
Obstructive sleep apnea versus central sleep apnea: prognosis in systolic heart failure
Introduction
Chronic heart failure (CHF) occurs in the general population with a prevalence of 2–3% (1). CHF is linked to significant mortality, morbidity and health care costs (2). After being diagnosed with CHF, 5 years mortality is estimated to be 50% and 10 years mortality to be 90% (3). In industrialized countries most patients with CHF are 65 years of age or over (4). CHF is predicted to be an increasing health problem in developed countries due to increasing elderly population (5).
Sleep-disordered breathing (SDB) is a common, chronic disorder with a major impact on morbidity and mortality in the general population (6). SDB is more prevalent in patients with CHF than in any other population (7). It is estimated that up to 80% of all patients with CHF have SDB (8). The disorder is most commonly presented as either obstructive sleep apnea (OSA) or central sleep apnea, the latter often presented in a clinical form known as Cheyne-Stokes respiration (CSR) (1,6). OSA is mainly caused by nocturnal upper airway collapse, but with preserved breathing efforts during apnea periods. CSR is characterized by complete cessation of respiratory effort and airflow alternating with profound hyperventilation and crescendo-decrescendo respiratory pattern (9,10). Both types are characterized by repetitive apneas and hypopneas during sleep, accompanied by intermittent hypoxia, re-oxygenation and recurrent arousals from sleep. This may lead to activation of the sympathetic nervous system, rise in blood pressure and inflammation (11,12). Overnight sympathetic nervous activity has been found to be significantly greater in CHF patients with CSR than CHF patients with OSA (13,14). It has been argued that OSA may lead to heart failure by altered loading conditions, hypoxia and adverse impact of increased sympathetic nervous system activity (9). Some investigators speculate that CSR might represent a compensatory mechanism with protective effects in heart failure (15) while others found the rationale for treatment to be directed specifically toward CSR is based on the premise that CSR may be detrimental in itself (16).
Since SDB often is associated with CHF, and CHF is a global problem, we therefore wanted to study the impact on mortality and morbidity of OSA and CSR. We used left ventricular ejection fraction (LVEF) as a marker of CHF. We investigated the prognostic effect of OSA and CSR in a population with similar systolic function at baseline (17-19).
Methods
Study population
This was a retrospective observational study, conducted at The Hospital of Oestfold between 2007 and 2012. We initially screened 148 CHF patients (18 women) from our outpatient clinic for SDB (20). Prior to screening, we excluded patients with unstable angina, myocardial infarction in the last 3 months, coronary intervention within 3 months, cardiac surgery within 6 months, severe valvular or pulmonary disease, or thoracic myopathies. We also excluded patients who were unable to comply for any reason. All screened patients were <85 years of age, were receiving optimal heart failure medication and clinically stable during the last month prior to screening, and were in New York Heart Association (NYHA) functional class II–IV.
Measurements
A careful clinical examination was performed prior to inclusion in our outpatient clinic. Blood pressure was measured on inclusion date at standard conditions: The patients were sitting in the chair for 15 minutes before measurements. The values were obtained from right arm and only one measurement was collected for each patient. The procedure was done by an experienced nurse known to the patients. Age, gender and body weight were recorded at study start. All included patients had an LVEF of ≤45% on echocardiography measured using the modified Simpson biplane rule (21) by an independent cardiologist unaffiliated with our study. The examinations were performed on VIVID 7, GE Vingmed, Norway. Patients who were ultimately included in this study had either refused machine therapy or had used positive airway pressure (PAP) less than 3 months before enrollment.
Sleep screening
Sleep screening data were collected on an ambulatory basis in the patients’ homes using polygraphy (PG) with the Embletta PDS (Embla, Corp., USA), a pocket-sized digital recorder and automatic analysis system (22,23). The Embletta has an actigraph that records activity and position. Patient position was an important channel in the scoring process. The results of automated analysis were reviewed and corrected by an independent experienced scorer, who was not connected to any commercial company or involved in patient treatment, using the applicable 2007 American Academy of Sleep Medicine (AASM) standards (24).
We determined the apnea-hypopnea index (AHI), describing the number of apnea and hypopnea episodes per hour of sleeping time. Apnea was defined as a cessation of inspiratory airflow lasting ≥10 seconds. Hypopnea was defined as either a 30% reduction in amplitude lasting ≥10 seconds, with at least a 4% decrease in saturation; or a 50% reduction in amplitude lasting ≥10 seconds, with at least a 3% decrease in saturation. Hypopneas were not scored as central or obstructive in this study. Baseline was defined as a normal breath within the vicinity of the hypopnea (24). An AHI value of 5–14 indicates mild SDB, 15–30 indicates moderate disease, and >30 indicates severe disease (6,8). For each patient, we also determined the obstructive apnea index (OAI) and central apnea index (CAI) (25), distinguished by ribcage movements and abdominal excursions. Obstructive apnea was defined as the absence of airflow and the presence of ribcage movements and abdominal excursions, whereas central apnea was defined as the absence of both airflow and of ribcage movement and abdominal excursions. CSR was defined by a minimum of three consecutive cycles of a crescendo-decrescendo pattern in the breathing signal, with periods of hyperventilation separated by central apneas and hypopneas (1). Those with a Cheyne-Stokes breathing pattern during >25% of total sleeping time were enrolled in the CSR group (n=43). Patients with an obstructive sleeping pattern, showing CSR for ≤25% of total sleeping time, and an AHI of ≥6 were included in the OSA group (n=19) (20) (Figure 1).

Endpoints
The primary end-point was all-cause mortality, and the secondary end-point was the combined death and readmission. End-points were determined based on information obtained from medical records and death certificates when available. For all patients, we recorded the numbers of hospitalizations and days in hospital. The two groups were observed for a median of 1,371 (range, 904–2,104) days.
Ethics statement
The patients were originally screened for possible inclusion in a recently published study (20). All included patients gave their informed consent, and the study was approved by the local ethical committee and the Norwegian social science data services.
Statistics
Continuous variables are reported as mean ± SD or median [interquartile range (IQR)] depending on the distribution, while categorical variables are reported as number (percentage). Between-group comparisons were investigated using Fisher’s exact test for categorical variables, and Student’s t-test or Mann-Whitney test for continuous variables depending on the distribution. All P values were two-tailed and considered significant if ≤0.05. To evaluate the association between outcome and the presence of CSR or OSA, we used the log-rank test and univariate Cox regression. Multivariate Cox regression analysis was performed with adjustment for the following confounding risk factors: age at screening, LVEF, NYHA class ≥ III, body weight, and use of β-blockers and angiotensin-converting enzyme (ACE) inhibitors or angiotensin II (ATII) blockers.
Results
The OSA and CSR groups were comparable in terms of gender, body weight, LVEF, NYHA classification, blood pressure, and CHF medication at inclusion (Table 1). On average, the patients in the CSR group were 7 years older and had an AHI value that was 2.4-times higher compared to the OSA group. In both groups, the majority of patients were in NYHA functional class III, and ischemic heart disease (IHD) was the main etiology.
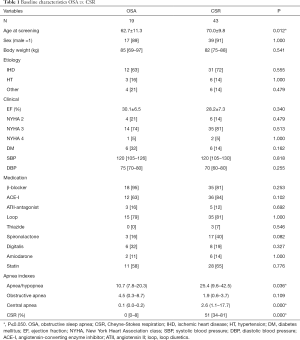
Full table
During follow-up, 23 CSR patients (53%) died, all men: 12 from heart failure (all had IHD), 1 from severe infection, 2 from cancer, and 8 from unknown causes (including 5 with no known illness other than ischemic heart failure). The malignancies were unknown at inclusion. In the OSA group, 5 patients died (26%), no women: 3 from heart failure due to IHD, 1 from severe infection, and 1 from unknown cause. A total of 40 patients with CSR (93%) and 14 patients with OSA (74%) met the secondary end-point of death or hospital admission during the follow-up period.
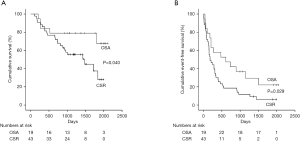
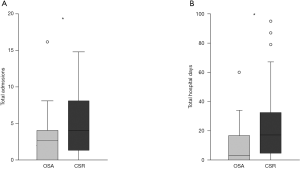
Compared to OSA, CSR was associated with a higher risk of death [CSR vs. OSA: log-rank P=0.040; HR, 2.70 (1.01–7.22); P=0.047] and of death or hospital admission [CSR vs. OSA: log-rank P=0.029; HR, 1.96 (1.06–3.63); P=0.032] during the study period (Figure 2). After adjustment for confounding risk factors, the association between CSR and death remained significant [HR, 4.73 (1.10–20.28); P=0.037], whereas the association between CSR and risk for death or readmission was no longer significant [HR, 2.15 (1.00–4.65); P=0.051] (Table 2). Compared to patients with CSR, patients with OSA needed less hospital admissions [OSA, median 2 (IQR, 0–3) vs. CSR, median 3 (IQR, 1–6); P=0.033] and hospitalization days [OSA, median 3 (IQR, 0–18) vs. median 117 (IQR, 4–33); P=0.010] (Figure 3).

Full table
Discussion
In this retrospective observational study, we directly compared patients with CSR and OSA, demonstrating that CSR was associated with higher mortality than OSA among CHF patients. CSR was also a marker of unfavorable outcome following adjustment for age, AHI, body weight, NYHA ≥ III, LVEF, and heart failure medication.
CHF patients with OSA also had fewer hospital readmissions than CHF patients with CSR, but the difference was not significant after adjustment. Our study implies that among patients with heart failure, CSR is associated with worse prognosis than OSA—highlighting the importance of identifying CSR in the CHF population (26). Several prior studies have shown increased incidence of cardiovascular disease and increased mortality in CHF patients suffering from SDB compared to those without SDB (27). Nakamura et al. performed a meta-analysis based on 11 studies and including almost 2,000 participants with CHF, and found that patients with SDB showed significantly increased mortality risk compared to those without SDB. Moreover, they observed significantly increased mortality with CSR vs. no SDB, but not with OSA vs. no SDB (28). This is consistent with our present findings that CSR was associated with poor prognosis in CHF patients. Supplemental treating options in patients with CHF and SDB, especially in patients with CHF and CSR are limited. Though PAP treatment remains standard treatment in OSA, this treatment is of limited use in CHF with CSR and reduced systolic function (29,30).
Reduced LVEF is associated with mortality in patients with systolic heart failure (19). However, baseline LVEF did not differ between the OSA and CSR groups in our study and mortality was not different when adjusted for LVEF. Therefore, differences in systolic function due to LVEF cannot explain the higher mortality observed in the CSR group. Moreover, the two groups did not significantly differ in NYHA functional class or in systolic or diastolic blood pressure at baseline.
Hospitalization for CHF increases with age (4). Interestingly, we found that patients with CSR were significantly older than those with OSA.
AHI was also significantly higher in CSR patients than OSA patients. This is in accordance with a prior report by Damy et al., which described a steep increase in mortality for an AHI of >5 per hour sleep (31).
Factors other than AHI may also play important roles in the poor outcome in the CSR group, since we found that the worse outcome in this group persisted even with adjustment for AHI values.
Sudden death occurred in six of our patients, including five patients with CSR, most likely caused by ventricular arrhythmias. Therefore, we speculate that an increased sympathetic tone, with subsequent increased risk for arrhythmias, may be one possible mechanism underlying the higher mortality in the CSR group. Hallmarks of both OSA and CSR include hypoxia and arousals from sleep, which are associated with sympathetic nerve stimulation (32). Increased sympathetic tone and heart rate are especially harmful in patients with CHF, potentially leading to myocyte injury and functional and structural abnormalities (6). CHF patients with CSR show higher synaptic nerve activity and higher overnight urinary norepinephrine compared to CHF patients with OSA (33). In particular, severe central sleep apnea is associated with impaired autonomic control, increased arrhythmias, daytime hypocapnia and enhanced ventilator response to exercise (1,34,35).
This may partly explain why hospital admission rates were not significantly different between the two groups after adjustment. Patients with CHF and CSR often die suddenly at home and are not admitted to hospital.
SDB may promote atherosclerosis and aggravate cardiac failure (36). Obesity is reportedly also a major risk factor in men with OSA, as the condition is worsened by layering of fat adjacent to the pharynx (9). Most patients in both the CSR and OSA groups of our study had CHF due to IHD; therefore, ischemia cannot explain the different results between the groups. Additionally, the two groups did not significantly differ in weight. Pulmonary capillary wedge pressure (PCWP) is reportedly elevated in CHF patients with CSR compared to in CHF patients with OSA and in CHF patients without SDB (37). This may support the involvement of some additional unknown mechanism influencing our results.
One challenge in interpreting our results relates to the selection process. OSA and CSR can co-exist, and an overnight shift from OSA to CSR has been documented (38). Moreover, some authors hypothesize that OSA could predispose CHF patients to CSR (10). We classified SDB as obstructive or central based on the dominant sleep pattern during the night. Polysomnography (PSG) would have been preferable compared to PG but was not available at our hospital. However, the high degree of agreement between AHIs obtained from the two scoring methods suggests that PG may be used as an alternative to PSG for SBD assessment among heart failure patients (23). But lack of PSG in our study is a limitation.
Limitations
Limitations of our study include its observational nature and the small number of participants. Additionally, the group balance was not optimal, with fewer patients in the OSA group. The mean AHI in the OSA group was 10.7, categorized as mild OSA. Mild OSA has not been convincingly shown to have adverse cardiovascular consequences. The results may have been influenced by the higher AHI and age in the CSR group; however, the association between CSR and poor outcome remained significant after adjustment for several confounders, including AHI and age.
Conclusions
Our observational study revealed that CSR was associated with higher mortality and risk of hospitalization compared to OSA among patients with similar degrees of CHF. However, differences in mortality and hospitalization were not related to age, and CSR was an age-independent predictor of unfavorable outcome. Hospital admissions rates were not significantly different between the two groups after adjustment. The search for supplemental treating options in patients with CHF and SDB must continue, especially regarding CHF patients with CSR.
Acknowledgments
Funding: The study was supported by The Hospital of Oestfold, Oestfold, Norway.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.03.02). MV reports other from License agreement with Paradigm Biopharma, outside the submitted work. In addition, MV has a patent Uses of enzyme inhibitors pending. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All included patients gave their informed consent, and the study was approved by the local ethical committee (No. 15470) and the Norwegian social science data services.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bitter T, Westerheide N, Prinz C, et al. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011;32:61-74. [Crossref] [PubMed]
- Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123-33. [Crossref] [PubMed]
- Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646-59. [Crossref] [PubMed]
- Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief 2012.1-8. [PubMed]
- Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014;1:4-25. [Crossref] [PubMed]
- Jaffe LM, Kjekshus J, Gottlieb SS. Importance and management of chronic sleep apnoea in cardiology. Eur Heart J 2013;34:809-15. [Crossref] [PubMed]
- Tsai M, Khayat R. Sleep apnea in heart failure. Curr Treat Options Cardiovasc Med 2018;20:33. [Crossref] [PubMed]
- Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007;9:251-7. [Crossref] [PubMed]
- Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation 2003;107:1671-8. [Crossref] [PubMed]
- Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation 2003;107:1822-6. [Crossref] [PubMed]
- Yumino D, Wang H, Floras JS, et al. Relationship between sleep apnoea and mortality in patients with ischaemic heart failure. Heart 2009;95:819-24. [Crossref] [PubMed]
- Lairez O, Legallois D, Agostini D. Sympathetic nervous system, systolic heart failure, and central sleep apnea: are we about to find the missing link? J Nucl Cardiol 2017;24:1938-40. [Crossref] [PubMed]
- Solin P, Kaye DM, Little PJ, et al. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003;123:1119-26. [Crossref] [PubMed]
- Mansfield D, Kaye DM, Brunner La Rocca H, et al. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation 2003;107:1396-400. [Crossref] [PubMed]
- Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax 2012;67:357-60. [Crossref] [PubMed]
- Javaheri S, Brown LK, Randerath W, et al. SERVE-HF: more questions than answers. Chest 2016;149:900-4. [Crossref] [PubMed]
- Edvardsen T, Haugaa KH. Imaging assessment of ventricular mechanics. Heart 2011;97:1349-56. [Crossref] [PubMed]
- Haugaa KH, Smedsrud MK, Steen T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 2010;3:247-56. [Crossref] [PubMed]
- Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42:736-42. [Crossref] [PubMed]
- Hetland A, Haugaa KH, Olseng M, et al. Three-month treatment with adaptive servoventilation improves cardiac function and physical activity in patients with chronic heart failure and cheyne-stokes respiration: a prospective randomized controlled trial. Cardiology 2013;126:81-90. [Crossref] [PubMed]
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358-67. [Crossref] [PubMed]
- Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J 2003;21:253-9. [Crossref] [PubMed]
- Pinna GD, Robbi E, Pizza F, et al. Can cardiorespiratory polygraphy replace portable polysomnography in the assessment of sleep-disordered breathing in heart failure patients? Sleep Breath 2014;18:475-82. [Crossref] [PubMed]
- Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester: American Academy of Sleep Medicine, 2007.
- Chami HA, Resnick HE, Quan SF, et al. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation 2011;123:1280-6. [Crossref] [PubMed]
- Parisot J, Damy T, Gellen B, et al. Sleep-disordered breathing in chronic heart failure: development and validation of a clinical screening score. Sleep Med 2015;16:1094-101. [Crossref] [PubMed]
- Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 2002;166:159-65. [Crossref] [PubMed]
- Nakamura S, Asai K, Kubota Y, et al. Impact of sleep-disordered breathing and efficacy of positive airway pressure on mortality in patients with chronic heart failure and sleep-disordered breathing: a meta-analysis. Clin Res Cardiol 2015;104:208-16. [Crossref] [PubMed]
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095-105. [Crossref] [PubMed]
- Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025-33. [Crossref] [PubMed]
- Damy T, Margarit L, Noroc A, et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail 2012;14:1009-19. [Crossref] [PubMed]
- Lorenzi-Filho G, Genta PR, Figueiredo AC, et al. Cheyne-Stokes respiration in patients with congestive heart failure: causes and consequences. Clinics (Sao Paulo) 2005;60:333-44. [Crossref] [PubMed]
- Naughton MT. Sleep disorders in patients with congestive heart failure. Curr Opin Pulm Med 2003;9:453-8. [Crossref] [PubMed]
- Lanfranchi PA, Somers VK, Braghiroli A, et al. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation 2003;107:727-32. [Crossref] [PubMed]
- Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med 1998;128:204-7. [Crossref] [PubMed]
- Man SF, Sin DD. Sleep-disordered breathing and heart disease: is it one big, vicious loop? Circulation 2011;123:1265-6. [Crossref] [PubMed]
- Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation 1999;99:1574-9. [Crossref] [PubMed]
- Tkacova R, Niroumand M, Lorenzi-Filho G, et al. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation 2001;103:238-43. [Crossref] [PubMed]


