the developing plaques and thereby promote intraplaque inflammation. Therefore, one would expect a decreased level of certain circulating leukocytes in those with unstable plaques. Indeed, studies have offered limited preliminary evidence of decreased leukocyte count with increased CEUS enhancement. Ammirati et al. have shown that patients with increased neovessels on CEUS were associated with significantly lower counts of circulating CD14+ and classical monocytes (P=0.039 and 0.029, respectively) (77). Interestingly, the authors did not find any significant correlation between CEUS enhancement and hs-CRP level. Similarly, Li et al. have found a negative correlation between circulating leukocytes and CEUS enhancement (r=−0.223, P<0.05) (78).
Endothelial biomarkers
Intraplaque inflammation can lead to an elevation of serum biomarkers, which correlate well with CEUS enhancement. Furthermore, intraplaque inflammation can also lead to endothelial biomarker expression, such as VCAM-1 and P-selectin, which facilitate the transport of pro-inflammatory cells into the plaque. A promising aspect of CEUS is the use of molecular imaging contrast microbubbles to target tissue-bound, endovascular inflammatory biomarkers. In pre-clinical experimental settings, studies have shown that VCAM-1 expression level was 24-fold higher in those with symptomatic plaques than those without (P<0.01) (79). Further, the authors have demonstrated that VCAM-1 tagged microbubbles can adhere to endothelium, even under high shear stress.
In animal studies, tissue VCAM-1 expression was significantly increased in an advanced atherosclerotic mouse model. In addition, in such animals, a significantly higher level of VCAM-1 tagged microbubble signal was observed as well (P=0.029) (80). Although similar studies have not been repeated in human subjects, molecular imaging of vulnerable carotid plaques may become a useful tool in stratifying the at-risk patient population.
Intraplaque enhancement on CEUS compared with neurological symptoms and cardiovascular disease
Past studies have shown that neovascularization and inflammation were major contributors of carotid plaque vulnerability. On the other hand, CEUS enhancement correlated well with histological vascular density and serum inflammatory biomarker level. One must ask whether such correlation has any clinical significance. Therefore, it is worth examining whether CEUS can evaluate plaque vulnerability and predict cerebrovascular outcomes in patients with carotid atherosclerotic disease. Indeed, research has shown some evidence that CEUS enhancement was indicative of past or current cerebrovascular events.
Xiong et al. studied the relationship between CEUS enhancement and clinical symptoms, namely a history of TIA and/or cerebrovascular ischemic stroke (52). CEUS enhancement was divided into two grades with grade 1 being no or limited enhancement and grade 2 being extensive enhancement. The authors found that there was no significant difference in plaque thickness or ulceration between symptomatic and asymptomatic patients. However, significant difference was observed in the enhancement pattern, intensity of enhancement, and the ratio of plaque to lumen enhancement on CEUS imaging between symptomatic and asymptomatic patients (P<0.001 for all three categories). Similarly, Staub et al. investigated the relationship between prior cardiovascular events or current cardiovascular disease and CEUS enhancement (81). CEUS studies were performed on 147 patients and intraplaque enhancement was graded into two categories. Grade 1 was defined as no or adventitial enhancement. Grade 2 was defined as clear intraplaque enhancement. On both univariate and multivariate analysis, higher grade intraplaque neovascularization was associated with an increased number of cardiovascular events (P=0.034 and 0.017).
Since then, multiple studies have shown comparable results. Xu et al. found a significant difference in plaque enhancement between patients with cerebral infarction and those without (P<0.001) (71). Li et al. also found a significant difference in contrast enhancement between patients with acute ischemic infarction and those without (P<0.01) (78). Huang et al. have conducted a semi-quantitative CEUS study (82). The authors found that there was a significant difference in stroke rates between the highest and the least CEUS enhancement grades (69% vs. 21%, P<0.001). Faggioli et al. conducted a study examining the relationship between CEUS enhancement intensity and patient symptomology (83). The authors found that patients with neurological symptoms (stroke, TIA, or amaurosis fugax) had higher CEUS peak enhancement intensity (7.4 vs. 3.5, P=0.006). In addition, an increased vessel density in those with a higher CEUS enhancement was detected (P=0.04). Interestingly, the authors also examined CT-evident ischemic lesions and its relationship to CEUS enhancement. In patients with CT-evident lesions, CEUS enhancement was significantly higher (5.96 vs. 3.0, P=0.01).
Aside from examining past cerebrovascular events, a number of studies have shown that CEUS is capable of identifying cerebrovascular risks and predicting future cerebrovascular events. Zhou et al. studied the risk of cerebral ischemic events by measuring microembolic signal (MES) on transcranial Doppler and plaque neovascularization on CEUS (84). In 46 patients with carotid stenosis >50%, the authors found that CEUS-evident plaque neovascularization is significantly associated with concurrently increased MES signal (50% vs. 12.5%, P=0.023). Further, Varetto et al. studied cerebral microembolization following carotid artery stenting and its relationship with pre-operative CEUS plaque enhancement (85). In patients with post-stenting, MRI-evident microembolism, the CEUS enhancement intensity was significantly higher (26 vs. 21, P=0.039). In 2018, Li et al. conducted a prospective study examining the relationship between CEUS assessment of carotid neovascularization and recurrent neurological symptoms in 112 patients with TIA (86). CEUS assessment followed a semi-quantitative method with grade 0 being no enhancement and grade 5 being diffuse intraplaque enhancement. After 24 months follow-up, subjects were divided into recurrent and non-recurrent groups based on the occurrence of TIA or stroke. Although there were significant intergroup differences in basic patient characteristics, it was found that the higher grade CEUS enhancement was associated with recurrent TIAs and strokes (P=0.01). However, the difference in patient characteristics may indicate the presence of confounding variables that can potentially interfere with the results. Other recent prospective study revealed that CEUS-assessed carotid intraplaque neovascularization was predictive of significant and complex coronary artery disease and future cardiovascular events (87).
Conclusions
In conclusion, CEUS is a valuable tool to evaluate sonographic markers of carotid plaque vulnerability by depicting plaque surface irregularities and ulceration as well as intraplaque neovascularization and adventitial vasa vasorum development (Table 2). The degree of intraplaque neovascularization on CEUS imaging closely correlates with histological microvessel density, a marker for plaque vulnerability. Preliminary evidence has also shown that CEUS could directly predict histologic grade of high-risk plaques. In addition, given the intricate interaction between intraplaque neovascularization and inflammation, CEUS can offer valuable insights into intraplaque inflammation. Indeed, past studies have shown a close association between CEUS enhancement and multiple serum inflammatory biomarkers. The use of tagged microbubbles can potentially enable CEUS examination of endothelial inflammatory biomarker. Lastly, apart from evaluating for markers of vulnerable carotid plaques, CEUS enhancement is directly associated with past cerebrovascular events. More importantly, preliminary evidence has shown that CEUS could be used to predict future cerebrovascular and cardiovascular events. Despite the progress in CEUS imaging for carotid atherosclerotic disease, past studies still suffer from the retrospective nature, small sample size, and a lack of matched well controlled prospective studies. In the future, large multi-center prospective studies addressing the relationship of CEUS findings with patient outcomes in carotid atherosclerotic disease are warranted.
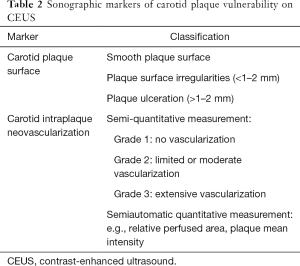
Full table
Acknowledgments
Funding: D Staub was supported by a research grant from the Swiss National Science Foundation (PZ00P3_142419). D Staub has also received an unrestricted research grant from Bracco SA.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.01.08). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. SP and DS serve as the unpaid editorial board members of Cardiovascular Diagnosis and Therapy from July 2019 to June 2021. DS reports grants from Bracco SA, outside the submitted work. PSS reports personal fees from Bracco SA, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Verhoeven B, Hellings WE, Moll FL, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg 2005;42:1075-81. [Crossref] [PubMed]
- Naghavi M, Falk E, Hecht H, et al. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol 2006;98:2H-15H. [Crossref] [PubMed]
- Staub D, Schinkel A, Coll B, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging 2010;3:761-71. [Crossref] [PubMed]
- Rafailidis V, Huang DY, Yusuf GT, et al. General principles and overview of vascular contrast-enhanced ultrasonography. Ultrasonography 2020;39:22-42. [Crossref] [PubMed]
- Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol 2004;14 Suppl 8:11-5. [Crossref] [PubMed]
- Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med 2018;39:154-80. [Crossref] [PubMed]
- Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006;32:1369-75. [Crossref] [PubMed]
- ten Kate GL, van Dijk AC, van den Oord SC, et al. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol 2013;112:292-8. [Crossref] [PubMed]
- Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography 2015;34:3-18. [Crossref] [PubMed]
- Dietrich CF, Averkiou M, Nielsen MB, et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open 2018;4:E2-15. [Crossref] [PubMed]
- Greis C. Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: tips and tricks. Clin Hemorheol Microcirc 2014;58:89-95. [Crossref] [PubMed]
- Marshall MM, Beese RC, Muiesan P, et al. Assessment of portal venous system patency in the liver transplant candidate: a prospective study comparing ultrasound, microbubble-enhanced colour Doppler ultrasound, with arteriography and surgery. Clin Radiol 2002;57:377-83. [Crossref] [PubMed]
- Nakamura J, Nakamura T, Deyama J, et al. Assessment of carotid plaque neovascularization using quantitative analysis of contrast-enhanced ultrasound imaging is useful for risk stratification in patients with coronary artery disease. Int J Cardiol 2015;195:113-9. [Crossref] [PubMed]
- Wilson SR, Jang HJ, Kim TK, et al. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol 2008;190:691-5. [Crossref] [PubMed]
- Eisenbrey JR, Daecher A, Kramer MR, et al. Effects of Needle and Catheter Size on Commercially Available Ultrasound Contrast Agents. J Ultrasound Med 2015;34:1961-8. [Crossref] [PubMed]
- Rafailidis V, Charitanti A, Tegos T, et al. Contrast-enhanced ultrasound of the carotid system: a review of the current literature. J Ultrasound 2017;20:97-109. [Crossref] [PubMed]
- Rafailidis V, Chryssogonidis I, Tegos T, et al. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017;8:213-25. [Crossref] [PubMed]
- Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304-8. [Crossref] [PubMed]
- Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke 2000;31:615-21. [Crossref] [PubMed]
- Saba L, Yuan C, Hatsukami TS, et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9-31. [Crossref] [PubMed]
- Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [Crossref] [PubMed]
- Saba L, Anzidei M, Marincola BC, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol 2014;37:572-85. [Crossref] [PubMed]
- Saba L, Caddeo G, Sanfilippo R, et al. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: potentialities and advantages of multidetector row CT angiography. AJNR Am J Neuroradiol 2007;28:1061-6. [Crossref] [PubMed]
- Sitzer M, Muller W, Siebler M, et al. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke 1995;26:1231-3. [Crossref] [PubMed]
- Brinjikji W, Rabinstein AA, Lanzino G, et al. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc Dis 2015;40:165-74. [Crossref] [PubMed]
- Eyding J, Geier B, Staub D. Current strategies and possible perspectives of ultrasonic risk stratification of ischemic stroke in internal carotid artery disease. Ultraschall Med 2011;32:267-73. [Crossref] [PubMed]
- Rafailidis V, Chryssogonidis I, Xerras C, et al. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur Radiol 2019;29:2137-45. [Crossref] [PubMed]
- de Bray JM BJ, Dauzat M. Consensus Concerning the Morphology and the Risk of Carotid Plaques. Cerebrovasc Dis 1997;7:289-96. [Crossref]
- Muraki M, Mikami T, Yoshimoto T, et al. New criteria for the sonographic diagnosis of a plaque ulcer in the extracranial carotid artery. AJR Am J Roentgenol 2012;198:1161-6. [Crossref] [PubMed]
- van den Oord SC, Akkus Z, Renaud G, et al. Assessment of carotid atherosclerosis, intraplaque neovascularization, and plaque ulceration using quantitative contrast-enhanced ultrasound in asymptomatic patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging 2014;15:1213-8. [Crossref] [PubMed]
- Rafailidis V, Charitanti A, Tegos T, et al. Swirling of microbubbles: Demonstration of a new finding of carotid plaque ulceration on contrast-enhanced ultrasound explaining the arterio-arterial embolism mechanism. Clin Hemorheol Microcirc 2016;64:245-50. [Crossref] [PubMed]
- Imbesi SG, Kerber CW. An experimental and angiographic explanation of why ulcerated carotid bulbs embolize. Interv Neuroradiol 1999;5:11-8. [Crossref] [PubMed]
- Furst H, Hartl WH, Jansen I, et al. Color-flow Doppler sonography in the identification of ulcerative plaques in patients with high-grade carotid artery stenosis. AJNR Am J Neuroradiol 1992;13:1581-7. [PubMed]
- Pelz JO, Weinreich A, Schob S, et al. Multiparametric 3D Contrast-Enhanced Ultrasound to Assess Internal Carotid Artery Stenosis: A Pilot Study. J Neuroimaging 2020;30:82-9. [Crossref] [PubMed]
- Fetzer DT, Rafailidis V, Peterson C, et al. Artifacts in contrast-enhanced ultrasound: a pictorial essay. Abdom Radiol (NY) 2018;43:977-97. [Crossref] [PubMed]
- Tegos TJ, Kalomiris KJ, Sabetai MM, et al. Significance of sonographic tissue and surface characteristics of carotid plaques. AJNR Am J Neuroradiol 2001;22:1605-12. [PubMed]
- Kanber B, Hartshorne TC, Horsfield MA, et al. Quantitative assessment of carotid plaque surface irregularities and correlation to cerebrovascular symptoms. Cardiovasc Ultrasound 2013;11:38. [Crossref] [PubMed]
- Kanber B, Hartshorne TC, Horsfield MA, et al. A Novel Ultrasound-Based Carotid Plaque Risk Index Associated with the Presence of Cerebrovascular Symptoms. Ultraschall Med 2015;36:480-6. [PubMed]
- Rafailidis V, Chryssogonidis I, Grisan E, et al. Does Quantification of Carotid Plaque Surface Irregularities Better Detect Symptomatic Plaques Compared to the Subjective Classification? J Ultrasound Med 2019;38:3163-71. [Crossref] [PubMed]
- Rafailidis V, Chryssogonidis I, Xerras C, et al. An Ultrasonographic Multiparametric Carotid Plaque Risk Index Associated with Cerebrovascular Symptomatology: A Study Comparing Color Doppler Imaging and Contrast-Enhanced Ultrasonography. AJNR Am J Neuroradiol 2019;40:1022-8. [Crossref] [PubMed]
- Hamada O, Sakata N, Ogata T, et al. Contrast-enhanced ultrasonography for detecting histological carotid plaque rupture: Quantitative analysis of ulcer. Int J Stroke 2016;11:791-8. [Crossref] [PubMed]
- Schinkel AF, Kaspar M, Staub D. Contrast-enhanced ultrasound: clinical applications in patients with atherosclerosis. Int J Cardiovasc Imaging 2016;32:35-48. [Crossref] [PubMed]
- Menon BK, Singh J, Al-Khataami A, et al. The donut sign on CT angiography: an indicator of reversible intraluminal carotid thrombus? Neuroradiology 2010;52:1055-6. [Crossref] [PubMed]
- Staub D. Atherosclerotic plaque neovascularization and inflammation - is there a link? Vasa 2015;44:163-5. [Crossref] [PubMed]
- Feinstein S. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol 2006;48:236-43. [Crossref] [PubMed]
- McCarthy M, Loftus I, Thompson M, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg 1999;30:261-8. [Crossref] [PubMed]
- Dunmore B, McCarthy M, Naylor A, et al. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg 2007;45:155-9. [Crossref] [PubMed]
- Partovi S, Loebe M, Aschwanden M, et al. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol 2012;198:W13-9. [Crossref] [PubMed]
- Rajaram V, Pandhya S, Patel S, et al. Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am J Cardiol 2004;93:32C-48C. [Crossref] [PubMed]
- Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology 2011;258:618-26. [Crossref] [PubMed]
- Schinkel AFL, Bosch JG, Staub D, et al. Contrast-Enhanced Ultrasound to Assess Carotid Intraplaque Neovascularization. Ultrasound Med Biol 2020;46:466-78. [Crossref] [PubMed]
- Xiong L, Deng Y, Zhu Y, et al. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology 2009;251:583-9. [Crossref] [PubMed]
- Hoogi A, Adam D, Hoffman A, et al. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol 2011;196:431-6. [Crossref] [PubMed]
- Akkus Z, Hoogi A, Renaud G, et al. New quantification methods for carotid intra-plaque neovascularization using contrast-enhanced ultrasound. Ultrasound Med Biol 2014;40:25-36. [Crossref] [PubMed]
- van den Oord SC, Akkus Z, Bosch JG, et al. Quantitative Contrast-Enhanced Ultrasound of Intraplaque Neovascularization in Patients with Carotid Atherosclerosis. Ultraschall Med 2015;36:154-61. [PubMed]
- Shah F, Balan P, Weinberg M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med 2007;12:291-7. [Crossref] [PubMed]
- Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol 2008;52:223-30. [Crossref] [PubMed]
- Li C, He W, Guo D, et al. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med Biol 2014;40:1827-33. [Crossref] [PubMed]
- Huang R, Abdelmoneim SS, Ball CA, et al. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J Am Soc Echocardiogr 2016;29:491-502. [Crossref] [PubMed]
- Vavuranakis M, Sigala F, Vrachatis DA, et al. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa 2013;42:184-95. [Crossref] [PubMed]
- Schmidt C, Fischer T, Ruckert RI, et al. Identification of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS One 2017;12:e0175331. [Crossref] [PubMed]
- Amamoto T, Sakata N, Ogata T, et al. Intra-Plaque Vessels on Contrast-Enhanced Ultrasound Sonography Predict Carotid Plaque Histology. Cerebrovasc Dis 2018;46:265-9. [Crossref] [PubMed]
- Giannoni M, Vicenzini E, Citone M, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg 2009;37:722-7. [Crossref] [PubMed]
- Iezzi R, Petrone G, Ferrante A, et al. The role of contrast-enhanced ultrasound (CEUS) in visualizing atherosclerotic carotid plaque vulnerability: which injection protocol? Which scanning technique? Eur J Radiol 2015;84:865-71. [Crossref] [PubMed]
- Michel JB, Martin-Ventura JL, Nicoletti A, et al. Pathology of human plaque vulnerability: Mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014;234:311-9. [Crossref] [PubMed]
- Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004;110:2843-50. [Crossref] [PubMed]
- Hermus L, Lefrandt JD, Tio RA, et al. Carotid plaque formation and serum biomarkers. Atherosclerosis 2010;213:21-9. [Crossref] [PubMed]
- Alvarez Garcia B, Ruiz C, Chacon P, et al. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J Vasc Surg 2003;38:1018-24. [Crossref] [PubMed]
- Kablak-Ziembicka A, Przewlocki T, Sokolowski A, et al. Carotid intima-media thickness, hs-CRP and TNF-alpha are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis 2011;214:185-90. [Crossref] [PubMed]
- Chang X, Feng J, Ruan L, et al. Positive correlation between neovascularization degree of carotid atherosclerosis determined by contrast-enhanced ultrasound and level of serum C-reactive protein. Vasa 2015;44:187-94. [Crossref] [PubMed]
- Xu R, Yin X, Xu W, et al. Assessment of carotid plaque neovascularization by contrast-enhanced ultrasound and high sensitivity C-reactive protein test in patients with acute cerebral infarction: a comparative study. Neurol Sci 2016;37:1107-12. [Crossref] [PubMed]
- Loftus IM, Naylor AR, Bell PR, et al. Plasma MMP-9 - a marker of carotid plaque instability. Eur J Vasc Endovasc Surg 2001;21:17-21. [Crossref] [PubMed]
- Molloy KJ, Thompson MM, Jones JL, et al. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation 2004;110:337-43. [Crossref] [PubMed]
- Owen D, Shalhoub J, Miller S, et al. Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology 2010;255:638-44. [Crossref] [PubMed]
- Shalhoub J, Monaco C, Owen DR, et al. Late-phase contrast-enhanced ultrasound reflects biological features of instability in human carotid atherosclerosis. Stroke 2011;42:3634-6. [Crossref] [PubMed]
- Kim HS, Woo JS, Kim BY, et al. Biochemical and clinical correlation of intraplaque neovascularization using contrast-enhanced ultrasound of the carotid artery. Atherosclerosis 2014;233:579-83. [Crossref] [PubMed]
- Ammirati E, Moroni F, Magnoni M, et al. Circulating CD14+ and CD14(high)CD16- classical monocytes are reduced in patients with signs of plaque neovascularization in the carotid artery. Atherosclerosis 2016;255:171-8. [Crossref] [PubMed]
- Li Z, Bai Y, Li W, et al. Carotid vulnerable plaques are associated with circulating leukocytes in acute ischemic stroke patients: a clinical study based on contrast-enhanced ultrasound. Sci Rep 2018;8:8849. [Crossref] [PubMed]
- Weinkauf CC, Concha-Moore K, Lindner JR, et al. Endothelial vascular cell adhesion molecule 1 is a marker for high-risk carotid plaques and target for ultrasound molecular imaging. J Vasc Surg 2018;68:105S-113S. [Crossref] [PubMed]
- Moccetti F, Weinkauf CC, Davidson BP, et al. Ultrasound Molecular Imaging of Atherosclerosis Using Small-Peptide Targeting Ligands Against Endothelial Markers of Inflammation and Oxidative Stress. Ultrasound Med Biol 2018;44:1155-63. [Crossref] [PubMed]
- Staub D, Patel M, Tibrewala A, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010;41:41-7. [Crossref] [PubMed]
- Huang PT, Chen CC, Aronow WS, et al. Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol 2010;2:89-97. [Crossref] [PubMed]
- Faggioli GL, Pini R, Mauro R, et al. Identification of carotid 'vulnerable plaque' by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg 2011;41:238-48. [Crossref] [PubMed]
- Zhou Y, Xing Y, Li Y, et al. An assessment of the vulnerability of carotid plaques: a comparative study between intraplaque neovascularization and plaque echogenicity. BMC Med Imaging 2013;13:13. [Crossref] [PubMed]
- Varetto G, Gibello L, Faletti R, et al. Contrast-enhanced ultrasound to predict the risk of microembolization during carotid artery stenting. Radiol Med 2015;120:1050-5. [Crossref] [PubMed]
- Li Z, Xu X, Ren L, et al. Prospective Study About the Relationship Between CEUS of Carotid Intraplaque Neovascularization and Ischemic Stroke in TIA Patients. Front Pharmacol 2019;10:672. [Crossref] [PubMed]
- Mantella LE, Colledanchise KN, Hetu MF, et al. Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. Eur Heart J Cardiovasc Imaging 2019;20:1239-47. [Crossref] [PubMed]


