Coronary steal due to ruptured right coronary aneurysm causing myocardial infarction in a patient with systemic lupus erythematosus
Introduction
It is well known that patients with systemic lupus erythematosus (SLE) are prone to develop coronary artery disease and myocardial infarction. This pre-disposition appears related to coronary arteritis, premature atherosclerosis, and thrombus formation related to coronary aneurysm or anti-phospholipid syndrome (APS) (1-6). We present a rare case of an acute inferior myocardial infarction caused by a coronary steal phenomenon due to a right coronary aneurysm ruptured into the right atrium in a patient with SLE.
Case report
A 34-year-old female was hospitalized for epigastric and left chest pain. The patient has been treated for SLE and APS for 22 years. She did not have any traditional cardiovascular risk factors such as hypertension, hyperlipidemia or diabetes mellitus. However, she had developed a posterior myocardial infarction 6 years prior to the current admission. At that time, a coronary angiography revealed ectatic changes of the proximal left anterior descending artery (LAD), proximal and mid-portion at the left circumflex artery (LCX), and segment #1-3 of the right coronary artery (RCA). A thrombotic occlusion of the mid-portion of the LCX was successfully re-canalized with a balloon dilatation. She has been treated with Aspirin and Warfarin since then, and her cardiovascular condition had been stable.
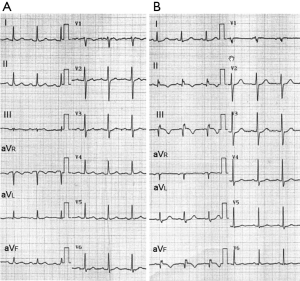
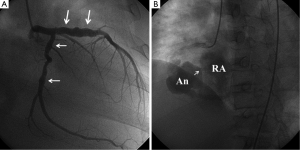
Initial vital signs showed the blood pressure of 130/88 mmHg, a heart rate of 100 beats per minute and the respiratory rate of 20/minute. She was started on heparin under the presumptive diagnosis of visceral ischemia related to APS. On 6th hospital day, she developed high fever and fatigue. Multiple blood cultures grew methicillin-resistant Staphylococcus Aureus (MRSA), for which Vancomycin was started. Panniculitis with a subcutaneous abscess of the left buttocks was considered the most likely site of bacterial entry. There were no peripheral manifestations suggestive of infective endocarditis. On the 10th day after admission, she developed anterior chest pain and dyspnea. A twelve-lead electrocardiogram (ECG) was normal at that time (Figure 1A). An echocardiography revealed a small pericardial effusion and a mass lesion in the lateral wall of the right atrium adjacent to atrioventricular groove, but there was no valvular vegetations. Over the next several days, the mass in the right atrium gradually became larger and the color Doppler showed blood flow signal in the mass. On the 22nd day after admission the patient developed anterior chest pain and dyspnea. A physical examination revealed a new continuous murmur at the lower sternal border and signs of heart failure. An electrocardiogram revealed ST segment elevation in II, III, and aVF, consistent with acute inferior myocardial infarction (Figure 1B). An emergency coronary angiography revealed a diffuse coronary aneurysm in the proximal LAD, but no stenosis was identified (Figure 2A). Mild ectasia was also seen in the proximal and middle portion of the LCX. A huge aneurysm (37 mm × 32 mm) was identified in the middle portion of the RCA, which had ruptured into the right atrium (Figure 2B, Figure 3). The distal RCA was visualized with some delay, but there was no significant stenosis in both proximal and distal edges of the aneurysm. An emergency catheter intervention was deferred and the patient was managed medically. After prolonged antibiotic control (55 days) of the MRSA sepsis, a coronary artery bypass graft surgery was performed and the aneurysm was resected on the 60th hospital day. At the time of the surgery, the cardiac surgeon noticed that there were thrombi and pus-like debris attached to the wall of the aneurysm but the lumen was not obstructed. A pathological examination of the resected aneurysm revealed findings consistent with arteritis characterized by predominant lymphocyte infiltration. Cultures of the wall of the aneurysm and debris were later reported to be negative. Vancomycin was continued for 16 days after the surgery (total of 71 days) and was switched to Teicoplanin due to skin eruption possibly due to allergy. Teicoplanin was continued for 9 days (total of 28 days after the surgery). The patient remained asymptomatic and hemodynamically stable. Infection was well controlled with prolonged period (9 weeks) of antibiotics.

Discussion
Coronary artery disease is common in patients with SLE and is an important cause of morbidity and mortality (1-6). Several pathophysiological causes have been identified. The most common cause is premature coronary atherosclerosis (1,2). Patients with SLE tend to have risk factors for atherosclerosis such as hyperlipidemia, hypertension and impaired glucose tolerance at younger age, related to renal disease manifestations and corticosteroid treatment. APS is also a risk factor for myocardial infarction not only in patients with atherosclerotic narrowing but also in those with angiographically normal coronary arteries (3,4). Vasculitis of the coronary artery has been known to cause myocardial infarction in patient with SLE but is considered to be less common than atherosclerosis (1). Although rare, coronary artery aneurysms have been known to develop and cause myocardial infarction in SLE (5,6). Coronary vasculitis, atherosclerosis and bacteremia (8) have been implicated in the etiology of coronary aneurysms in patient with SLE.
Our patient had a remote history of posterior myocardial infarction due to thrombotic occlusion of an ectatic left circumflex artery. In the presence of APS, the etiology was considered to have been slow flow in the ectatic lesion combined with coagulopathy related to APS resulting in the formation of obstructive thrombi. A cardiac catheterization at that time had demonstrated diffuse, ectatic lesions in the proximal LAD, middle portion of the LCX, and proximal/mid RCA. These were considered to be related to coronary arteritis.
The rapid enlargement of the mass in the right atrium during the current admission, appears most consistent with an infected aneurysm. The inflammatory response of the wall of the pre-existing aneurysm might have contributed to the rapid growth and eventual rupture (8).
Regarding the pathophysiology of the myocardial infarction, there was neither obstructive stenosis by coronary angiography, nor evidence of obstructive thrombus formation in spite of the history of APS. However, the huge ruptured aneurysm in the mid-portion of the right coronary artery with communication into the right atrium was associated with significant shunt. Even with a forceful injection of the contrast dye, most of the contrast dye leaked into the right atrium, and the right coronary artery distal to the aneurysm was difficult to visualize. Therefore, a “coronary steal” phenomenon was the most likely pathophysiological explanation of the inferior myocardial infarction in our patient.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Karrar A, Sequeira W, Block JA. Coronary artery disease in systemic lupus erythematosus: A review of the literature. Semin Arthritis Rheum 2001;30:436-43. [PubMed]
- Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003;349:2407-15. [PubMed]
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002;346:752-63. [PubMed]
- Asherson RA, Khamashta MA, Baguley E, et al. Myocardial infarction and antiphospholipid antibodies in SLE and related disorders. Q J Med 1989;73:1103-15. [PubMed]
- Matayoshi AH, Dhond MR, Laslett LJ. Multiple coronary aneurysms in a case of systemic lupus erythematosus. Chest 1999;116:1116-8. [PubMed]
- Sumino H, Kanda T, Sasaki T, et al. Myocardial infarction secondary to coronary aneurysm in systemic lupus erythematosus. An autopsy case. Angiology 1995;46:527-30. [PubMed]
- Hirata K, Yagi N, Wake M, et al. Left anterior oblique view of the right coronary artery showing a huge aneurysm which ruptured into the right atrium. Asvide 2014;1:261. Available online: http://www.asvide.com/articles/274
- Howe HS, Wong JS, Ding ZP, et al. Mycotic aneurysm of a coronary artery in SLE—a rare complication of salmonella infection. Lupus 1997;6:404-7. [PubMed]


