
Ephrin B2 mediates high glucose induced endothelial-to-mesenchymal transition in human aortic endothelial cells
Introduction
Diabetes is a growing epidemic worldwide. As one of the most serious manifestations of the disease, cardiovascular complications has received more and more attention (1,2). Endothelial cell (EC) damage is an initiating factor in the process of vascular complication in diabetes, and high glucose (HG) is involved in the onset and progression of EC damage (3-5). Using transgenic lineage-tracing techniques, previous studies have demonstrated that HG could induce EC damage via endothelial-to-mesenchymal transition (EndMT) (6,7). EndMT could be characterized as the EC lose their endothelial characteristics and gain the mesenchymal phenotype, accompanied by the reduction of the EC markers, such as CD31 and overexpression of mesenchymal cell markers, such as fibroblast-specific protein 1 (FSP1) (8). Multiple studies have demonstrated that EndMT contributed to EC damage (9,10). However, the underlying mechanism of HG-induced EndMT remains unclear.
Ephrin B2 is a member of the largest receptor tyrosine kinases, which plays an important role in vascularization, development, nervous system patterning, cancer and angiogenesis (11). Ephrin B2 is expressed in both endothelial and pericyte cells (12,13), and is important for cell-cell communication (14). Recently, Ephrin B2 has been further found to be associated with EC damage (15). In addition, numerous studies have reported that inhibition of Ephrin B2 might prevent epithelial injury via epithelial-to-mesenchymal transition (EMT) (15,16). As we known, EndMT is classified as a specialized form of EMT (17). These findings suggested the participation of Ephrin B2 in EC damage. Interestingly, it remains unclear whether Ephrin B2 was involved in HG induced EC damage.
Therefore, we hypothesized that HG could induce EC damage via an Ephrin B2-dependent pathway in human aortic ECs (HAECs). In this study, we examine the effects of HG on EndMT in vivo and investigate the underlying mechanism of Ephrin B2 regulation.
Methods
Cell culture and reagents
Primary HAECs were purchased from ScienCell Research Laboratories (No. 6100) and cultured as previously described (18). Cells at passage 2–5 were selected for this study. At approximately 75% confluence, the culture medium was changed to a serum-free solution for 12 hours prior to their further experiments. To examine the effects of HG on Ephrin B2 and EndMT in HAECs, cells were treated with normal glucose (NG, 5.5 mM), 15 mM D-glucose, 30 mM HG and mannitol (MN, 5.5 mM NG +24.5 mM mannitol) for 48 hours, respectively. Obviously, MN was used as a control hyperosmolarity.
Western blot
Equal amounts of protein (20 µg) were obtained for cell lysates, electrophoresed on 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane (Pall) by electroblotting. Blots were incubated overnight with primary antibodies against CD31 (sc-376764, Senta Cruz, 1:1,000 dilution), FSP1 (ab-197896, Abcam, 1:800 dilution), Ephrin B2 (ab-131536, Abcam, 1:1,000 dilution), focal adhesion kinase (FAK) (ab-40794, Abcam, 1:1,000 dilution), phospho-FAK (ab-4792, Abcam, 1:1,000 dilution) and GAPDH (ab-181602, Abcam, 1:1,000 dilution) followed by the horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (7074s, CST, 1:2,000 dilution) or HRP-conjugated anti-mouse IgG (7076s, CST, 1:2,000 dilution). Signals were detected by an ECL system (GE Healthcare). The bands were measured by Image J software (NIH, Bethesda).
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted using RNAiso Plus according to the manufacture’s directions (TAKARA). The concentrations and purity of the samples were detected by Nanodrop 2000 (Thermo). A relative absorbance ratio at 260/280 nm between 1.8–2.0 were used. GAPDH was used as reference for normalization. The relative expression of mRNA was detected by the 2–ΔΔCt methods. The primers were presented in Table 1.
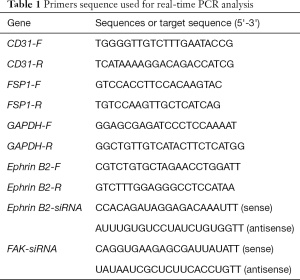
Full table
Immunofluorescence staining
Coverslips were fixed with 4% paraformaldehyde, and blocked with 5% BSA in PBS at room temperature for 30 minutes. Cells were then immunostained with primary antibodies against CD31 (sc-376764, Senta Cruz, 1:250 dilution) at 4 °C overnight. Then the cells were incubated with the fluorescent secondary antibodies (A-31570, Invitrogen, 1:300 dilution) for 1 hour in the dark and finally counterstained with DAPI (G1012, Servicebio).
Small interfering RNA (siRNA) transfection
siRNA targeting Ephrin B2 and FAK were purchased from Genepharm. Transfection reagent Lipo 2000 were purchased from Invitrogen (No. 12566014). On the day before the transfection, HAECs that were in the phase of logarithmic growth were seeded on 6-well plate. Transfections were conducted when the HAECs were cultured approximately 75% confluence. And the cells were transfected based on the transfection reagent manufacturer’s protocol. Briefly, the siRNA and Lipo 2000 were mixed with Opti-MEM (31985070, Gibco) respectively at room temperature before seeding in a well containing serum-free medium. Replacement of Opti-MEM by complete growth media was done at 6 hours post-transfection.
Statistical analysis
Data were expressed as the means ± standard deviation (SD) and analyzed by one-way analysis of variance (ANOVA) using SPSS version 20.0. Results were considered significant if P<0.05. Experiments were repeated at least three times.
Results
HG triggered EndMT in cultured HAECs
To determine the effects of HG on EndTM, we treated HAECs with HG (30 mM, 48 hours). We observed that HAECs exposed to HG acquired a spindle-shaped morphology reminiscent of the phenotypic changes typically associated with EndMT (Figure 1A). Parallel with the findings above, we performed qTR-PCR and western blot analysis. We found that the endothelial marker CD31 was reduced while the myofibroblast marker FSP1 decreased after HG treatment (Figure 1B,C,D), which was characteristic of EndMT. In addition, we performed immunofluorescence labeling experiments, and observed that HAECs exhibited round cellular morphology. In contrast, cells in the HG group exhibited a spindle-shaped morphology and the expression of CD31 were decreased (Figure 1E). The above results confirmed that HG triggered EndMT in HAECs.
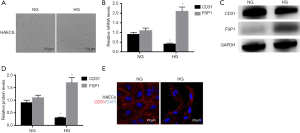
HG upregulated Ephrin B2 expression in HAECs
We next examined the expression of Ephrin B2 after HG incubation. As shown in Figure 2A,B,C, the results of the qRT-PCR and western blot indicated that the mRNA and protein levels of Ephrin B2 was up-regulated in HG group.

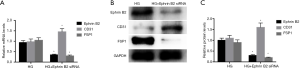
Blocking Ephrin B2 inhibited HG-induced EndMT in HAECs
To confirmed the role of Ephrin B2 in HG induced EndMT, HAECs were incubated with HG in the presence of absence of the Ephrin B2 siRNA. As shown in Figure 3A,B,C, the results of the qRT-PCR and western blot revealed that Ephrin B2 siRNA transfection could effectively decreased the expression of Ephrin B2. In addition, we found a significant decrease in CD31 expression while an increase in FSP1 in HG+Ephrin B2 siRNA group compared to the HG group. These data suggested that HG-induced EndMT might be partially mediated by Ephrin B2.
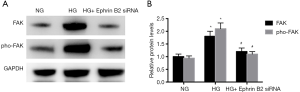
Ephrin B2 blockade suppressed the HG-induced FAK pathway
To gain further insight into the mechanism involved in HG-induced EndMT, we examined the expression of FAK and phosphorylated FAK (pho-FAK) in HAECs treated with HG and Ephrin B2 siRNA. As shown in Figure 4A,B, the protein levels of FAK and pho-FAK were increased in HG group compared with the NG group. Moreover, the administration of Ephrin B2 siRNA largely blocked the FAK and pho-FAK expression after HG incubation. These results suggest that FAK pathway could be activated after HG incubation, while the blocking of Ephrin B2 suppressed the HG-induced FAK pathway.
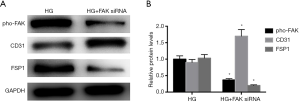
FAK siRNA inhibits the HG-induced EndMT
We further evaluated the influence of FAK pathway on the markers related to EndMT in HAECs. As shown in Figure 5A,B, the FAK siRNA significantly decreased the FAK expression. In addition, FAK siRNA largely prevented the protein levels of FSP1 and improved CD31 expression. That’s to say, the inhibition of FAK prevented HG-induced EndMT of HAECs.
Discussion
EC damage is a hallmark of vascular complications in diabetes. In the current study, we showed that Ephrin B2 mediated the HG-induced EndMT in HAECs. Since EndMT is recognized as EC damage in vascular disease, our study provides a novel mechanism for vascular disease in diabetes.
Hyperglycemia induces endothelial injury by producing lots of multiple protein, which serve roles in EndMT. Yu et al. identified that HG-induced EndMT in HAECs was mediated by TGF-β (19). Shang et al. proved that NOD2 promoted EndMT in glomerular ECs in diabetes (20). The results of our study demonstrated that HG-induced EndMT was accompanied by increased Ephrin B2 expression. In addition, blockade of Ephrin B2 by siRNA were found to significantly inhibit EndMT. These results came in agreement with the study that showed an increased expression of Ephrin B2 in proliferative angiogenesis as in diabetic retinopathy and tumors (12). Recently, Xiong et al. found Ephrin B2 inhibition markedly attenuated EC damage (15). Taken together, these findings showed a crucial role for Ephrin B2 activation in EC injury in diabetes.
Previous studies demonstrated that Ephrin B2 activation triggers different intracellular pathways. Liu et al. showed that PI3K/AKT and PLC-γ/PKC signaling pathway is involved in Ephrin B2 activation in tumor cells (21). Vrahnas et al. proved that Ephrin B2 suppresses autophagy in osteocytes through a Rho-ROCK-dependent pathway (22). Das et al. found that Ephrin B2 promote chemotaxis of ECs through Erk phosphorylation and KLF2 up-regulation (23). In our current study, we further evaluated whether the FAK pathway is implicated in Ephrin B2-mediated ECs in HAECs treated with HG. As the data show, we observed that Ephrin B2 siRNA could partially attenuate the HG-induced FAK activation in HAECs. Then, we found that blockage of FAK could further inhibit EndMT with HG. FAK was a widely expressed non-receptor protein tyrosine kinase, which regulated several biological processes (e.g., cell adhesion, migration and angiogenesis) and controlled the epithelial morphology (24). Previous studies showed that activated FAK pathway could promote EMT in gastric carcinoma cells and colorectal carcinoma cells, which accelerated the cancer progression (16,25). In our present study, FAK might be a downstream effector of the HG-Ephrin B2 pathway and drive EndMT in HAECs. To our knowledge, this is the first study linking HG to Ephrin B2/FAK in vascular disorders under hyperglycemia condition.
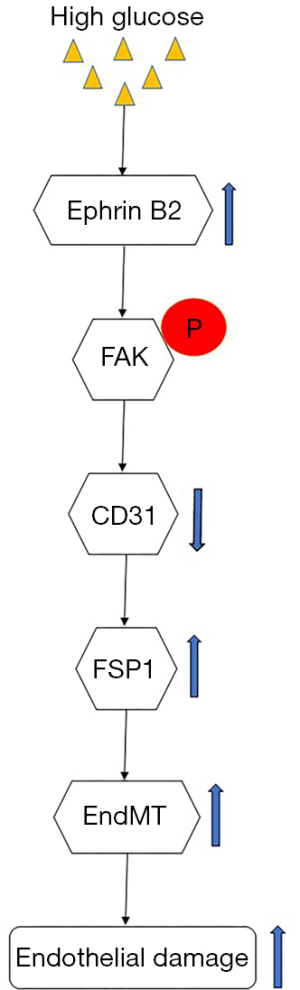
In summary, our work showed that Ephrin B2 expression was up-regulated after HG treatment. HG-induced Ephrin B2 signaling triggered EndMT and endothelial damage, which mediated by the downstream FAK pathway (Figure 6). These findings suggest that an improved understanding of the Ephrin B2-dependent pathway might lead to a novel therapeutic target for vascular disease in diabetes.
Acknowledgments
Funding: This work was supported by grants from the Fundamental Research Funds for the Central University (2042020kf0137), Hubei Province Health and Family Planning Scientific Research project (WJ2019MB103), Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (znpy2017044), the Clinical Research Project for Wu Jieping Medical Foundation (320.6750.19089-58), the Research Fund from Medical Sci-Tech Innovation Platform of Zhongnan Hospital, Wuhan University (PTXM2020028) and the National Natural Science Foundation of China (81270184).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-299
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-299). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was not required, because this study does not involve patients or animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: Determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis 2019;62:306-14. [Crossref] [PubMed]
- Sharma AN, Deyell JS, Sharma SN, et al. Role of and Recent Evidence for Antiplatelet Therapy in Prevention of Cardiovascular Disease in Diabetes. Curr Cardiol Rep 2019;21:78. [Crossref] [PubMed]
- Gonzalez-Blazquez R, Alcala M, Fernandez-Alfonso MS, et al. Relevance of control diet choice in metabolic studies: impact in glucose homeostasis and vascular function. Sci Rep 2020;10:2902. [Crossref] [PubMed]
- Wang T, Zhu H, Hou Y, et al. Ketamine attenuates high-glucose-mediated endothelial inflammation in human umbilical vein endothelial cells. Can J Physiol Pharmacol 2020;98:156-61. [Crossref] [PubMed]
- Liu J, Chen S, Biswas S, et al. Glucose-induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol Rep 2020;8:e14331. [Crossref] [PubMed]
- Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 2008;19:2282-7. [Crossref] [PubMed]
- Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 2009;175:1380-8. [Crossref] [PubMed]
- Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev 2019;99:1281-324. [Crossref] [PubMed]
- Wesseling M, Sakkers TR, de Jager SCA, et al. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vascul Pharmacol 2018;106:1-8. [Crossref] [PubMed]
- Hao YM, Yuan HQ, Ren Z, et al. Endothelial to mesenchymal transition in atherosclerotic vascular remodeling. Clin Chim Acta 2019;490:34-8. [Crossref] [PubMed]
- Theofanous SA, Florens MV, Appeltans I, et al. Ephrin-B2 signaling in the spinal cord as a player in post-inflammatory and stress-induced visceral hypersensitivity. Neurogastroenterol Motil 2020;32:e13782. [Crossref] [PubMed]
- Coucha M, Barrett AC, Elgebaly M, et al. Inhibition of Ephrin-B2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats. PLoS One 2019;14:e0210523. [Crossref] [PubMed]
- Ghori A, Freimann FB, Nieminen-Kelha M, et al. EphrinB2 Activation Enhances Vascular Repair Mechanisms and Reduces Brain Swelling After Mild Cerebral Ischemia. Arterioscler Thromb Vasc Biol 2017;37:867-78. [Crossref] [PubMed]
- Heroult M, Schaffner F, Pfaff D, et al. EphB4 promotes site-specific metastatic tumor cell dissemination by interacting with endothelial cell-expressed ephrinB2. Mol Cancer Res 2010;8:1297-309. [Crossref] [PubMed]
- Xiong C, Wen Y, Zhao J, et al. Targeting Forward and Reverse EphB4/EFNB2 Signaling by a Peptide with Dual Functions. Sci Rep 2020;10:520. [Crossref] [PubMed]
- Alam SK, Yadav VK, Bajaj S, et al. DNA damage-induced ephrin-B2 reverse signaling promotes chemoresistance and drives EMT in colorectal carcinoma harboring mutant p53. Cell Death Differ 2016;23:707-22. [Crossref] [PubMed]
- Cruz-Solbes AS, Youker K. Epithelial to Mesenchymal Transition (EMT) and Endothelial to Mesenchymal Transition (EndMT): Role and Implications in Kidney Fibrosis. Results Probl Cell Differ 2017;60:345-72. [Crossref] [PubMed]
- Ni LH, Tang RN, Yuan C, et al. Cinacalcet attenuated bone loss via inhibiting parathyroid hormone-induced endothelial-to-adipocyte transition in chronic kidney disease rats. Ann Transl Med 2019;7:312. [Crossref] [PubMed]
- Yu CH. High glucose induced endothelial to mesenchymal transition in human umbilical vein endothelial cell. Exp Mol Pathol 2017;102:377-83. [Crossref] [PubMed]
- Shang J, Zhang Y, Jiang Y, et al. NOD2 promotes endothelial-to-mesenchymal transition of glomerular endothelial cells via MEK/ERK signaling pathway in diabetic nephropathy. Biochem Biophys Res Commun 2017;484:435-41. [Crossref] [PubMed]
- Liu R, Yu R, Cui Y, et al. Inhibitory effect of taspine derivative TAD1822-7 on tumor cell growth and angiogenesis via suppression of EphrinB2 and related signaling pathways. Acta Pharm 2019;69:423-31. [Crossref] [PubMed]
- Vrahnas C, Blank M, Dite TA, et al. Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone. Nat Commun 2019;10:3436. [Crossref] [PubMed]
- Das A, Shergill U, Thakur L, et al. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk-dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol 2010;298:G908-15. [Crossref] [PubMed]
- Serrels A, Canel M, Brunton VG, et al. Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell Adh Migr 2011;5:360-5. [Crossref] [PubMed]
- Wu J, Long Z, Cai H, et al. Homeobox B7 accelerates the cancer progression of gastric carcinoma cells by promoting epithelial-mesenchymal transition (EMT) and activating Src-FAK pathway. Onco Targets Ther 2019;12:3743-51. [Crossref] [PubMed]


