
Performance of cardiovascular magnetic resonance strain in patients with acute myocarditis
Introduction
Acute myocarditis is associated with high rates of morbidity and mortality, accounting for 2–42% of sudden cardiac death cases in young people. It is the underlying etiology in 9–16% of adult idiopathic dilated cardiomyopathy cases (1,2). The clinical manifestations of acute myocarditis are highly variable; the condition can be asymptomatic or manifest with severe heart failure and even cause sudden death. Early clinical diagnosis is challenging but extremely important for determination of treatment strategy and evaluation of prognosis.
Cardiac magnetic resonance (CMR) is a noninvasive examination modality with excellent spatial, temporal and contrast resolution, and is the gold standard for precise assessment of atrial and ventricular function. It further allows evaluation of myocardial edema, hyperaemia, necrosis and fibrosis, using T2-weighted imaging (T2WI) and early and late gadolinium enhancement (EGE and LGE, respectively) (3). Currently, the “Lake Louise Criteria” is widely used for the diagnosis of myocarditis with CMR. LGE evidence of necrosis/fibrosis is an important criterion and also correlates with prognosis (4-6).
Left ventricular ejection fraction (LVEF) is the parameter most commonly used to describe LV function and predict prognosis. However, in early stages of LV dysfunction, EF is preserved and therefore of limited value. Feature tracking imaging (FTI) is a novel technique that allows quantitative segmental and global strain analysis based on standard cine CMR images. Previous studies (7,8) demonstrated good inter- and intra-observer reproducibility of FTI-derived strain indices and a robust correlation with strain parameters derived from CMR tagging (9,10). Recently, CMR feature tracking was recently reported to be sensitive in detecting early LV dysfunction by strain deformation and twist analysis (11-15).
This study aims to explore the value of CMR feature tracking for detection of early LV dysfunction in acute myocarditis and correlations with the LVEF and LGE. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-221).
Methods
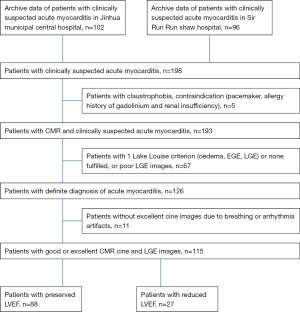
Patient population
The study included 198 patients with clinically suspected acute myocarditis from two centres evaluated between January 2013 to July 2018. The CMR studies were performed with a Siemens scanner in 66 patients, with a GE scanner in 59 patients, and with a Philips scanner in 11 patients. The inclusion criteria were developed based on the 2013 ESC guidelines (2). Clinically suspected acute myocarditis was diagnosed when the patients had acute chest pain and met at least one of the following criteria: new ECG abnormalities, myocardial cytolysis markers, and wall motion abnormalities on echocardiography. In patients without chest pain, who met 2 or more criteria, additional requirements included absence of angiographically detectable coronary artery disease (CAD) (or age <30 years and a low risk of CAD) and an absence of known pre-existing cardiovascular disease or secondary conditions. The definite diagnosis was made when 2 or more Lake Louise criteria (oedema, EGE, LGE) were fulfilled. Exclusion criteria included poor cine image quality caused by respiratory motion, arrhythmia, and poor LGE image due to poor myocardium nulling. A total of 115 patients were finally enrolled (Figure 1). The patients were divided into EF preserved group (EF ≥50%) and EF reduced group (EF <50%). In all, 50 normal volunteers were enrolled as the control group; the age range of the volunteers was 15–64 years, with an average age of 38.76±11.67 years, and 26 volunteers were female. The normal volunteers underwent non-contrast CMR. The research was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). This study was approved by ethics committee of Jinhua Hospital, Zhejiang University School of Medicine (2018-96), and informed consent was taken from all the patients and volunteers.
CMR protocols
CMR images were obtained with three systems, including two 1.5-T systems and one 3-T system. The 1.5-T Siemens system (Avanto, Germany) used an 8-channel phased-array body coil. True fast imaging with steady precession (True FISP) cine was used to obtain two-, three-, and four-chamber view as well as short axis (SA) views, with the following parameters: slice thickness =8 mm; gap =2 mm; FOV =340 mm × 276 mm; TR =39.75 ms; TE =1.1 ms; flip angle =42°; cardiac phases =25. Ten minutes after the injection of 0.2 mmol/kg gadodiamide (Omniscan, GE Healthcare, Ireland), LGE imaging consisting of magnitude and phase-sensitive inversion recovery (PSIR) images was performed, including two-, three-, and four-chamber views as well as SA views with the following parameters: TR =700 ms; TE =3.36 ms; flip angle =25°; TI =200–300 ms; slice thickness =8 mm; and gap =2 mm. The coverage of the SA was the same as that of the SA cine. The 1.5-T GE system (Signa CV/i, GE Healthcare, Milwaukee, WI, USA) used an 8-channel phased-array cardiac coil. All patients underwent examination by a standard protocol that included two-, three-, four-chamber, and an SA bright-blood cine sequence (fast imaging employing steady-state acquisition, FIESTA) covering the entire left ventricle with the following parameters: slice thickness =8 mm; gap =2 mm; TR/TE =35/1.5 ms; flip angle =45°; FOV =360 mm × 280 mm; views per segment (VPS) =14, and slice-reconstructed cardiac phases =20. Myocardial delayed-enhancement magnitude images were acquired approximately 10 minutes after the injection of gadodiamide, with TR =6.5 ms, TE =3.0 ms, flip angle =20°, FOV =360 mm × 270 mm, slice thickness =8 mm, gap =2 mm, and TI =170–280 ms, including two-, three- and four-chamber views, as well as SA images (approximately 6–10 slices from the apex to base). The 3.0-T system (Achieva, Philips, Netherlands), used a 16-channel phased-array torso coil. The CMR protocol included SA, two-,three-,and four-chamber views for sense-balanced turbo field echo (sBTFE) sequences, with FOV =320 mm × 300 mm, TR/TE =3/1 ms, flip angle =45°, slice-reconstructed cardiac phases =25, slice thickness =8 mm, and gap =2 mm. The coverage of the SA view was the whole left ventricle from the base to the apex. LGE images were acquired 10 minutes after the injection of 0.2 mmol/kg of gadodiamide using a PSIR turbo field echo (PSIR-TFE) sequence, with FOV =300 mm × 300 mm, TR/TE =3/1 ms, TI =300–450 ms, flip angle =25°, slice thickness =8 mm, and gap =2 mm and including two-, three- and four-chamber and SA views. The coverage of the SA view was the same as that of the SA cine.
CMR analysis
CMR image analysis included three parts: global LV function and volume analysis; LGE analysis; and feature tracking analysis. All parts were performed using CVI42 version 5.9 (Circle, Canada) by an experienced radiologist (more than 5 years’ experience in CMR).
Global LV function and volume analysis
Automated segmentation of the left ventricle was performed using the Short 3D Module of the LV Function Module. The indices obtained included the end systolic volume (ESV) and index (ESVi), the end diastolic volume (EDV) and index (EDVi), the stroke volume (SV) and index (SVi), the LVEF, the peak ejection rate (PER), the peak filling rate (PFR), and the LV volume, which was defined as the range from the apex to the annulus of the mitral valve. The papillary muscle was excluded from the mass and included in the LV volume (16). At the base, the slices were deemed to be within the left ventricle when the volume was encircled by 50% or more of ventricular myocardium (17); otherwise, they were considered to be within the left atrium and were excluded.
LGE analysis
Quantitative evaluation of LGE was performed using the Tissue Characterization Module. A region of interest (ROI) approximately 20 mm2 in size was placed at the remote myocardium in each slice of axis LGE images, and the signal intensity was acquired [mean ± standard deviation (SD)]. The extent of LGE images acquired by scanners was calculated automatically in each slice, as the mass of LGE and the proportion of the total LV mass, according to the formula (threshold = mean +3 SD). An experienced radiologist (with more than 5 years of experience in CMR) checked the extent of LGE and made minor manual adjustments when necessary. Finally LGE and LGE proportion (LGE%) were obtained.
Feature tracking analysis
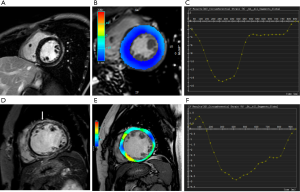
Feature tracking analysis of the left ventricle was performed using the Tissue Tracking Module to obtain LV strain data (Figure 2). The SA cine was loaded together with the two, three- and four-chamber cines to perform 3D LV strain analysis. In the SA view, segmentation of left ventricle was the same as that in the global LV function and volume analysis. In the two-, three- and four-chamber views, the base and apex were defined manually and required adjustment of the automatically computed endo- and epicardial contours. The parameters obtained included the peak strain radial (PSR), peak strain circumferential (PSC) and peak strain longitudinal (PSL), peak systolic strain rate radial (PSSRR), peak systolic strain rate circumferential (PSSRC), peak systolic strain rate longitudinal (PSSRL), peak diastolic strain rate radial (PDSRR), peak diastolic strain rate circumferential (PDSRC), peak diastolic strain rate longitudinal (PDSRL). Intraobserver and interobserver agreement was tested using intraclass correlation coefficients in a subset of 10 subjects.
Statistical analysis
The quantitative data are presented as the mean ± SD or percentage. The normal distribution was assessed by Kolmogorov-Smirnov test. The independent samples t-test was used for parametric data between groups, and Mann-Whitney U test was used for non-parametric data. The chi-squared test or the fisher exact test was used to analyse the categorical variables. Correlation analysis between EF, LGE, LGE% and strain analysis parameters were performed by Spearman correlation analysis. The discriminatory power of various parameters for evaluating LV function was tested according to the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Reproducibility was evaluated by inter- and intraobserver agreement and analyzed by intraclass correlation coefficients. Statistical analysis was performed using SPSS for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA) and Graphpad Prism for Windows (version 7.00; Graphpad Software, Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
Inter- and intraobserver agreement
Before FTI was used to do the strain analysis, a subset of 10 cases were used to test the intraobserver and interobserver agreement. The intraobserver and interobserver agreement were good for all global strain. The intraclass correlation coefficients of intraobserver (ICC >0.90) and interobserver (ICC >0.85) were good.
Clinical characteristics of acute myocarditis
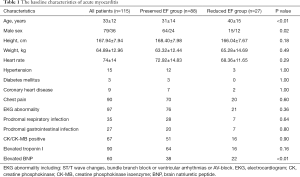
The baseline characteristics of EF reduced and preserved myocarditis patients groups are shown in Table 1. There were more male patients in EF preserved myocarditis patients group than the patients in EF reduced group (64 males and 24 females vs. 15 males and 12 females), and the patients were elder in EF reduced myocarditis patients group (31±14 vs. 40±15 years).The proportion of brain natriuretic peptide (BNP) elevated patients were higher in EF reduced myocarditis patients group (38/88 vs. 22/27), the differences were statistically significant. And the differences in the remaining parameters such as elevated troponin I patients, elevated creatine phosphokinase isoenzyme (CK/CK-MB) patients, electrocardiogram (EKG) abnormality patients, prodromal patients, etc. were not statistically significant.
Full table
Comparisons of groups, EF preserved myocarditis patients group vs. control group, EF reduced myocarditis patients group vs. EF preserved myocarditis patients group
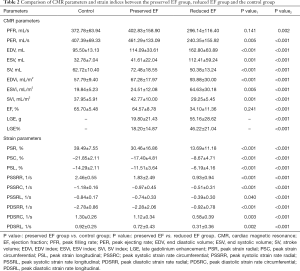
The comparison of acute myocarditis patients and healthy control group showed statistically significant differences in CMR parameters and strain parameters except PER and PFR. The left ventricular chamber were dilated in acute myocarditis patients group, LVEF and strain parameters were decreased.
Compared with the control group (Table 2, Figure 3), the EF preserved myocarditis patients group showed an increased PER, EDV, ESV, SV, EDVi, ESVi, SVi and all decreased strain indices, but the differences in the LVEF and PFR were not significant. Among the EF reduced and preserved patients groups, the comparison indicated significant differences in all CMR parameters and strain parameters, the EF reduced myocarditis patients group showed dilated left ventricular chamber (EDV, ESV, SV, EDVi, ESVi, SVi), decreased left ventricular function (EF, PER, PFR), strain (PSR, PSC, PSL) systolic strain rate (PSSRR, PSSRC, PSSRL) and diastolic strain rate (PDSRR, PDSRC, PDSRL), as well as more LGE and LGE%.
Full table
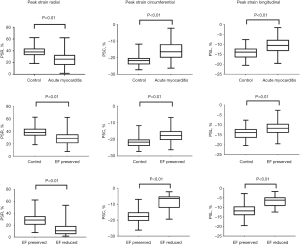
Correlation of strain parameters with EF and LGE
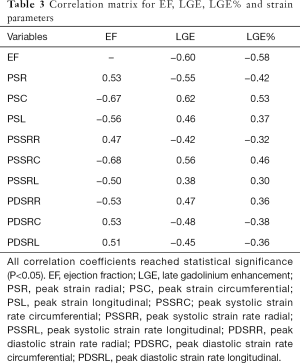
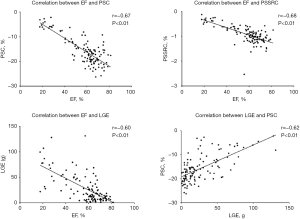
The correlation analysis (Table 3, Figure 4) showed that the EF was well correlated with the LGE, PSC, PSSRC (r>0.6). LGE correlated with PSC well (r=0.62), but the correlation between LGE and other strain indices was not strong (r<0.6) although all correlation coefficients reached statistical significance. LGE% was not well correlated with the EF and all strain parameters neither (r<0.6).
Full table
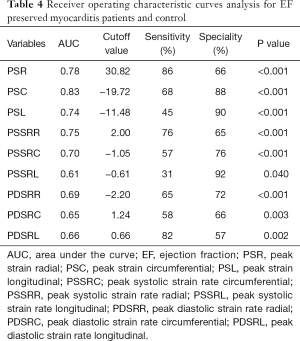
Diagnostic performance of strain parameters in EF preserved myocarditis patients, strain parameters and LGE in EF reduced myocarditis patients
When using cardiac strain to do the differentiation between EF preserved myocarditis patients and healthy controls, the ROC curve analysis (Table 4, Figure 5) indicated that PSR was the most sensitive parameter (86%), the PSSRL was the most specific parameter (92%); The AUC of the PSC was optimal when the cutoff was set at −19.72%, with a sensitivity of 68% and a specificity of 88%.
Full table
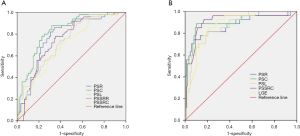
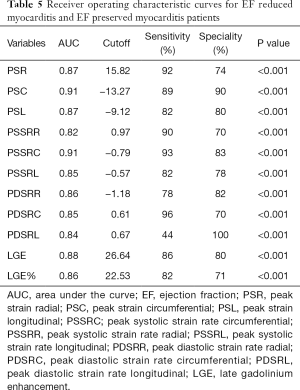
The ROC curve analysis also showed good diagnostic value of strain indices and LGE for differentiation between EF preserved myocarditis patients and EF reduced myocarditis patients (Table 5, Figure 5), which indicated that PDSRC was the most sensitive parameter (96%), the PDSRL was the most specific parameter (100%); The AUC of the PSC and PSSRC were optimal when the cutoffs were set at −13.27% and −0.79/s, the sensitivity of PSSRC was better (93% vs. 89%), the specificity of PSC was better (90% vs. 83%). The diagnostic performance of other strain indices was also good (all AUC ≥0.82), so did LGE and LGE%. When using LGE to differentiate these two groups, the AUC of LGE was 0.88 when the cutoff was set at 26.64 g, with a sensitivity of 86% and a specificity of 80%, and the AUC of LGE% was 0.86 when the cutoff was set at 22.53%,with a sensitivity of 82% and a specificity of 71%.
Full table
Discussion
This study aimed to explore LV strain characteristics in patient with myocarditis and their correlations with the EF and LGE. Major results include: (I) the feature tracking technique allowed robust left ventricular myocardium strain analysis; (II) strain indices were helpful to detect left ventricular dysfunction in myocarditis patients, EF preserved and EF reduced myocarditis patients; (III) strain parameters correlated closely with EF and LGE, especially PSC; (IV) strain parameters had good diagnostic performance in EF preserved myocarditis patients and healthy controls, and strain indices, LGE, LGE% were found to be useful to distinguish EF preserved and reduced myocarditis patients.
The intraobserver and interobserver agreement were good for all global strain parameters in our study. In previous studies (7-10,18), the feature tracking technique was proven robust to do strain analysis. Compared with myocardial tagging techniques, strain can be calculated from traditional cine images using FTI without need for additional sequences, which is more efficient. Furthermore, the results of feature tracking and myocardial tagging correlated well. Compared with speckle tracking echocardiography, CMR has better image quality because of its excellent spatial and contrast resolution. Additionally, CMR is the gold standard for evaluating cardiac structure and function, and allows detection of myocardial edema, hyperemia and necrosis.
Difference in CMR parameters and strain indices
Comparison of the healthy control and acute myocarditis patients groups revealed differences in structure, function and strain, with LV dilation, impaired LV function and reduced strain in the acute myocarditis groups, which is similar to the results of some previous studies (14,19,20). The PER and PFR calculated from the LV volume-time curve showed no significant differences between the control and acute myocarditis patients groups. However, the comparisons showed that the PER and PFR were lower in EF reduced myocarditis patients group than that in EF preserved myocarditis patients group. One possible reason may be functional reserve when myocardial damage is not severe in patient with myocarditis and maintained LVEF.
In addition to increased left ventricular volume, EF preserved myocarditis patients group also showed a reduced strain, systolic strain rate and diastolic strain rate, which has also been reported by other studies (12,21) when compared with healthy controls. Therefore, CMR feature tracking is more sensitive than EF in detecting early LV dysfunction and can be applied clinically, especially when the LVEF is still preserved. The EF reduced myocarditis patients group revealed dilated left volume, increased LGE, reduced strain and strain rate, in comparison to EF preserved myocarditis patients group. EF is widely used in clinical assessment, but many studies have indicated a poor prognosis even if EF is preserved (22-24), LGE is also an independent predictor of combined endpoint (4-6). Therefore, not only early LV dysfunction should be carefully considered in clinical evaluations, but also late LV dysfunction when EF is already reduced, particular if LGE is present. CMR myocardial strain based on feature tracking can provide more detailed information, which could contribute to the detection LV dysfunction whether EF is preserved and reduced.
Correlation of strain parameters with EF and LGE
More advanced LGE was observed in EF reduced myocarditis patients than that in EF preserved myocarditis group. It is reasonable that the EF was severely reduced when there was a greater proportion of LGE, and the correlation analysis showed a good correlation (r=−0.60) between LGE and the EF. The correlation between the EF and LGE was negative, and a reduced EF was significantly related to increased LGE. A previous study by Andre et al. (11) showed that the EF was well correlated with myocardial strain and the strain rate but was not correlated with LGE; however, these results may be limited by the relatively small sample size of 36. In our study, the correlation analysis indicated that the EF was correlated with not only myocardial strain and the strain rate but also LGE, and LGE%. Further correlations were also good between EF and PSC (r=−0.67), EF and PSSRC (r=−0.68), LGE and PSC (r=0.62). The correlation results indicated EF closely correlated with strain, especially PSC and PSSRC. Increased LGE was well correlated with reduced EF and PSC, which indicated an impaired PSC in acute myocarditis. LGE has already been proved as an independent risk factor of a poor prognosis for acute myocarditis patients (4-6). Thus, LGE is important in acute myocarditis diagnosis and prognosis. However, acute myocarditis may appear as diffuse oedema or little fibrosis occasionally, such as fulminant myocarditis, and conventional LGE would be normal (25), and limited in revealing diffuse myocardium injury at this point. At this point, T1 mapping would be helpful and important and is the subject of our future research.
Diagnostic performance of strain parameters and LGE
The LVEF is the most common parameter of LV function, but it cannot detect early LV dysfunction. Therefore, it is important to choose an appropriate method for evaluating early LV dysfunction, especially when the EF is preserved. In our study, LV dysfunction was detected through CMR feature tracking analysis in patients with preserved EF. Similar to previous studies (11,20,21), the diagnostic performance of strain indices was good, for detecting early LV dysfunction in EF preserved myocarditis patients group and healthy controls. The ROC curve analysis (Figure 5) indicated that the PSC was the best strain parameter, when the cutoff was set at −19.72%, with a sensitivity of 68% and a specificity of 88%. Although CMR feature tracking analysis can detect subclinical LV dysfunction, it still needs standardizations because the value may be affected by spatial resolution, temporal resolution, post-processing software (algorythm). Therefore, more clinical research on CMR feature tracking should be performed in the future.
For EF preserved myocarditis patients and EF reduced myocarditis patients, the AUC of PSC and PSSRC were were superior to the other strain indices (AUC >0.82), demonstrating that myocardium strain can provide additional assessment of LV dysfunction. The AUC of LGE and LGE% were good (AUC =0.88, 0.86) as well. LGE is important in the diagnosis of myocarditis, and is one criterion of “Lake Louise Criteria”. In our study, for EF preserved and EF reduced myocarditis patients, the diagnostic value of LGE was also good.
Limitation
This was a retrospective study, including 115 cases from two centres. Although the sample size was relatively large compared with that of prior studies (11-13), and the results may be more reliable, differences in the parameters and image quality among different scanners cannot be neglected, using three different platform in a small sample size could could have some influence on diagnostic accuracy. This included different reconstructed cardiac phases among the different scanners. In all, 25 cardiac phases were obtained using the Siemens and Philips systems, while 20 were obtained using the GE system. When there are more phases in cine images, the cine provides more details of cardiac function and strain. There is no guideline or consensus on the cardiac phases of the cine sequence, although there were studies on normal strain values (7,8). A second limitation is the difference in image quality; images obtained using a 3-T system may have high spatial and contrast resolution but may have more artefacts, such as banding artefacts on balanced SSFP sequences, and there may be some effects on postprocessing. Another limitation may be the lack of data from T1 mapping and T2 mapping, which are accurate and quantitative techniques that have proven to be helpful in making diagnoses by CMR (26-30). This is another future direction of our work.
Conclusions
CMR is important for evaluating suspected myocarditis in clinical practice, not only for making the diagnosis but also for evaluating cardiac function and viability. Furthermore, myocardial strain analysis using CMR feature tracking can be used to detect ventricular dysfunction when the LVEF is reduced or still preserved. The diagnostic performance of strain indices was good when EF was preserved or reduced. PSC showed best diagnostic value in two diagnostic tests, and closely correlated with LGE. In addition to the LVEF, myocardial strain analysis, LGE assessment are related to left ventricular dysfunction.
Acknowledgments
We thank Professor Paul Schoenhagen for his language and copyediting assistance.
Funding: This study is funded by the commonweal project of Jinhua Municipal Science and Technology Bureau (2018-4-009), and the project of Jinhua Municipal Central Hospital (JY2018-2-08).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-221
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-221
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-221). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). This study was approved by ethics committee of Jinhua Hospital, Zhejiang University School of Medicine (2018-96), and informed consent was taken from all the patients and volunteers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Basso C, Calabrese F, Corrado D, et al. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res 2001;50:290-300. [Crossref] [PubMed]
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-2648d.
- Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. [Crossref] [PubMed]
- Aquaro GD, Perfetti M, Camastra G, et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: Itamy Study. J Am Coll Cardiol 2017;70:1977-87. [Crossref] [PubMed]
- Gräni C, Eichhorn C, Bière L, et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol 2017;70:1964-76. [Crossref] [PubMed]
- Grün S, Schumm J, Greulich S, et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis. J Am Coll Cardiol 2012;59:1604-15. [Crossref] [PubMed]
- Taylor RJ, Moody WE, Umar F, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 2015;16:871-81. [Crossref] [PubMed]
- Andre F, Steen H, Matheis P, et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2015;17:25. [Crossref] [PubMed]
- Augustine D, Lewandowski AJ, Lazdam M, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 2013;15:8. [Crossref] [PubMed]
- Onishi T, Saha SK, Ludwig DR, et al. Feature tracking measurement of dyssynchrony from cardiovascular magnetic resonance cine acquisitions: comparison with echocardiographic speckle tracking. J Cardiovasc Magn Reson 2013;15:95. [Crossref] [PubMed]
- André F, Stock FT, Riffel J, et al. Incremental value of cardiac deformation analysis in acute myocarditis: a cardiovascular magnetic resonance imaging study. Int J Cardiovasc Imaging 2016;32:1093-101. [Crossref] [PubMed]
- Kostakou PM, Kostopoulos VS, Tryfou ES, et al. Subclinical left ventricular dysfunction and correlation with regional strain analysis in myocarditis with normal ejection fraction. A new diagnostic criterion. Int J Cardiol 2018;259:116-21. [Crossref] [PubMed]
- Weigand J, Nielsen JC, Sengupta PP, et al. Feature Tracking-Derived Peak Systolic Strain Compared to Late Gadolinium Enhancement in Troponin-Positive Myocarditis: A Case-Control Study. Pediatr Cardiol 2016;37:696-703. [Crossref] [PubMed]
- Baeßler B, Schaarschmidt F, Dick A, et al. Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol 2016;85:218-27. [Crossref] [PubMed]
- Claus P, Omar A, Pedrizzetti G, et al. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging 2015;8:1444-60. [Crossref] [PubMed]
- Guerra M, Sampaio F, Bras-Silva C, et al. Left intraventricular diastolic and systolic pressure gradients. Exp Biol Med (Maywood) 2011;236:1364-72. [Crossref] [PubMed]
- Alfakih K, Plein S, Thiele H, et al. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 2003;17:323-9. [Crossref] [PubMed]
- van Everdingen WM, Zweerink A, Nijveldt R, et al. Comparison of strain imaging techniques in CRT candidates: CMR tagging, CMR feature tracking and speckle tracking echocardiography. Int J Cardiovasc Imaging 2018;34:443-56. [Crossref] [PubMed]
- Wisotzkey BL, Soriano BD, Albers EL, et al. Diagnostic role of strain imaging in atypical myocarditis by echocardiography and cardiac MRI. Pediatr Radiol 2018;48:835-42. [Crossref] [PubMed]
- Luetkens JA, Schlesinger-Irsch U, Kuetting DL, et al. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 2017;27:4661-71. [Crossref] [PubMed]
- Doerner J, Bunck AC, Michels G, et al. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol 2018;104:120-8. [Crossref] [PubMed]
- Kanagala P, Cheng A, Singh A, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction - implications for clinical trials. J Cardiovasc Magn Reson 2018;20:4. [Crossref] [PubMed]
- Duca F, Kammerlander AA, Zotter-Tufaro C, et al. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study. Circ Cardiovasc Imaging 2016;9:e005277. [Crossref] [PubMed]
- Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014;168:721-30. [Crossref] [PubMed]
- Mavrogeni S, Bratis K, Terrovitis J, et al. Fulminant myocarditis. Can cardiac magnetic resonance predict evolution to heart failure? Int J Cardiol 2012;159:e37-8. [Crossref] [PubMed]
- Baeßler B, Treutlein M, Schaarschmidt F, et al. A novel multiparametric imaging approach to acute myocarditis using T2-mapping and CMR feature tracking. J Cardiovasc Magn Reson 2017;19:71. [Crossref] [PubMed]
- Bohnen S, Radunski UK, Lund GK, et al. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 2015;8:e003073. [Crossref] [PubMed]
- Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [Crossref] [PubMed]
- Ammirati E, Sormani P, Moroni F, et al. Changes of late gadolinium enhancement extension compared with native T1 mapping early after acute myocarditis. Int J Cardiol 2018;257:227. [Crossref] [PubMed]
- Kotanidis CP, Bazmpani MA, Haidich AB, et al. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging 2018;11:1583-90. [Crossref] [PubMed]


