
Vessel wall MR imaging of intracranial atherosclerosis
Introduction
Intracranial atherosclerotic disease (ICAD) is one of the leading causes of stroke world-wide (1). Despite intensive vascular risk factor control and optimal antiplatelet therapy, risk of recurrent strokes is as high as 25–30% after the initial event in patients with symptomatic ICAD (2). Furthermore, ICAD has been shown to be associated with long term sequelae such as cognitive decline and vascular dementia that can have a huge economic burden (3-5). Given its high risk of recurrent subclinical and clinical ischemic events as well as its association with dementia, investigations studying the development and progression of ICAD have become a priority in cardiovascular research.
Imaging studies report a prevalence of 3.5% to 12.9% of ICAD in asymptomatic populations. These estimates are derived from luminal imaging exams, such as magnetic resonance angiography (MRA) (6) and transcranial Doppler ultrasound (7,8), which use stenosis as a surrogate for ICAD. However, autopsy studies suggest luminal exams may underestimate true prevalence. Pathological evidence of intimal lesions in a study of 193 cases acquired from the Brain Arterial Remodeling Study reported a prevalence of greater than 40% at age 60 and above (9). In a French study, among 259 patients who had fatal brain infarctions, the prevalence of intracranial plaques was as high as 62.2% (10). Differences in these prevalence rates between neuroimaging and autopsy studies may be due to methods of plaque detection by these lumen-based imaging techniques. A need for more sensitive methods of in vivo plaque detection are needed.
High resolution vessel wall MR imaging (VW-MR) permits visualization of the vessel wall enabling direct imaging of plaque, including angiographically occult plaque that has not yet led to detectible stenosis. In the population-based Atherosclerosis Risk in Communities (ARIC) study, the prevalence of ICAD representative of a US community-based population was 34.4% using VW-MR (11,12). These results suggest ICAD prevalence rates in asymptomatic populations using VW-MR more closely approximate estimates reported from autopsy studies (9,10,12). As intracranial VW-MR becomes a technique that is increasingly used among investigators, it is important to be aware of the diagnostic utility and limitations of the technique. In this review, we provide an update on the imaging technique, imaging features of ICAD by VW-MR, technical considerations and interpretive pitfalls. We also describe how studying ICAD with VW-MR may improve the diagnostic work-up of ischemic stroke. Finally, we discuss future directions and how multiparametric data on plaque features generated from intracranial VW-MR investigations may be used to develop methods for automatic lesion detection and assessment of radiomic features may aid patient risk-stratification for large scale assessments.
Intracranial VW-MR
Our ability to image beyond the lumen with VW-MR has allowed us to address diagnostic dilemmas in differentiating steno-occlusive vasculopathies, including ICAD (13). Previously, neuroimaging has largely been limited to lumen-based techniques such as computed tomography angiography (CTA) and MRA. These angiographic methods lack the ability to resolve intracranial vasculopathies as they only detail secondary luminal changes (13). Digital subtraction angiography (DSA) presents luminal and hemodynamic information with high spatial resolution but is invasive with risks of neurologic complications and radiation exposure (14). CTA is noninvasive, widely available, and fast but requires intravenous contrast administration and also exposes the patient to ionizing radiation. Time-of-flight MRA (TOF-MRA) addresses the limitations of DSA and CTA with no ionizing radiation or intravenous contrast administration but is susceptible to flow-artifacts, can overestimate stenosis measurements, and may be limited in detecting vascular pathology in the absence of luminal stenosis (15-17). With optimized contrast and spatial resolution, VW-MR imaging permits visualization of the vessel wall at the site of vascular injury. Studies comparing VW-MR with other angiographic techniques are shown in Table 1. Stenosis measurements by VW-MR have been shown to be comparable to measurements by DSA and have stronger correlations with DSA than measurements between DSA and CTA maximum intensity projections (19,21). Also, compared to 3D TOF-MRA, VW-MR sources images and VW-MR black blood luminal angiography show higher agreement with CTA for measuring degree of stenosis (22). As a noninvasive imaging technique that does not necessitate ionizing radiation, VW-MR may offer improved stenosis measurements that could complement conventional luminal techniques.
Full table
Intracranial applications of VW-MR followed technical developments of VW-MR for carotid arteries. Carotid VW-MR was used to characterize atherosclerotic plaque, efforts of which were partly driven by endarterectomy specimen availability (24). Atherosclerotic plaque features from carotid arteries could be identified with histopathologic confirmation and related to stroke risk (25). Extrapolating what we have learned from carotid plaque imaging, investigators have applied these concepts to interpreting VW-MR of intracranial arteries (26,27).
Intracranial VW-MR is a valuable diagnostic adjunct to conventional angiographic imaging to help distinguish intracranial vasculopathies. Studies report on its use for identifying cerebral vasculitis, reversible cerebral vasoconstriction syndrome (RCVS), arterial dissection, and ICAD (13,17,28-32). These studies describe imaging features of the vessel wall that improve the specificity of conventional angiographic imaging. For example, vasculitis is typically characterized by smooth, homogeneous, concentric wall thickening and enhancement in contrast with eccentric, and often heterogeneous wall thickening and enhancement with ICAD. Distinguishing vasculitis and RCVS can be challenging based on clinical and angiographic presentations but is especially important as management strategies differ (31). While both diseases can result in wall thickening, the vessel wall often shows no or only mild enhancement with RCVS, in contrast to avid wall enhancement seen with inflammatory vasculopathies (31). Intracranial VW-MR has also been shown to distinguish arterial dissections from other steno-occlusive vasculopathies by visualization of an intimal flap separating the true and false lumens and the presence of an intramural hematoma (33). These imaging features can differ from atherosclerotic plaque, which has a tendency to show focal, eccentric wall thickening. Given histologic verification of intracranial vasculopathies is largely unavailable, VW-MR imaging offers the most accurate assessment of in vivo vessel wall pathology available in the clinical domain.
VW-MR of ICAD
The advent of intracranial VW-MR has enabled a shift from studying luminal stenosis to intracranial vessel wall and plaque characteristics as predictors of plaque rupture and ischemic stroke. Qualitative and quantitative imaging features of ICAD include, but are not limited to, degrees and patterns of vessel wall thickening and enhancement, wall remodeling and eccentricity indices, signal intensities of plaque on pre-contrast T1- and T2-weighted acquisitions, plaque distribution/quadrant, and metrics of plaque length, volume, and per-patient burden. Which features are most strongly associated with symptomatic plaque is still poorly understood and the focus of recent meta-analyses (34,35). One such study evaluating VW-MR imaging features of culprit plaque and ischemic stroke reported plaque enhancement (OR, 10.09; 95% CI, 5.38–18.93), positive wall remodeling (OR, 6.19; 95% CI, 3.22–11.92), and plaque surface irregularity (OR, 3.94; 95% CI, 1.90–8.16) to be strongly associated with stroke events (34). Limitations of this meta-analysis, however, included methodological heterogeneity among studies in design, patient selection, and reporting outcomes. For example, patient selection was based on either lesion, symptom, or both among the 20 included articles (34) and the inclusion criterion for stenosis varied from any stenosis (36), >30% stenosis (37), ≥50% stenosis (38), to >70% stenosis (39). Also, among the 11 studies pooled to estimate the effect of degrees in plaque enhancement characteristics, the intravenous contrast injection-to-scan interval time ranged from 1.3 minutes (40) to “within 20 minutes” (41). Better understanding symptomatic plaque features to use as imaging biomarkers of ICAD would be helpful to increase diagnostic confidence in image interpretation and also provide guidelines for future trials.
Imaging biomarkers of ICAD
Vessel wall enhancement
Intracranial plaque enhancement has been shown to strongly correlate with ischemic stroke independent of the degree of stenosis (34,35,42,43). Both degree and persistence of enhancement at follow-up imaging strengthen diagnostic confidence in identifying culprit plaque and future risk for stroke recurrence (42,44). If plaque at baseline shows no enhancement or a decrease in the degree of enhancement at follow-up imaging, the plaque is unlikely to be a culprit lesion (42). Though likely multifactorial, mechanisms thought to underlie vessel wall enhancement in symptomatic plaque include neovascularization and inflammation, similar to unstable carotid plaque (45). An ultrastructural study of ruptured coronary plaques also showed increased density of thin-walled microvessels with compromised endothelial cell junctions extending from the adventitia, which can be extrapolated to gadolinium leakage in plaque (46). While mechanisms of intracranial plaque enhancement may be similar, enhancement may be a stronger biomarker of disease in intracranial arteries, considering their relative lack of vasa vasorum, compared with coronary and carotid arteries (47). This paucity of vasa vasorum in normal intracranial arteries is thought to be a consequence of surrounding cerebrospinal fluid (CSF) from which nutrients are thought to diffuse (48). Thus, vessel wall enhancement in healthy intracranial arteries is not expected though there are noteworthy exceptions. The intracranial internal carotid arteries and vertebral arteries, at the site of dural penetration, may show mild enhancement as vasa vasorum extend from the extracranial segments of the arteries (47,49). Thus, a mild degree of wall enhancement in these segments may be a normal finding. Moreover, mild vessel wall enhancement has been reported as part of aging, further limiting its specificity as an imaging marker (50). Autopsy studies support this finding and show vasa vasorum is more likely to be found in intracranial arteries with advancing age.
Arterial wall remodeling
The adaptability of arteries to disease makes VW-MR particularly advantageous to study ICAD. To compensate for atherosclerotic wall thickening, the vessel can remodel outwardly, preserving the lumen and blood flow, until this mechanism is overcome leading to angiographically detectable stenosis (51,52). Notably, in the population-based ARIC study, up to 10.8% of identified lesions by VW-MR were nonstenotic (12). The threshold for stenosis of intracranial vessels in response to atherosclerotic plaque accumulation has been estimated at approximately 55.3%, and the posterior circulation seems to have a greater ability to accommodate plaque formation (52). Possible explanations for this include differences in sympathetic innervation and flow dynamics. While investigations strive to better understand differences between the anterior and posterior circulations, having a higher index of suspicion may be warranted in patients with posterior circulation strokes when angiographic exams are negative. This is especially important given nearly 20–25% of ischemic strokes involve the posterior circulation (53-55) and there is a 33% 90-day risk of stroke after a first event from a vertebrobasilar stenosis (56).
Vessels may also show inward remodeling in which there is contraction of the outer wall with plaque development. A number of studies have reported associations between outward remodeling of intracranial arteries and increased plaque vulnerability (57-60). A possible explanation for this is the higher content of lipids and inflammatory cells (61). These inflammatory cells are often concentrated at the plaque shoulders (e.g., margins) where the fibrous cap is most prone to rupture (62). Understanding arterial wall remodeling is an important step to establish the biologic underpinnings of disease progression. VW-MR may play an important role in helping to characterize this mechanism.
Vessel wall thickening
Morphologic features of concentric and eccentric wall thickening and enhancement have been described to distinguish between plaque and other vasculopathies. While earlier studies have used eccentric vessel wall thickening patterns to identify intracranial plaque, an increasing number of studies report an overlap of eccentric and concentric wall thickening as plaque features (63-65). A meta-analysis pooling 7 studies to assess eccentric versus concentric features to identify culprit plaque also did not identify eccentricity to be significantly associated with culprit lesions (OR, 1.22; 95% CI, 0.51–2.91) (34). Histology also supports both eccentric and concentric wall thickening for atherosclerotic plaque features (66,67), suggesting a lack of specificity of this morphologic imaging biomarker. For example, one histologic study reported that while 69% of middle cerebral artery (MCA) plaques were eccentric, 75% of vertebral and 62% of basilar artery (BA) plaques were concentric (67). Another histology study showed concentric plaques in 63.9% and eccentric plaques in 26.1% of MCAs (66). T2-weighted imaging, which can show eccentric heterogeneous or hyperintense T2 signal in plaque, may also be a useful adjunct for identifying these lesions (28,68,69). However, T2-weighted imaging can require lengthy acquisition times considering its relatively low signal profile and this may be prohibitive especially for stroke patients.
Components of intracranial atherosclerosis
Although much of our understanding of the clinical implications of intracranial atherosclerotic plaque components detected by VW-MR originated from investigations of carotid plaque, the extent of the similarity between carotid and intracranial plaques is unclear. This is partly because histological correlation of in vivo VW-MR of ICAD is difficult to obtain with only a few case reports available (70,71). Moreover, adequate spatial resolution to image ICAD components in vivo is difficult to achieve. Intravascular ultrasound (IVUS) of in vitro circle of Willis (COW) specimens has been reported to have a 73.5% and 96.6% sensitivity and specificity, respectively, for plaque detection using histopathology as a reference standard. However, IVUS underestimated quantitative estimates of necrotic core and fibrofatty areas of plaque. In comparison, in vitro imaging showed 7 Tesla (7T) MR to have a positive predictive value of 88% and 93% for detecting fibrous and attenuated calcium deposits, respectively (72). In another study, T1, T2, T2*, and proton density signal characteristics of ex vivo intracranial plaque specimens were measured on ultra-high resolution 7T MR to identify lipid, fibrous tissue, fibrous cap, and calcifications (73). However, distinguishing these components in vivo using VW-MR at 3T poses a greater challenge given acquisition time and spatial resolution constraints relevant to the size of these structures. Several studies have used intrinsic T1 hyperintensity on VW-MR as a surrogate imaging biomarker for intraplaque hemorrhage (72,73). Xu and colleagues reported the prevalence of intraplaque hemorrhage in ICAD to be 19.6% in symptomatic and 3.2% in asymptomatic MCA plaque (39). Yu and colleagues reported a prevalence of 80.0% vs. 48.8% of VW-MR-identified intraplaque hemorrhage in symptomatic versus asymptomatic BA stenoses, respectively (74). However, autopsy studies suggest a far lower prevalence of intraplaque hemorrhage in intracranial plaque (67,75). One autopsy study reported a prevalence of 12% among middle cerebral arteries, vertebral arteries, and basilar arteries (67). This is in contrast to an 81% prevalence of intraplaque hemorrhage reported in a histologic study of carotid endarterectomy specimens (76), questioning our ability to directly extrapolate prevalence rates from extracranial carotid studies to ICAD.
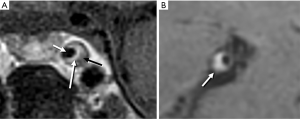
Multiple patterns of intracranial plaque by VW-MR have been reported. As seen with extracranial carotid plaques, intracranial plaque can present with a thin rim of enhancement overlying a hypoenhancing core with hypointense foci of calcification (Figure 1A). However, more often intracranial plaques are too small to identify these distinct features and instead show eccentric wall thickening and enhancement on T1-weighted imaging due to spatial resolution constraints (Figure 1B) (13).
Technical considerations for VW-MR
The small size of intracranial arteries challenges spatial resolution constraints to resolve vessel wall changes and plaque components by VW-MR. First, adequate spatial resolution is important for discriminating pathologic wall thickening. Prior work in carotid artery imaging showed wall thickening can be greatly exaggerated with seemingly small changes in spatial resolution (27,77). This artifactual thickening can mimic ICAD. Recognizing this pitfall, the Vessel Wall Imaging Study Group of the American Society of Neuroradiology issued an expert consensus recommending 0.5 mm isotropic resolution for intracranial VW-MR (13). This recommendation considered what was felt to be achievable at most institutions at reasonable acquisition times for in vivo imaging. However, vessel walls of the COW can vary in both healthy and diseased segments and may be below the acquired spatial resolution of 0.5 mm. COW specimens imaged using 7T VW-MR showed mean vessel wall thicknesses ranging between 0.45 to 0.66 mm with slightly thicker measurements in patients with symptomatic cerebrovascular disease (78). Thus, one should be wary of partial volume effects when interpreting these exams.
Also contributing to misidentifying ICAD is artifactual eccentric wall thickening due to an oblique orientation of a 2D image slice or reconstruction relative to the vessel wall, which is frequently encountered when imaging the tortuous intracranial arteries. Vessel segments should be viewed in orthogonal planes to distinguish circumferential and eccentric thickening. Acquiring 3D isotropic sequences and creating multiplanar reformats with thin slice thicknesses oriented orthogonal to the vessel axis can help minimize these volume-averaging effects. 3D isotropic resolution sequences also enable imaging of large fields of view within a reasonable scan time (79). Specifically, 3D variable flip angle turbo spin echo (VFA-TSE) pulse sequences have been increasingly used for intracranial VW-MR due to its scan efficiency compared to 2D TSE techniques (80). Vendor labels for single-slab 3D VFA-TSE pulse sequences include Sampling Perfection with Application optimized Contrasts by using different flip angle Evolutions (SPACE, Siemens Healthcare), Volumetric Isotropic TSE Acquisition (VISTA, Philips Healthcare), and CUBE (GE Healthcare); however, these sequences are not optimized for intracranial VW-MR and are not recommended without appropriate modifications.
Incomplete CSF and blood suppression artifacts are important to be aware of as these can be mistaken for wall thickening or enhancement on postcontrast imaging (81). Slow flow artifacts are particularly common near the vessel walls and in veins due to slower laminar flow along the walls. CSF suppression is particularly challenging since CSF flow is slow and nulling slow CSF flow signal which has a long T1 is particularly challenging. Acquiring imaging of the vessel segment in at least 2 planes may help confirm whether there is true wall enhancement though this is not always practical. Another strategy to avoid this artifact is to shorten the echo train (>60 turbo factor) to minimize larger free induction decay artifacts that can grow with longer echo train lengths (82). To minimize CSF and blood suppression artifacts, preparation pulses such as delayed alternating with nutation for tailored excitation, improved motion-sensitized driven-equilibrium, trailing magnetization flip-down, and anti-DRIVE preparations have been used (83-86). However, these preparation pulses may reduce vessel wall signal-to-noise (87) and further development to optimize vessel wall conspicuity would be advantageous.
Considering the lengthy acquisition time needed for the high spatial resolution of intracranial VW-MR imaging, poor image quality due to motion degradation is often reported. Up to 17% of cases are reported to be excluded due to motion degradation, highlighting a need for improved scan efficiency (80). Some sites perform only postcontrast imaging to minimize the burden of a lengthy exam (88-90). However, reports on intrinsic T1 hyperintense signal in plaque as a risk feature suggest pre-contrast imaging may be of value (74,91). Technical developments to improve scan efficiency while maintaining a high spatial resolution are ongoing with techniques such as compressed sensing (92,93).
ICAD and ischemic stroke
There are several mechanisms of ischemic stroke that result from ICAD. A primary mechanism is plaque rupture, which leads to occlusion of small penetrating arteries and hypoperfusion. Plaque rupture exposes the thrombogenic core and results in an in-situ thrombus, which can either occlude the artery locally or embolize distally. A second mechanism includes the development and growth of plaque over penetrating artery origins known as branch atheromatous disease. Third, severe stenosis or occlusion of the lumen may lead to hemodynamic impairment and hypoperfusion. These mechanisms can be inferred by infarct patterns on imaging (94) and combining information from both infarct patterns and intracranial plaque features by VW-MR may improve diagnostic specificity and patient selection for interventions (Figure 2).

The use of VW-MR to identify patients with plaques who will benefit from stenting versus medical management may be an important application in future clinical trials. The Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Arterial Stenosis (SAMMPRIS) trial concluded that patients with transient ischemic attacks or ischemic strokes due to 70–99% intracranial arterial stenosis have increased stroke or death outcomes within 30 days after treatment with percutaneous angioplasty and stenting compared to medical therapy alone (14.7% vs. 5.8%, P=0.06) (95). This led to a revision of the 2014 American Heart Association guidelines for managing patients with stroke due to severe intracranial arterial stenosis. Yet even with aggressive medical therapy, the SAMMPRIS trial reported a 12.2% annual risk of stroke recurrence though there were ensuing concerns about the study’s design and conclusions, including patient selection. The use of VW-MR to help stratify treatment groups has yet to be tested in interventional trials, but its ability to identify risk features beyond luminal narrowing supports its role in personalizing treatment strategies and improving outcomes particularly in this era of precision medicine.
Intracranial VW-MR may also have a role in managing patients with cryptogenic strokes. Up to 30–40% of strokes have no identifiable cause despite a comprehensive work-up (96-98). The work-up for these patients is often long and costly, including multiple imaging exams of the brain and heart and outpatient care with Holter monitoring. Nonstenotic intracranial plaque, given the possibility of outward remodeling, remains a potential explanation for cryptogenic strokes. The potential diagnostic value of VW-MR for assessing cryptogenic stroke has not been fully established but has shown promise for identifying culprit lesions not detectible by conventional imaging techniques.
Future directions
The clinical implementation of VW-MR for risk stratification and treatment of ICAD depends not only on availability of optimized sequences across different scanner platforms, but also on accurate and reliable interpretation of the images. Reliability estimates for qualitative assessment of intracranial VW-MR for plaque presence and stenosis degree have been shown to range from fair to good on non-contrast exams acquired on 3T MR scanners (12). Notably, reliability estimates for quantitative feature assessments generally range from good to excellent with semi-automated processing tools (12). Measures such as remodeling index, wall thickness measures, plaque area and wall area are well suited to support data-driven methods for lesion detection through semi-automated methods. Innovative investigations exploring these possibilities include automated intracranial vessel wall segmentation methods using convolutional neural networks and radiomic texture analysis of plaque (99,100). These techniques are heavily reliant on the quality of the provided data and interpretations. Future investigations to improve scan efficiency and studies on diagnostic accuracy are needed to facilitate clinical adoption. Image refinement and standardization of radiomic feature definitions and extraction methods are also needed in this era of increasing digitization. With the accumulation of large amounts of multiparametric data, artificial intelligence will facilitate maximizing diagnostic and prognostic yield from these acquired images. Analysis of large population-based studies will be able to leverage these innovative methods to acquire robust data on intracranial atherosclerosis and stroke-risk assessments.
Acknowledgments
Funding: This work was supported by the RSNA Research & Education Foundation, through grant number RSCH1929 and the Institute for Translational Medicine and Therapeutics/Thomas B. McCabe and Jeannette E. Laws McCabe Fund (JWS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the RSNA R&E Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in the Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-470). The series “Advanced Imaging in the Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. BAW discloses a patent (Patent No. 13/922,111). The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014;383:984-98. [Crossref] [PubMed]
- Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555-63. [Crossref] [PubMed]
- Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: The atherosclerosis risk in communities (ARIC) study. Neurology 2017;88:1556-63. [Crossref] [PubMed]
- Cantarero-Prieto D, Leon PL, Blazquez-Fernandez C, et al. The economic cost of dementia: a systematic review. Dementia (London) 2019. [Epub ahead of print].
- Gustavsson AM, van Westen D, Stomrud E, et al. Midlife atherosclerosis and development of alzheimer or vascular dementia. Ann Neurol 2020;87:52-62. [Crossref] [PubMed]
- Uehara T, Tabuchi M, Mori E. Risk factors for occlusive lesions of intracranial arteries in stroke-free japanese. Eur J Neurol 2005;12:218-22. [Crossref] [PubMed]
- Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large-vessel occlusive disease: the role of diabetes. J Neuroimaging 2003;13:224-7. [Crossref] [PubMed]
- Wong KS, Ng PW, Tang A, et al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 2007;68:2035-8. [Crossref] [PubMed]
- Gutierrez J, Elkind MS, Virmani R, et al. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke 2015;10:1074-80. [Crossref] [PubMed]
- Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008;39:1142-7. [Crossref] [PubMed]
- The atherosclerosis risk in communities (ARIC) study: design and objectives. the ARIC investigators. Am J Epidemiol 1989;129:687-702. [Crossref] [PubMed]
- Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016;280:860-8. [Crossref] [PubMed]
- Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the american society of neuroradiology. Am J Neuroradiol 2017;38:218-29. [Crossref] [PubMed]
- Kaufmann TJ, Huston J, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007;243:812-9. [Crossref] [PubMed]
- Mühlenbruch G, Das M, Mommertz G, et al. Comparison of dual-source CT angiography and MR angiography in preoperative evaluation of intra- and extracranial vessels: a pilot study. Eur Radiol 2010;20:469-76. [Crossref] [PubMed]
- Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: Evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005;26:1012-21. [PubMed]
- Song JW, Obusez EC, Raymond SB, et al. Vessel wall MRI added to MR angiography in the evaluation of suspected vasculopathies. J Neuroimaging 2019;29:454-7. [Crossref] [PubMed]
- Klein IF, Lavallée PC, Touboul PJ, et al. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology 2006;67:327-9. [Crossref] [PubMed]
- Liu Q, Huang J, Degnan AJ, et al. Comparison of high-resolution MRI with CT angiography and digital subtraction angiography for the evaluation of middle cerebral artery atherosclerotic steno-occlusive disease. Int J Cardiovasc Imaging 2013;29:1491-8. [Crossref] [PubMed]
- Lou X, Ma N, Shen H, et al. Noninvasive visualization of the basilar artery wall and branch ostia with high-resolution three-dimensional black-blood sequence at 3 tesla. J Magn Reson Imaging 2014;39:911-6. [Crossref] [PubMed]
- Lee NJ, Chung MS, Jung SC, et al. Comparison of high-resolution MR imaging and digital subtraction angiography for the characterization and diagnosis of intracranial artery disease. AJNR Am J Neuroradiol 2016;37:2245-50. [Crossref] [PubMed]
- Bai X, Lv P, Liu K, et al. 3D black-blood luminal angiography derived from high-resolution MR vessel wall imaging in detecting MCA stenosis: a preliminary study. AJNR Am J Neuroradiol 2018;39:1827-32. [Crossref] [PubMed]
- Kim DK, Verdoorn JT, Gunderson TM, et al. Comparison of non-contrast vessel wall imaging and 3-D time-of-flight MRA for atherosclerotic stenosis and plaque characterization within intracranial arteries. J Neuroradiol 2020;47:266-71. [Crossref] [PubMed]
- Wasserman BA, Smith WI, Trout HH, et al. Carotid artery atherosclerosis: In vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 2002;223:566-73. [Crossref] [PubMed]
- Qiao Y, Etesami M, Astor BC, et al. Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. AJNR Am J Neuroradiol 2012;33:755-60. [Crossref] [PubMed]
- Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009;72:627-34. [Crossref] [PubMed]
- Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011;34:22-30. [Crossref] [PubMed]
- Mossa-Basha M, Hwang WD, De HA, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 2015;46:1567-73. [Crossref] [PubMed]
- Mossa-Basha M, Shibata DK, Hallam DK, et al. Added value of vessel wall magnetic resonance imaging for differentiation of nonocclusive intracranial vasculopathies. Stroke 2017;48:3026-33. [Crossref] [PubMed]
- Song JW, Ojeda S, Romero JM. High resolution vessel wall MRI and vasculopathy related to herpes zoster ophthalmicus. Clin Imaging 2018;50:336-9. [Crossref] [PubMed]
- Obusez EC, Hui F, Hajj-Ali RA, et al. High-resolution MRI vessel wall imaging: Spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 2014;35:1527-32. [Crossref] [PubMed]
- Song JW, Guiry SC, Shou H, et al. Qualitative assessment and reporting quality of intracranial vessel wall MR imaging studies: a systematic review. AJNR Am J Neuroradiol 2019;40:2025-32. [PubMed]
- Obusez EC, Jones SE, Hui F. Vessel wall MRI for suspected isolated basilar artery dissection. J Clin Neurosci 2016;27:177-9. [Crossref] [PubMed]
- Lee HN, Ryu CW, Yun SJ. Vessel-wall magnetic resonance imaging of intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. Front Neurol 2018;9:1032. [Crossref] [PubMed]
- Gupta A, Baradaran H, Al-Dasuqi K, et al. Gadolinium enhancement in intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. J Am Heart Assoc 2016;5:e003816. [Crossref] [PubMed]
- Ryu CW, Jahng GH, Kim EJ, et al. High resolution wall and lumen MRI of the middle cerebral arteries at 3 Tesla. Cerebrovasc Dis 2009;27:433-42. [Crossref] [PubMed]
- Zhao DL, Deng G, Xie B, et al. Wall characteristics and mechanisms of ischaemic stroke in patients with atherosclerotic middle cerebral artery stenosis: a high-resolution MRI study. Neurol Res 2016;38:606-13. [Crossref] [PubMed]
- Yang WQ, Huang B, Liu XT, et al. Reproducibility of high-resolution MRI for the middle cerebral artery plaque at 3T. Eur J Radiol 2014;83:e49-55. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: Prevalence and clinical relevance. Ann Neurol 2012;71:195-8. [Crossref] [PubMed]
- Lu SS, Ge S, Su CQ, et al. MRI of plaque characteristics and relationship with downstream perfusion and cerebral infarction in patients with symptomatic middle cerebral artery stenosis. J Magn Reson Imaging 2018;48:66-73. [Crossref] [PubMed]
- Vakil P, Vranic J, Hurley MC, et al. T1 gadolinium enhancement of intracranial atherosclerotic plaques associated with symptomatic ischemic presentations. AJNR Am J Neuroradiol 2013;34:2252-8. [Crossref] [PubMed]
- Kwee RM, Qiao Y, Liu L, et al. Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI. Neuroradiology 2019;61:651-7. [Crossref] [PubMed]
- Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534-42. [Crossref] [PubMed]
- Kim JM, Jung KH, Sohn CH, et al. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke 2016;11:171-9. [Crossref] [PubMed]
- Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012;43:3023-8. [Crossref] [PubMed]
- Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol 2009;53:1517-27. [Crossref] [PubMed]
- Portanova A, Hakakian N, Mikulis DJ, et al. Intracranial vasa vasorum: Insights and implications for imaging. Radiology 2013;267:667-79. [Crossref] [PubMed]
- Zervas NT, Liszczak TM, Mayberg MR, et al. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg 1982;56:475-81. [Crossref] [PubMed]
- Takaba M, Endo S, Kurimoto M, et al. Vasa vasorum of the intracranial arteries. Acta Neurochir (Wien) 1998;140:411-6. [Crossref] [PubMed]
- Harteveld AA, van der Kolk AG, van der Worp HB, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol 2017;27:1585-95. [Crossref] [PubMed]
- Astor BC, Sharrett AR, Coresh J, et al. Remodeling of carotid arteries detected with MR imaging: atherosclerosis risk in communities carotid MRI study. Radiology 2010;256:879-86. [Crossref] [PubMed]
- Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke 2016;47:434-40. [Crossref] [PubMed]
- Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. [Crossref] [PubMed]
- Markus HS, van der Worp HB, Rothwell PM. Posterior circulation ischaemic stroke and transient ischaemic attack: diagnosis, investigation, and secondary prevention. Lancet Neurol 2013;12:989-98. [Crossref] [PubMed]
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988;19:1083-92. [Crossref] [PubMed]
- Gulli G, Marquardt L, Rothwell PM, et al. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: Pooled data analysis from prospective studies. Stroke 2013;44:598-604. [Crossref] [PubMed]
- Zhao DL, Deng G, Xie B, et al. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci 2015;22:700-4. [Crossref] [PubMed]
- Chung GH, Kwak HS, Hwang SB, et al. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol 2012;81:4069-74. [Crossref] [PubMed]
- Ryoo S, Lee MJ, Cha J, et al. Differential vascular pathophysiologic types of intracranial atherosclerotic stroke: a high-resolution wall magnetic resonance imaging study. Stroke 2015;46:2815-21. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010;212:507-11. [Crossref] [PubMed]
- Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105:939-43. [Crossref] [PubMed]
- Pasterkamp G, Schoneveld AH, van der Wal AC, et al. Inflammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unruptured plaques of femoral and coronary arteries. Arterioscler Thromb Vasc Biol 1999;19:54-8. [Crossref] [PubMed]
- Dieleman N, Yang W, Abrigo JM, et al. Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis. Stroke 2016;47:1797-802. [Crossref] [PubMed]
- Dieleman N, van der Kolk AG, van Veluw SJ, et al. Patterns of intracranial vessel wall changes in relation to ischemic infarcts. Neurology 2014;83:1316-20. [Crossref] [PubMed]
- Zhu X, Liu L, He X, et al. Wall thickening pattern in atherosclerotic basilar artery stenosis. Neurol Sci 2016;37:269-76. [Crossref] [PubMed]
- Yang WJ, Chen XY, Zhao HL, et al. In vitro assessment of histology verified intracranial atherosclerotic disease by 1.5T magnetic resonance imaging: Concentric or eccentric? Stroke 2016;47:527-30. [Crossref] [PubMed]
- Yang WJ, Fisher M, Zheng L, et al. Histological characteristics of intracranial atherosclerosis in a chinese population: a postmortem study. Front Neurol 2017;8:488. [Crossref] [PubMed]
- Yu YN, Liu MW, Villablanca JP, et al. Middle cerebral artery plaque hyperintensity on T2-weighted vessel wall imaging is associated with ischemic stroke. AJNR Am J Neuroradiol 2019;40:1886-92. [PubMed]
- Li ML, Xu W, Song L, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3 T. Atherosclerosis 2009;204:447-52. [Crossref] [PubMed]
- Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: Correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014;237:460-3. [Crossref] [PubMed]
- Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 2014;9:E19. [Crossref] [PubMed]
- Majidi S, Sein J, Watanabe M, et al. Intracranial-derived atherosclerosis assessment: An in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259-64. [Crossref] [PubMed]
- Harteveld AA, Denswil NP, Siero JCW, et al. Quantitative intracranial atherosclerotic plaque characterization at 7T MRI: An ex vivo study with histologic validation. AJNR Am J Neuroradiol 2016;37:802-10. [Crossref] [PubMed]
- Yu JH, Kwak HS, Chung GH, et al. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke 2015;46:2768-72. [Crossref] [PubMed]
- Denswil NP, van der Wal AC, Ritz K, et al. Atherosclerosis in the circle of Willis: Spatial differences in composition and in distribution of plaques. Atherosclerosis 2016;251:78-84. [Crossref] [PubMed]
- Derksen WJ, Peeters W, van Lammeren GW, et al. Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: A large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis 2011;218:369-77. [Crossref] [PubMed]
- Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med 2008;60:1020-8. [Crossref] [PubMed]
- Harteveld AA, Denswil NP, Van HW, et al. Ex vivo vessel wall thickness measurements of the human circle of willis using 7T MRI. Atherosclerosis 2018;273:106-14. [Crossref] [PubMed]
- Eiden S, Beck C, Venhoff N, et al. High-resolution contrast-enhanced vessel wall imaging in patients with suspected cerebral vasculitis: Prospective comparison of whole-brain 3D T1 SPACE versus 2D T1 black blood MRI at 3 Tesla. PLoS One 2019;14:e0213514. [Crossref] [PubMed]
- Song JW, Moon BF, Burke MP, et al. MR intracranial vessel wall imaging: a systematic review. J Neuroimaging 2020;30:428-42. [Crossref] [PubMed]
- Cornelissen BMW, Leemans EL, Coolen BF, et al. Insufficient slow-flow suppression mimicking aneurysm wall enhancement in magnetic resonance vessel wall imaging: a phantom study. Neurosurg Focus 2019;47:E19. [Crossref] [PubMed]
- Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging 2014;39:745-67. [Crossref] [PubMed]
- Cho SJ, Jung SC, Suh CH, et al. High-resolution magnetic resonance imaging of intracranial vessel walls: Comparison of 3D T1-weighted turbo spin echo with or without DANTE or iMSDE. PLoS One 2019;14:e0220603. [Crossref] [PubMed]
- Yang H, Zhang X, Qin Q, et al. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging 2016;44:665-72. [Crossref] [PubMed]
- Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017;77:1142-50. [Crossref] [PubMed]
- Choi JW, Han M, Hong JM, et al. Feasibility of improved motion-sensitized driven-equilibrium (iMSDE) prepared 3D T1-weighted imaging in the diagnosis of vertebrobasilar artery dissection. J Neuroradiol 2018;45:186-91. [Crossref] [PubMed]
- Cogswell PM, Siero JCW, Lants SK, et al. Variable impact of CSF flow suppression on quantitative 3.0T intracranial vessel wall measurements. J Magn Reson Imaging 2018;48:1120-8. [Crossref] [PubMed]
- Lindenholz A, van der Kolk A G, Zwanenburg JJM, et al. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology 2018;286:12-28. [Crossref] [PubMed]
- Ryu CW, Jahng G, Shin HS. Gadolinium enhancement of atherosclerotic plaque in the middle cerebral artery: relation to symptoms and degree of stenosis. AJNR Am J Neuroradiol 2014;35:2306-10. [Crossref] [PubMed]
- Jang J, Kim T, Hwang E, et al. Assessment of arterial wall enhancement for differentiation of parent artery disease from small artery disease: comparison between histogram analysis and visual analysis on 3-dimensional contrast-enhanced T1-weighted turbo spin echo MR images at 3T. Korean J Radiol 2017;18:383-91. [Crossref] [PubMed]
- Wu F, Song H, Ma Q, et al. Hyperintense plaque on intracranial vessel wall magnetic resonance imaging as a predictor of artery-to-artery embolic infarction. Stroke 2018;49:905-11. [Crossref] [PubMed]
- Guggenberger K, Krafft AJ, Ludwig U, et al. High-resolution compressed-sensing T1 black-blood MRI: a new multipurpose sequence in vascular neuroimaging? Clin Neuroradiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Zhu C, Tian B, Chen L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). MAGMA 2018;31:457-67. [Crossref] [PubMed]
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106-14. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]
- Kolominsky-Rabas PL, Weber M, Gefeller O, et al. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735-40. [Crossref] [PubMed]
- Petty GW, Brown RD, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30:2513-6. [Crossref] [PubMed]
- Marnane M, Duggan CA, Sheehan OC, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the north Dublin population stroke study. Stroke 2010;41:1579-86. [Crossref] [PubMed]
- Shi Z, Zhu C, Degnan AJ, et al. Identification of high-risk plaque features in intracranial atherosclerosis: Initial experience using a radiomic approach. Eur Radiol 2018;28:3912-21. [Crossref] [PubMed]
- Shi F, Yang Q, Guo X, et al. Vessel wall segmentation using convolutional neural networks. IEEE Trans Biomed Eng 2019;66:2840-47. [Crossref] [PubMed]


