
Identification of characteristics of overt myocarditis in adult patients with idiopathic inflammatory myopathies
Introduction
It is well known that inflammatory myocardial damage may be associated with idiopathic inflammatory myopathies (IIMs), such as polymyositis (PM), dermatomyositis (DM) and overlap syndrome (1). Percentages of cardiac involvement range 6–75% depending on case selection and investigations employed (2). The common clinical presentation of IIMs includes proximal muscle weakness, myalgia and heliotrope sign (3), while the most frequently reported cardiac abnormalities consist of rhythm, conduction abnormalities and left ventricular (LV) diastolic dysfunction in patients with IIMs (4). Although cardiac complication is a major contributing factor for morbidity and mortality in patients with IIMs, available data remain limited (4).
Myocarditis is commonly defined as an inflammatory injury to the myocardium caused by various diseases. The most common etiology is acute viral infection, as well as systemic inflammatory diseases such as IIMs (5). Diagnosis of myocarditis has to be based on the typical myocardial inflammation in endomyocardial biopsy and/or cardiovascular magnetic resonance (CMR) (6,7), both of which can not be obtained easily in clinical application, and the detection of myocarditis in many underlying lesions or subclinical cases is limited subsequently. Although myocarditis is a well-known disease, the reports of its manifestation of IIMs are rare in the literatures (4). The clinical characteristics implacable to identify myocarditis related to IIMs remain unclear. However, cardiac symptoms in IIMs are similar to those in acute viral myocarditis, including heart failure, arrhythmia and chest pain (6). Furthermore, autopsy series have shown that histology proven myocarditis was found to be present in 25–30% of patients with IIMs (8,9). Therefore, myocarditis related to IIMs is probably underestimated (5).
Although endomyocardial biopsy is the gold standard for diagnosis of myocarditis, the application is limited due to its invasion and limited sensitivity (10). CMR is an imaging modality, which can be used for evaluating not only cardiac morphology and function, but also myocardial inflammation with high resolution, more sensitivity and no invasion (1,10). These features of CMR lead to growing interest in evaluation of myocardium (11). Besides, CMR T2-weighted and late gadolinium-enhancement (LGE) sequences could be used for the detection of myocarditis in 50% of patients (12,13). However, by using CMR, whether the features of myocarditis related to IIMs are similar to those of viral myocarditis has not been concluded.
It is necessary to find suspected cases with myocarditis related to IIMs, even if symptoms of heart failure or arrhythmia remain absent. Our current study aimed to identify the clinical characteristics of patients with myocarditis related to IIMs, further to provide clinical basis for diagnosis and treatment for these patients.
Methods
Design and patients
Sixty-two patients with muscular biopsy-proven IIM were analyzed in this observational, single-center retrospective cohort. These patients were first diagnosed and treated in the Peking Union Medical College Hospital (PUMCH) between January 2014 and January 2019. 31 patients with myocarditis accommitant with IIMs (case group) and 31 patients without cardiac involvement (control group) were enrolled in this study. Myocarditis secondary to IIMs was defined based on following definitions of IIMs and myocarditis and excluding acute viral myocarditis. The clinical, biological, electrocardiographic, echocardiographic, CMR data and initial treatment strategies of these two groups were retrieved from electrical medical records and analyzed subsequently. The procedures of this study were approved by the Institutional Review Board of PUMCH, and informed consents were obtained from all patients.
Diagnosis and exclusion
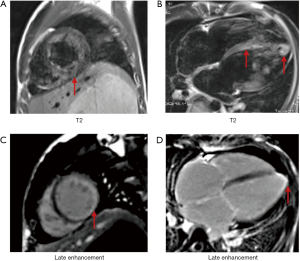
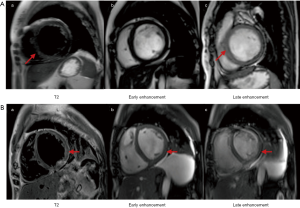
IIMs were defined and classified into subtypes of PM and DM based on the 2017 European League Against Rheumatism American College of Rheumatology classification criteria (14). Muscle biopsies were assessed in the neuropathological laboratory of PUMCH with accepted histopathological diagnostic criteria (15). To identify the myocardial injury, the strategy for early detection of heart involvement in patients with IIMs was obeyed (16), followed by excluding other common cardiovascular etiologies. CMR and/or EMB was recommended for certification in uncertain cases. The criteria of EMB to diagnose myocarditis is in accordance with the “Dallas criteria”, which means “inflammatory cells such as lymphocytes infiltrate in myocardium, accompanying with nearby myocardial fiber necrosis or degeneration, or interstitial edema”. In all 31 cases, 5 of them underwent EMB, for uncertain results in CMR and clinical characteristics. These pathologic characteristics were seen in all of the 5 patients with EMB. Meanwhile, to diagnose myocarditis related to IIMs, patient suspected of virus infection was excluded (N=1), then patients who encountered the 2013 ESC diagnostic criteria for clinically suspected myocarditis were involved (N=34) (6). Finally, myocarditis was defined by CMR in accordance with the Lake Louise criteria (at least meeting two of the following criteria): (I) myocardial edema indicated by regional or global myocardial signal intensity increase in T2-weighted images; (II) hyperemia and capillary leak indicated by increased global myocardial early gadolinium-enhancement (EGE) ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images; (III) necrosis and fibrosis indicated by at least one focal lesion with non-ischemic regional distribution in LGE (7) (e.g., Figures S1,S2). Thirty-one patients with myocarditis accommitant with IIMs were enrolled in our study (Figure 1).
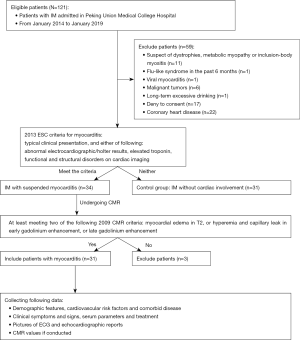
The following concomitant diseases were also excluded: inclusion-body myositis, necrotizing autoimmune myopathy, dystrophies, metabolic myopathy, long-term excessive alcohol consuming, mild to serious kidney dysfunction (estimated GFR <60 mL/min), malignant tumors, coronary heart disease, flu-like syndrome in the past 6 months.
Baseline measures of IIM
The results of all patients were evaluated by at least one experienced rheumatologist before diagnosis of IIMs. Detailed symptoms of IIMs and heart failure, and the levels of routine serum biomarkers of inflammation (including white blood cells, blood sedimentation and high-sensitivity C-reactive protein) were recorded. The data of cardiac parameters were also collected, including cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP). The levels of muscular enzymes (including lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase and creatine kinase-isozyme), antinuclear antibodies [including anti-mitochondrial antibody subtype M2 (AMA-M2), anti-centromere antibody, anti-proliferating-cell nuclear antigen antibody, anti-nucleosome antibody, anti-histone antibody, anti-SSB antibody, anti-RNP antibody, anti-Scl-70 antibody, anti-SM antibody, anti-rRNP antibody, and anti-double-stranded deoxyribonucleic acid antibody],myositis-specific antibodies (including antibody of Jo-1, PL-7, PL-12, OJ, EJ, signal recognition particle, Mi-2, TIF1γ, MDA5, NXP2 and SAE) and myositis-associated autoantibodies (including antibody of PM-Scl 75, PM-Scl 100, Ro-52 and Ku), anti-cardiolipin antibody, SP 100 antibody and GP120 antibody were also recorded.
Electrocardiogram and echocardiography
Standard 12-lead electrocardiogram was performed as an initial detection for all patients. Data from the holter monitoring were also stored if available. Electrocardiogram and holter data were analyzed by two experienced cardiologists in accordance with standard published criteria (17). According to the American College of Cardiology/American Heart Association/European Society of guidelines for ventricular arrhythmias (18), the data of ventricular arrhythmias were collected. Besides, the manifestations of atrial arrhythmias were recorded.
Echocardiography was performed as an initial detection of the patients with IIMs using machine of GE Vivid 7 and 9 with S5 transducer (GE Medical Systems, USA). Based on the current cardiovascular imaging guidelines (19), the echocardiographic parameters were measured and recorded, including left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular fractional shortening (FS), left ventricular ejection fraction (LVEF), areas of each cardiac chambers, left ventricular diastolic function, pulmonary arterial systolic pressure (SPAP), tricuspid regurgitation, right ventricular systolic function, left ventricular wall motions and pericardial effusion.
CMR
If patients with IIM had chest pain, elevated serum levels of cTnI, ventricular arrhythmia, decreased LVEF in echocardiography but normal coronary arteries, CMR was conducted to provide sufficient evidence for myocardial inflammation, which was performed using a 1.5T machine (Magnetom Avanto; Siemens-Healthcare, Germany). Patients were examined in a supine position with the whole heart being covered for assessing all 17 LV segments according to previous recommendations (20). The characteristics of myocarditis were recorded (7). Besides, the data of functional and morphological abnormalities were recorded, including enlargement of four cardiac chambers, left ventricular end-diastolic volume index (LVEDVI), left ventricular cardiac output (LVCO), LVEF, right ventricular ejection factor (RVEF), right ventricular end-diastolic volume index (RVEDVI), right ventricular cardiac output (RVCO) and pericardial effusion. To evaluate all the 17 left ventricular segments in T1-, T2-weighted images, myocardial regions were drawn in the precontrast images and copied to the postcontrast images. The levels of myocardial edema were evaluated in T2-weighted images, with myocardial signal intensity increase, and the signal ratio between myocardium and skeletal muscle >2. Zero-point one mmol/kg Gd-DTPA was given twice, EGE was calculated within 1 min of first-time Gd-DTPA injection and was considered abnormal according to following criterias: EGE ratio between myocardium and skeletal muscle >4, or early postcontrast myocardial signal >50% higher than the precontrast while accompanying with muscular inflammation. LGE was calculated 15 min later of the second Gd-DTPA injection, and was considered abnormal when at least one non-ischemic regional distribution in inversion recovery-prepared gadolinium-enhanced T1-weighted images.
In addition to quantifying LGE, CMR can also detect a variety of characteristics of myocarditis, including inflammatory hyperemia and edema, changes in ventricular size and cardiac structure, segmental and wall motion abnormalities. CMR is also the gold standard for quantitative assessment of ventricular systolic function. Measurement parameters include LVEF, RVEF, LVCO, RVCO, etc. In addition, CMR also can identify the pericardial effusion.
Only patients in case group were tested for CMR. The control group did not perform CMR. As a retrospective study, IIM patients in the control group had no heart-related symptoms, NYHA heart function was normal, myocardial enzymes, BNP and echocardiography were normal. These patients have no indication for CMR.
Statistical analysis
The data were analyzed by SPSS 19.0 (SPSS Inc., IL, USA) in this study. All the measurement data were expressed as mean ± standard deviation (SD) or median (interquartile range) if they were normally or non-normally distributed, respectively. Enumeration data were expressed as percentages. The independent sample t-test or Mann-Whitney U test was performed in the comparison of enumeration data. The χ2 or Fisher’s exact test was used to evaluate the association between echocardiographic parameters and potential risk factors of myocarditis. Furthermore, the remaining significant biomarkers and echocardiographic variables were included in analysis of receiver operating curve to detect possible cutoffs for the indications of myocarditis related to IIM. P<0.05 was considered statistically significant.
Results
Clinical characteristics of patients with IIMs
The present cohort consisted of 62 patients with IIMs (25 males and 37 females) with average age of 47.2±13.8 years, and median disease course of 3.5 years. Compared with the control group, disease course was shorter and PM was more common to be reported in the case group [6 (3.0–10.0) vs. 2 (1.0–6.0) years, and 11/31 vs. 23/31, P<0.05, respectively]. Other rheumatic diseases such as lupus, Sjogren syndrome and primary biliary cirrhosis was showed in 17 patients. Proximal muscle weakness was the most common symptom in patients with IIMs (82.3%), while the patients in case group exhibited higher percentages of symptoms of myalgia, proximal muscle weakness, polyarthralgia, DM rash, pleural effusion, ascites, hepatomegaly, splenomegaly and pulmonary hypertension than that in the control group (P<0.05). There was no difference in peak values of creatine kinase, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, blood sedimentation or high-sensitivity C-reactive protein between two groups. Different titers of positive antinuclear antibodies were showed in more than 70% of the patients, while only positive AMA-M2 was presented more frequently in the case group than that in the control group (25.8% vs. 3.2%, P<0.05). There was no significant difference in MSA/MAA antibodies and myocarditis between two groups (Table 1).
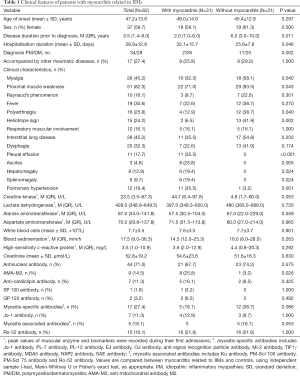
Full table
Descriptions of cardiac involvement
Cardiac symptoms (related to HF, arrhythmia and ischemia) were showed in nearly half of the cases with myocarditis (15/31). There were no differences in the traditional cardiovascular risk factors between two groups, including hypertension, diabetes, atherosclerosis or hyperlipidemia. All patients with myocarditis exhibited elevated levels of cTnI at least once during the active period of disease. Meanwhile, the levels of creatine kinase-isozyme, cTnI and NT-proBNP were higher in case group than that in control group (Table 2).
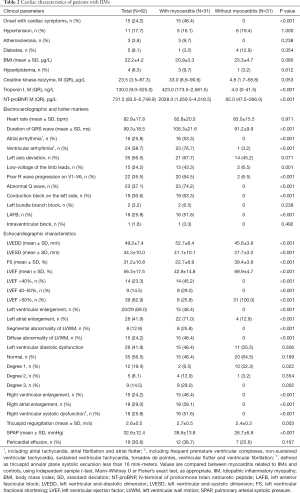
Full table
Characteristics of electrocardiogram
Ventricular and atrial arrhythmia was found in 76.7% and 53.3% of patients with myocarditis respectively. The duration of QRS wave was longer in the case group than that in control group (108.3 vs. 91.2 ms, P<0.01). Meanwhile, the percentages of low-voltage of the limb leads, poor R wave progression on chest leads, abnormal Q wave and left anterior fascicular block (LAFB) were significant higher in the case group than that in control group (P<0.01) (Table 2).
Characteristics of echocardiography
Compared with control group, case group was characterized as LV enlargement (LVEDD: 52.7 vs. 45.6 mm; LVESD: 41.1 vs. 27.7 mm). Besides, left atrial, right atrial and right ventricular enlargement were found in 71.0%, 58.1% and 48.4% of IIM patients with myocarditis, respectively. Furthermore, left ventricular function was significantly decreased in the case group compared with control group (P<0.001). In terms of comparison of diastolic function, more events of decreased function of relaxation (left ventricular diastolic dysfunction of degree 1) were found in the control group (6.5% vs. 32.3%), whereas restrictive pattern (degree 3 of diastolic function on echocardiography) was more common in the case group (29% vs. 0%). The systolic pulmonary artery pressure was also significantly higher in the case group than that in control group (P<0.05) (Table 2).
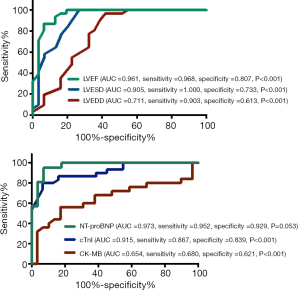
In addition, serum biomarkers and echocardiographic LV parameters were further evaluated in the receiver operating characteristic curve analysis to explore their indicative values in patients with myocarditis (Figure 2). The AUC for LVEDD, LVESD, and LVEF was 0.711 (95% CI: 0.649–0.894), 0.905 (95% CI: 0.825–0.985), and 0.961 (95% CI: 0.913–1.008), respectively; meanwhile, the AUC for CK-MB, cTnI, and NT-proBNP was 0.654 (95% CI: 0.497–0.810), 0.915 (95% CI: 0.845–0.984) and 0.973 (95% CI: 0.935–1.011), respectively.
CMR and LGE in myocarditis related to IIMs
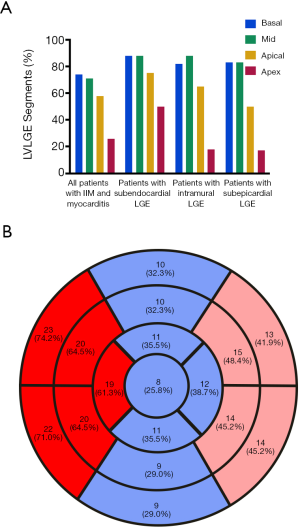
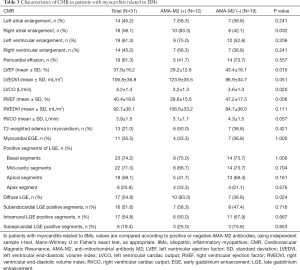
The CMR data of case group showed the events of LV enlargement were found in 61.3% of them with an average LVEF of 37.9%, while pericardial effusion was present in more than 60% of involved patients (Figure 3A). The myocardium has been divided into 17 segments to evaluate the range of LGE, and our results showed that no matter in the sub-endocardium, mid-myocardium or sub-epicardium, the basal and mid-cavity segments were the most frequently involved areas, while the apex segment was the least damaged section (Figure 3B). If more than 8 segments of LGE was defined as diffuse LGE, there were more than half of cases characterized as diffuse LGE, and the rates of intramural and subendocardial LGE were higher than that of subepicardial LGE (Table 3).
Full table
Association of AMA-M2 antibody and myocarditis in IIMs
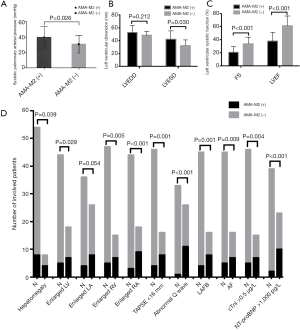
Our results found higher systolic pulmonary arterial pressure, larger LV dimension, lower LVEF, higher percentages of patients with enlarged LA, RV, RA, and decreased RV systolic function were presented in the patients with positive AMA-M2 antibody compared with those patients with negative AMA-M2 antibody (Figure 4A,B,C). Meanwhile, more events of hepatomegaly, abnormal Q wave, LAFB and AF, cTnI elevation (>0.5 µg/L) and NT-proBNP elevation (>1,000 pg/L) were found in patients with positive AMA-M2 antibody (Figure 4D). Additionally, the CMR data demonstrated that compared with patients with negative AMA-M2 antibody, decreased LVEF and RVEF, and more cases with diffuse LGE were found in the group of positive AMA-M2 antibody (Table 3).
Discussion
Herein, the patients with IIMs concomitant with myocarditis was presented, and the myocardial involvement in patients with IIMs was indicated by typical clinical abnormalities, including atrial fibrillation, ventricular arrhythmia, abnormal cTnI, elevated NT-proBNP and impaired cardiac systolic function. As we know, this is the first article systemically exhibiting clinical and imaging characteristics of myocarditis related to IIMs.
There are some clinical or subclinical features of cardiac involvement in myocarditis related to IIMs. Although the reported rate of myocarditis related to IIMs was 38% (21), only less than 50% of patients with IIMs and myocarditis had cardiac symptoms in our study. Furthermore, myocarditis, arrhythmia, cardiomyopathy, congestive heart failure and pericarditis may be isolated or concurrent cardiac manifestations of IIMs (1,21). Heart failure was the most frequent characteristics of these patients. Studies have found that heart failure could be an important lethal and prognostic factor for myocarditis (22,23).
Previous studies had confirmed the diagnostic and prognostic values of NT-proBNP in heart failure, as well as reflection of preclinical hemodynamic and structural cardiovascular changes and predictive value in cardiovascular events (24-26). Similarly, our study found that myocarditis related to IIMs was associated with higher level of NT-proBNP compared with control group. Furthermore, 531 pg/L (cut-off value) of NT-proBNP could demonstrated a high sensitivity and specificity in the identification of myocarditis related to IIM. The diagnostic value of NT-proBNP was proved in patients with myocarditis related to IIMs for the first time, and it is necessary to detect NT-proBNP when suspecting about cardiac involvement in IIMs.
As a quantitative biomarker to evaluate myocardial injury, elevated level of cTnI was found in all the patients of case group. However, there were no differences in CK-MB, CK or other muscular enzymes between two groups. Compared with cardiac troponin T, which was found in more than 40% of IIMs patients and also expressed in skeletal muscle and fetal heart muscle (27,28), cTnI was found to be positive in only 2–2.5% of IIMs patients and only expressed in myocardium of adult (29-31). Hence, elevated level of cTnI could indicate the myocardial injury specifically, which can be considered as a biomarker to reflect the scale of ischemic myocardial infarction (32). Abnormal cTnI usually exists in myocarditis related to IIM, and the detection of cTnI is beneficial for identification of myocardium damage in the early stage (9,33-35). In our study, 100 ng/L of cTnI could demonstrate a high sensitivity and specificity, indicating its diagnostic value and cTnI can be recommended to detect and quantify damage in patients with myocarditis related to IIMs.
It is reported that about 30% to 70% of patients with PM/DM had abnormal electrocardiogram (9,36,37). In our study, ventricular and atrial arrhythmia were found in 76.7% and 53.3% of the patients with myocarditis respectively, which were higher than that in a systematic review (31%) (21). Some other electrocardiogram characteristics were even more prevalent in patients with myocarditis related to IIM, including LAFB, poor R wave progression on chest leads, low-voltage of the limb leads, pathological Q-wave and longer duration of QRS wave. These events of arrhythmia and conduction disorders could be attributed to heart remodeling and inflammation and fibrosis in the conduction system. Besides, the diastolic function of patients with IIMs was evaluated by echocardiography, and it was found in our study that the high frequency of left ventricular systolic dysfunction coexisted with left ventricular diastolic dysfunction, which is consistent with previous studies (38-41). Although the underlying mechanisms of cardiac abnormalities in IIMs have not been clarified, myocarditis was considered as the leading cause of diastolic dysfunction in IIMs (42). However, the detailed changes of diastolic function based on the ASE guideline classification have never been reported. To the best of our knowledge, this is the first cohort to announce the grade III diastolic dysfunction as the echocardiographic characteristic in patients with myocarditis related to IIMs.
Compared with echocardiography, CMR is more accurate and sensitive to detect myocardial injury in IIMs (13). There are some characteristics as follows: (I) CMR is the gold standard of evaluation for LV and RV anatomy and functions; (II) CMR has the unique capability to detect inflammation, necrosis, fibrosis and tissue edema, which is essential for identification of various etiologies (43-45); (III) it might be a quantifiable tool for evaluating cardiac involvement in IIMs. In our study, 74.2% patients (23/31) had echocardiographic wall motion abnormalities in the case group. Similarly, in a case series about acute viral myocarditis, 91.3% of patients had epicardial inflammation indicated by LGE, whereas only 34.8% of patients were diagnosed with wall motion abnormalities in terms of echocardiography (46). Generally, there were three major characteristics of CMR indicating myocarditis: myocardial edema on T2-weighted sequences, capillary leak and LGE (7). The semi-quantitative method was used correspondingly to calculate involved areas of LGE, by dividing myocardium into 17 segments, and our results demonstrated that septum was the most affected segment, following with lateral and anterior segments. Especially, compared with apex segment, LGE was prone to be found in basal and mid-cavity segments. Herein, our results proved that CMR was capable to quantify the damage of myocarditis in IIMs.
AMA-M2 antibody plays an important role in diagnosis of IIMs and indication of underlying cardiac complications. Prevalence of AMA-M2 antibody in IIMs was higher in East Asia (from 5.2% to 19.5%) and France (7.80%), but lower in the USA (0.6%) (47-50). Previous case series had described a growingly frequency of cardiac involvement in AMA-associated IIMs (49-54), varying from approximately 30% to 70%. Similarly, our results demonstrated a significant correlation between AMA-M2 antibody and atrial arrhythmia in patients with IIMs, as well as the correlation between AMA-M2 antibody and following echocardiographic parameters: dimensions of four chambers, LVEF, TAPSE, and pulmonary artery pressure. Furthermore, positive antibodies of AMAs were presented in 25.8% of patients (8/31) with myocarditis related to IIMs in our study, while the percentage was only 3.2% in control group. Strikingly, if dividing these patients into two groups according to the AMA-M2, the group of positive AMA-M2 antibody exhibited enlarged right atrium, decreased LVEF, LVCO and RVEF. Above results suggested the characteristics of patients with AMA-positive myocarditis for the first time, including decreased LV and RV systolic function, LV enlargement and higher pulmonary artery pressure. More importantly, in patients with myocarditis related to IIM and positive antibodies of AMA-M2, more than 80% of them (10/12) presented diffuse LGE, indicating a strong association between antibody of AMA-M2 and diffuse LGE, which may result in serious myocardial injuries and worse cardiac outcomes. For 6 out of 12 patients with myocarditis and positive AMA-M2 antibody concomitant with PBC, the presence of AMA-M2 in myocarditis related to IIMs could be partly explained by the association between PBC and increased cardiovascular disease risk (47,55-57). On the other side, antibodies of AMAs could bind to protein on the inner mitochondrial membrane, leading to block of particular site of the carrier protein, and reduction of ADP/ATP exchange in myocardial mitochondria (58,59). In conclusion, coexistence with PBC and imbalance of energy metabolism in the heart mitochondria may be associated with the mechanisms of autoimmune-mediated myocardial dysfunction in patients with IIMs and positive AMA-M2 antibody.
Limitations
There were some limitations in our study as follows:
- Considering this was a single-center respective study, our sample size was limited. A prospective study with a larger sample size will be conducted to investigate the histological subtypes of IIMs and whether it is benefit to early intervention by immunosuppression or anti-heart failure therapy;
- CMR examination was not conducted in the control group to exclude subclinical myocardial involvement. There might be some slight myocardial injury in patients with IIMs who were assigned mistakenly into the control group. However, there was an ethical problem to conduct enhanced CMR in IIM patients without clinical clues of myocardial abnormalities;
- Some new CMR sequences such as T1 mapping, T2 mapping, extra-cellular volume and T2 STAR were not conducted, which might be potential assessment to evaluate myocardial inflammation, and more data need to explore their values in IIMs;
- Though diffuse LGE is a marker of myocardial inflammation, whether it is associated with poor prognosis in IIMs remains unknown because of lacking of middle-long term data about outcome and mortality;
- The endomyocardial biopsy was conducted in only 5 patients, and there was no sufficient histologic confirmation of myocarditis in our study. This procedure is invasive and its result is limited in sensitivity by the localized and patchy feature of myocarditis in IIMs.
Conclusions
The discovery of clinical clues indicating heart failure and arrhythmias and detection of AMA-M2 antibody play an important role in the identification of myocarditis in IIMs. Notably, cTnI was recommended to detect and quantify myocardial damage in patients with IIMs. NT-proBNP could also help to identify myocarditis in IIMs. Echocardiography is a useful imaging tool in measurement and gradation of diastolic functions in IIMs. Compared with other sequences, LGE in CMR is reliable and essential to detect myocardial inflammation and confirm the diagnosis of myocarditis in IIMs. The most frequently involved areas of LGE were found in the ventricular septal, basal and mid-cavity segments, as well as in the sub-endocardium and intramural myocardium. Diffuse LGE is common to be detected, which is correlated with AMA-M2 antibody in patients with myocarditis related to IIMs.
Acknowledgment
Funding: This research was supported by the National Key Research and Development Program: National Rare Diseases Registry System of China (NRDRS, 2016YFC0901500).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The procedures of this study were approved by the Institutional Review Board of Peking Union Medical College Hospital (No. ZS-1790), and informed consents were obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schwartz T, Diederichsen LP, Lundberg IE, et al. Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open 2016;2:e000291. [Crossref] [PubMed]
- Danieli MG, Gelardi C, Guerra F, et al. Cardiac involvement in polymyositis and dermatomyositis. Autoimmun Rev 2016;15:462-5. [Crossref] [PubMed]
- Rai SK, Choi HK, Sayre EC, et al. Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology (Oxford) 2016;55:461-9. [PubMed]
- Diederichsen LP. Cardiovascular involvement in myositis. Curr Opin Rheumatol 2017;29:598-603. [Crossref] [PubMed]
- Huber AT, Bravetti M, Lamy J, et al. Non-invasive differentiation of idiopathic inflammatory myopathy with cardiac involvement from acute viral myocarditis using cardiovascular magnetic resonance imaging T1 and T2 mapping. J Cardiovasc Magn Reson 2018;20:11. [Crossref] [PubMed]
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013 Sep;34:2636-48, 2648a-d.
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475-87. [Crossref] [PubMed]
- Haupt HM, Hutchins GM. The heart and cardiac conduction system in polymyositis-dermatomyositis: a clinicopathologic study of 16 autopsied patients. Am J Cardiol 1982;50:998-1006. [Crossref] [PubMed]
- Denbow CE, Lie JT, Tancredi RG, et al. Cardiac involvement in polymyositis. Arthritis Rheum 1979;22:1088-92. [Crossref] [PubMed]
- Pohost GM. The history of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2008;1:672-8. [Crossref] [PubMed]
- Ohata S, Shimada T, Shimizu H, et al. Myocarditis associated with polymyositis diagnosed by gadolinium-DTPA enhanced magnetic resonance imaging. J Rheumatol 2002;29:861-2. [PubMed]
- Assomull RG, Lyne JC, Keenan N, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 2007;28:1242-9. [Crossref] [PubMed]
- Rosenbohm A, Buckert D, Gerischer N, et al. Early diagnosis of cardiac involvement in idiopathic inflammatory myopathy by cardiac magnetic resonance tomography. J Neurol 2015;262:949-56. [Crossref] [PubMed]
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 2017;69:2271-82. [Crossref] [PubMed]
- Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord 2004;14:337-45. [Crossref] [PubMed]
- Chen F, Peng Y, Chen M. Diagnostic approach to cardiac involvement in idiopathic inflammatory myopathies. Int Heart J 2018;59:256-62. [Crossref] [PubMed]
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA Guidelines for Ambulatory Electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999;34:912-48. [Crossref] [PubMed]
- European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006;48:e247-346. [Crossref] [PubMed]
- Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:1301-10. [Crossref] [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology at the American Heart Association. Circulation 2002;105:539-42. [Crossref] [PubMed]
- Gupta R, Wayangankar SA, Targoff IN, et al. Clinical cardiac involvement in idiopathic inflammatory myopathies: a systematic review. Int J Cardiol 2011;148:261-70. [Crossref] [PubMed]
- Guerra F, Gelardi C, Capucci A, et al. Subclinical cardiac dysfunction in polymyositis and dermatomyositis: a speckle-tracking case-control study. J Rheumatol 2017;44:815-21. [Crossref] [PubMed]
- Dankó K, Ponyi A, Constantin T, et al. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine (Baltimore) 2004;83:35-42. [Crossref] [PubMed]
- Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardio- vascular diseases: current use and perspectives. Eur Heart J 2014;35:419-25. [Crossref] [PubMed]
- Jin M, Wei S, Gao R, et al. Predictors of long-term mortality in patients with acute heart failure. Int Heart J 2017;58:409-15. [Crossref] [PubMed]
- Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655-63. [Crossref] [PubMed]
- Humphreys JE, Cummins P. Regulatory proteins of the myocardium. Atrial and ventricular tropomyosin and troponin-I in the developing and adult bovine and human heart. J Mol Cell Cardiol 1984;16:643-57. [Crossref] [PubMed]
- Bodor GS, Porterfield D, Voss EM, et al. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal-muscle tissue. Clin Chem 1995;41:1710-5. [Crossref] [PubMed]
- Aggarwal R, Lebiedz-Odrobina D, Sinha A, et al. Serum cardiac troponin T, but not troponin I, is elevated in idiopathic inflammatory myopathies. J Rheumatol 2009;36:2711-4. [Crossref] [PubMed]
- Erlacher P, Lercher A, Falkensammer J, et al. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin Chim Acta 2001;306:27-33. [Crossref] [PubMed]
- Cox FM, Delgado V, Verschuuren JJ, et al. The heart in sporadic inclusion body myositis: a study in 51 patients. J Neurol 2010;257:447-51. [Crossref] [PubMed]
- Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care (dagger). Eur Heart J 2010;31:2197-204. [Crossref] [PubMed]
- Lynch PG. Cardiac incolvement in chronic polymyositis. Br Heart J 1971;33:416-9. [Crossref] [PubMed]
- Lightfoot PR, Bharati S, Lev M. Chronic dermatomyositis with intermittent trifascicular block. An electrophysiologic-conduction system correlation. Chest 1977;71:413-6. [Crossref] [PubMed]
- Khoo T, Stokes MB, Teo K, et al. Cardiac involvement in idiopathic inflammatory myopathies detected by cardiac magnetic resonance imaging. Clin Rheumatol 2019;38:3471-6. [Crossref] [PubMed]
- Stern R, Godbold JH, Chess Q, et al. ECG abnormalities in polymyositis. Arch Intern Med 1984;144:2185-9. [Crossref] [PubMed]
- Gottdiener JS, Sherber HS, Hawley RJ, et al. Cardiac manifestations in polymyositis. Am J Cardiol 1978;41:1141-9. [Crossref] [PubMed]
- Gonzalez-Lopez L, Gamez-Nava JI, Sanchez L, et al. Cardiac manifestations in dermato-polymyositis. Clin Exp Rheumatol 1996;14:373-9. [PubMed]
- Lu Z, Wei Q, Ning Z, et al. Left ventricular diastolic dysfunction: early cardiac impairment in patients with polymyositis/dermatomyositis: a tissue Doppler imaging study. J Rheumatol 2013;40:1572-7. [Crossref] [PubMed]
- Wang H, Liu HX, Wang YL, et al. Left ventricular diastolic dysfunction in patients with dermatomyositis without clinically evident cardiovascular disease. J Rheumatol 2014;41:495-500. [Crossref] [PubMed]
- Schwartz T, Sanner H, Husebye T, et al. Cardiac dysfunction in juvenile dermatomyositis: a case-control study. Ann Rheum Dis 2011;70:766-71. [Crossref] [PubMed]
- Diederichsen LP, Simonsen JA, Diederichsen AC, et al. Cardiac abnormalities in adult patients with polymyositis or dermatomyositis as assessed by noninvasive modalities. Arthritis Care Res (Hoboken) 2016;68:1012-20. [Crossref] [PubMed]
- American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;55:2614-62. [Crossref] [PubMed]
- Allanore Y, Vignaux O, Arnaud L, et al. Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Ann Rheum Dis 2006;65:249-52. [Crossref] [PubMed]
- Mavrogeni S, Bratis K, Karabela G, et al. Myocarditis during acute inflammatory myopathies: evaluation using clinical criteria and cardiac magnetic resonance imaging. Int J Cardiol 2013;164:e3-4. [Crossref] [PubMed]
- Goitein O, Matetzky S, Beinart R, et al. Acute myocarditis: noninvasive evaluation with cardiac MRI and transthoracic echocardiography. AJR Am J Roentgenol 2009;192:254-8. [Crossref] [PubMed]
- Maeda MH, Tsuji S, Shimizu J. Inflammatory myopathies associated with anti-mitochondrial antibodies. Brain 2012;135:1767-77. [Crossref] [PubMed]
- Mauhin W, Mariampillai K, Allenbach Y, et al. Anti-mitochondrial antibodies are not a hallmark of severity in idiopathic inflammatory myopathies. Joint Bone Spine 2018;85:375-6. [Crossref] [PubMed]
- Albayda J, Khan A, Casciola-Rosen L, et al. Inflammatory myopathy associated with anti-mitochondrial antibodies: a distinct phenotype with cardiac involvement. Semin Arthritis Rheum 2018;47:552-6. [Crossref] [PubMed]
- Uenaka T, Kowa H, Ohtsuka Y, et al. Less limb muscle involvement in myositis patients with anti-mitochondrial antibodies. Eur Neurol 2017;78:290-5. [Crossref] [PubMed]
- Fujimoto M, Sato S, Ihn H, et al. Autoantibodies to mitochondrial 2-oxo-acid dehydrogenase complexes in localized scleroderma. Clin Exp Immunol 1996;105:297-301. [Crossref] [PubMed]
- Suzuki S, Yonekawa T, Kuwana M, et al. Clinical and histological findings associated with autoantibodies detected by RNA immunoprecipitation in inflammatory myopathies. J Neuroimmunol 2014;274:202-8. [Crossref] [PubMed]
- Kassardjian CD, Lennon VA, Alfugham NB, et al. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015;72:996-1003. [Crossref] [PubMed]
- Kao AH, Lacomis D, Lucas M, et al. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 2004;50:209-15. [Crossref] [PubMed]
- Selmi C, Bowlus CL, Gershwin ME, et al. Primary biliary cirrhosis. Lancet 2011;377:1600-9. [Crossref] [PubMed]
- Jackson H, Solaymani-Dodaran M, Card TR, et al. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology 2007;46:1131-7. [Crossref] [PubMed]
- Jones DE, Hollingsworth K, Fattakhova G, et al. Impaired cardiovascular function in primary biliary cirrhosis. Am J Physiol Gastrointest Liver Physiol 2010;298:G764-73. [Crossref] [PubMed]
- Schulze K, Becker BF, Schultheiss HP. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ Res 1989;64:179-92. [Crossref] [PubMed]
- Schulze K, Becker BF, Schauer R, et al. Antibodies to ADP-ATP carrier–an autoantigen in myocarditis and dilated cardiomyopathy–impair cardiac function. Circulation 1990;81:959-69. [Crossref] [PubMed]


