MiR-181c protects cardiomyocyte injury by preventing cell apoptosis through PI3K/Akt signaling pathway
Introduction
In recent years, heart failure has been a serious clinical problem all over the world, which threatens the life of a large population of patients with cardiovascular heart diseases (1,2). It has been well-established that cardiomyocyte apoptosis is associated with the various cellular events in heart failure (3,4). Programmed cell death (apoptosis) of cardiac muscle cells has been identified as an essential process in the progression to heart failure. Specifically, cardiomyocyte apoptosis, that is closely related to the clinical severity of dilated cardiomyopathy, could act as a feature of heart failure (5,6). Accumulative evidences suggest the critical role of cell apoptosis in heart failure, which urges us to explore the therapeutic methods to prevent apoptosis.
In recent years, researchers are paying growing efforts in the identification of various microRNAs (miRNAs) in the treatment or diagnostics of heart failure. miRNAs are small non-coding NA with 22 to 25 nucleotides, which inhibit the target protein translation expression via binding complementary sequences in mRNAs for post-transcriptional regulation (7,8). For instance, Xu et al. demonstrated that miR-19b attenuated H2O2-induced apoptosis in rat H9C2 cardiomyocytes via targeting PTEN (9). Zhou et al. reported that miRNA-183 could regulate the cardiomyocyte apoptosis in heart failure (10). Shen et al. demonstrated that down-regulated microRNA-195-5p and up-regulated CXCR4 attenuates the heart function injury of heart failure mice via inactivating JAK/STAT pathway (11). MiRNA-181 family is a miRNA that attracted the interest of many scientist in recent 2 years (12,13). Previously, the miR-181 family (including miR-181b and miR-181c) expression pattern was changed in the glioblastoma multiforme (GBM) in which they could be used to predict the outcome of diseases or the therapeutic responses (14,15). In addition, there are also studies reporting the role of miRNA-181 in heat diseases (16,17). For example, HN Banavath showed that miR-181c activated mitochondrial calcium uptake by regulating MICU 1 in the heart (16). Further study showed that miR-181c could modulate the neuronal apoptosis through binding and inhibiting the TNF (18). Accordingly, we supposed this subtype of the microRNA, miR-181c, might regulate apoptosis in different organs not only in the brain but also in the heart.
In recent years, it has been confirmed that PI3K/Akt signaling pathway components are frequently activated in cell proliferation, apoptosis and survival, which are essential in human cancers (19,20). Studies also presented that PI3K/AKT pathway was also involved in the pathogenesis of cardiomyocyte apoptosis (21,22).In this study, we will be focused to elucidate the functions and mechanisms of miR-181c in cardiomyocyte apoptosis, and identify its interaction with PI3K/Akt cascade. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-490).
Methods
Model of DOX-induced heart failure
Sixty male C57 mice (4-week old, ~15 gram) were purchased from Hunan SJA Laboratory Animal Co., Ltd (Hunan, China). Doxorubicin (DOX) induced heart failure animal model was established according to the protocol described previously (23). Adriamycin (ADR, DOX hydrochloride) was injected intraperitoneally (i.p.) at ADR 2.5 mg/kg (mice weight) for 6 times. The total duration was 14 days and total dosage was 15 mg/kg body weight. After final injection, mice were observed for 4 weeks for their general appearance, behavior, and mortality. Mice in the control group received saline in the same regimen with adriamycin treatment. This study was approved by Hunan SJA Laboratory Animal Co., Ltd. (No. SJA1906055: the license number) and conducted in strict accordance with the national institutes of health guidelines for the care and use of experimental animals. After experiments, the mice were sacrificed by pentobarbital sodium injection intraperitoneally (150 mg/kg).
RNA extraction and real-time polymerase chain reaction (RT-PCR)
RNA extraction and RT-PCR were performed as previously described (24). Total RNA was extracted from tissue and cell using Trizol (Invitrogen), according to the manufacturer’s instructions. Reverse transcription (RT) was performed using an RT kit (Takara) with miRNA-specific primers (Ribo) or oligo dT (Takara), respectively. Real-time RNA quantification was conducted on an ABI StepOne Plus Detection System (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems) and specific primers.
Detection of cell apoptosis by flow cytometry
Flow cytometry was performed as previously described (9,25). The H9c2 cells were collected and then labeled using an Annexin V-PI apoptosis kit (Dijindo). The cells were incubated with fluorescein isothiocyanate (FITC)-labeled Annexin V and propidium iodide (PI) solution in the dark for 15 min. Flow cytometry was conducted using FACS Calibur FL1 (530 nm) bandpass filters (Becton Dickinson), and the data were analyzed using Cell Quest software (BD).
Western blotting analysis
The Western blotting analysis was performed as previously described (26,27). H9c2 cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Pierce) supplemented with protease inhibitor cocktail (Roche). The protein samples were subjected to routine sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane and incubated with primary antibodies overnight. After incubation with appropriate secondary antibodies for 2 h at room temperature, ECL System (Bio-Rad, USA) was used to visualize the protein bands.
Cell culture and H/R treatment
Rat cardiomyocyte H9c2 cell line was purchased from the Cell Bank of Chinese Academy of Science (Shanghai, China) and cultured in Dulbecco’s modified eagle’s medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% streptomycin/penicillin in a humidified atmosphere containing 5% CO2 at 37 °C. H9C2 cardiomyocytes were treated with H/R (16 hours’ hypoxia/2 hours’ reoxygenation) to induce cell apoptosis mimicking I-R injury in vitro.
TUNEL assay
The TUNEL assay was performed according to the manufacturer’s instructions (Chemicon). In brief, the heart tissues were fixed in formaldehyde and embedded in paraffin. 5 µm-thick tissue sections were deparaffinized, rehydrated, and rinsed with PBS. Normal heart tissue treated with DNase I (10 U/mL, 10 min at room temperature) is a positive control. Sections pretreated with 3.0% H2O2 were subjected to TdT enzymes reaction for 37 °C for 1 hour and incubated in digoxigenin-conjugated nucleotide substrate at 37 °C for 30 min. 3,3-diaminobenzidine treatment for 5 min was used to exhibiting DNA fragmentation in nuclei. Apoptotic cardiomyocyte’s nuclei were stained dark brown. Lastly, sections were counterstained with methyl green and cover-slipped. The sections were observed by light microscopy.
Statistical analysis
Statistical analysis was conducted utilizing SPSS 21.0. Results are represented as mean ± SD. Difference between the 2 groups were analyzed by Student’s t-test, and >2 groups were evaluated by one-way ANOVA. A value of <0.05 was statistically significant.
Results
miR181c was down-regulated in apoptotic myocardium induced by doxorubicin-induced heart failure in mice and hypoxia/reoxygenation (H/R) induced H9C2 cardiomyocyte
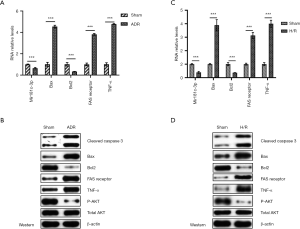
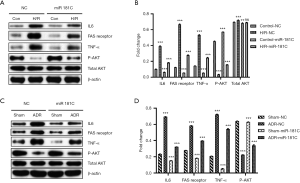
Firstly, we established the animal model of doxorubicin-induced heart failure and cardiomyocyte apoptosis in myocardium to determine the effect of miR-181c and underlying mechanisms. Figure S1 provides the experimental flow diagram. ADR, a broad-spectrum anticancer drug, is particularly toxic to the heart tissue. The cardiotoxicity from ADR can cause myocardial damage with increased oxidative stress and apoptosis. A animal model was induced by i.p. injection of ADR and relative expressions of members of miR-181 family were evaluated. In our results, miR-181c was suppressed on the heart tissue at 48 h post ADR inducement in vivo, while miR-181a/b had no significant difference (Figure 1A). Figure 1B showed that the expressions of apoptosis signaling molecule Fas, inflammation marker TNF-α and PI3K/AKT pathway related protein under the DOX exposure. Figure 1C demonstrated that the expression of miR-181c was also suppressed in H/R induced H9C2 cardiomyocyte injury. Figure 1D showed that the expressions of apoptosis signaling molecule Fas, inflammation marker TNF-α and PI3K/AKT pathway related protein under the H/R exposure. As Fas is closely related to cell apoptosis, we measured the primary molecules regulating cardiomyocyte apoptosis including Bax, Bcl2, and cleaved caspase-3. It is obvious that DOX treatment and H/R induction both resulted to the increased expressions of cleaved caspase-3, bax, fas receptor and TNF-α. Conversely, it reduced the expressions of Bcl2 and p-AKT. In summary, these results confirmed that miR-181c was down-regulated in myocardium from DOX-induced heart failure and H/R induced H9C2 cardiomyocyte.
Overexpression of miR181c inhibited hypoxia-induced cardiomyocyte apoptosis in vitro
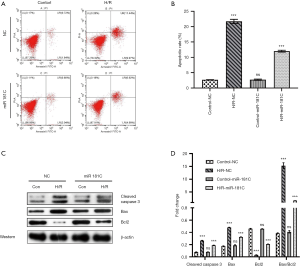
To further investigate the role of miR-181c on cardiomyocyte apoptosis, we cultured the H9c2 cell lines in vitro. As a result, flow cytometry indicated that H/R treatment significantly increased cell apoptosis, but miR-181c inhibited the H/R induced apoptosis in vitro (Figure 2A,B). Figure 2C,D showed that miR-181c reversed the protein expression of caspase 3, BAX, and BCL2 induced by H/R. As a summary, it is obvious that miR-181c overexpression could inhibit the H/R induced apoptosis and inflammation reaction in vitro.
Intravenous injection of miR181c resists DOXs-induced tissue apoptosis in mice in vivo
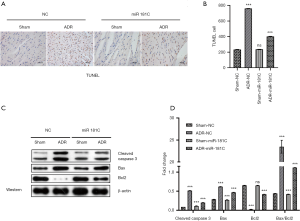
MiR181c was injected intravenously to treat heart injury induced by intravenous injection of ADR in the mice. Sixty male C57 mice were used for experiment, with 15 mice per group. The TUNEL staining demonstrated that the apoptosis was reduced, suggesting that miR-181c might contribute to the anti-apoptotic activity (Figure 3A,B). Furthermore, we measured the primary molecules regulating myocardial tissue apoptosis including cleaved caspase-3, Bax and Bcl2 (Figure 3C,D). The results showed that miR-181c suppressed the cleavage mediated activation of caspase-3, proving the anti-apoptosis function of miR-181c (Figure 3D). Meanwhile, miR-181c mimic inhibited the Bax expression while enhanced the antiapoptotic Bcl2 expression under DOX treatment, preventing myocardial tissue apoptosis in vivo (Figure 3D). It is highly consistent with the H/R treatment results in vitro.
miR-181c protected cardiomyocytes from apoptosis and injury partly through the PI3K/Akt pathway
It was known that the PI3K/Akt pathway plays an essential role in cell apoptosis. Thus, we speculated whether the miR-181c regulated cell apoptosis through the PI3K/Akt pathway. Based on western blot analysis, we found that miR-181c could promote the phosphorylation cascades in the classic PI3K/Akt pathway under the H/R (Figure 4A,B) or DOX-induced apoptosis condition (Figure 4C,D). In summary, miR-181c protected cardiomyocytes from apoptosis and injury partly through the PI3K/Akt pathway based on its effect on protein expression as well as protein phosphorylation .
Discussion
Previous studies indicated a variety of miRNAs that could regulate the apoptosis of cardiomyocyte apoptosis (28-30). For instance, Zhao et al. reported that miR-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1β in 2020 (31). Zhou et al. demonstrated that miR-182 regulated the cardiomyocyte apoptosis in heart failure (10). Das et al. demonstrated the divergent effects of miR-181 family members on myocardial function through protective cytosolic and detrimental mitochondrial microRNA targets (32). However, very limited information has been revealed regarding its role in cardiomyocyte apoptosis. In our study, we established the role of miR-181c in the inhibition of cardiomyocyte apoptosis through in vitro and in vivo experiments. This may provide a new therapeutic direction for the treatment of apoptosis-related heart diseases.
In oncology study, miR-181c was identified using miRNA profiling for prediction and monitoring of osteosarcoma development and therapeutic outcome (33,34). It was confirmed that miR-181c was expressed in the myocardial cells (35). In this study, we directly investigated the roles of miR-181c on series of apoptosis parameters using the miR-181c mimics and inhibitors. miR-181c transcriptionally activated the normal mice apoptosis molecules including Fas and TNF. miR-181c inhibited cardiomyocyte apoptosis with downregulated Bax but upregulated Bcl2 expression in vivo in the Adriamycin induced CHF model. It is different from its effect on the Ventricular myocytes isolated from Kunming mice described previously (36). In our experiment, we detected Bax/Bcl2 expression on cultured cardiomyocyte H9c2 in vitro. MiRNA-181c made direct effect on the expression of apoptosis protein Bax and Bcl2.
Alternative mechanisms of miR-181c on myocardial cell apoptosis were deciphered from the aspects of the signaling pathway. It was known that PI3K/Akt was functionally important in cell apoptosis (37,38). miR-181c was also reported to target Akt1 with involvement in the lipopolysaccharide-induced macrophage inflammatory reaction (39). It was possible that miR-181c might be involved in the signaling events of PI3K/Akt pathway. Although miR-181c did not change the expression of key component Akt, it promoted PI3K phosphorylation for the activation of PI3K-Akt signaling chain. PI3K/Akt pathway is closely involved in cell proliferation, cell cycle and cell apoptosis especially in different cancer cells, such as cervical cancer, lung cancer, or renal cancer (20,40). However, PI3K/Akt activation could either promote or prevent cell apoptosis as a cancer chemotherapy target (38,41). In our experiments, we found miR-181c mimic could confer the resistance to apoptosis in PI3K/Akt pathway. In consistence, we found that miR-181c attenuated apoptosis through PI3K/Akt phosphorylation.
miRNAs could mediate gene expression by influence on mRNA translation or stability (42,43). miR-181c has been report to target ST8SIA4 in chronic myelocytic leukemia (44), Smad7 in neuroblastoma (45), and Wnt inhibition factor 1 in non-small lung cancer cells (46). It is widely known that the regulation mechanisms of miRNA on heart diseases are very complicated (47). Previous studies by Das et al. (48) and Wang et al. (35) have shown that miR-181c can cause cardiac dysfunction by regulating mitochondrial morphology. In our experiments, cardiomyocyte apoptosis was induced by H/R (in vitro) or ADR (in vivo), which is different from the incentives of the previous two researches. In our results, miR-181c reversed the apoptosis induced by ADR, of which the mechanism might be relying on the targeting of Bcl2. Our regulatory mechanisms are different. Due to funding and time limitations, we did not focus on the different effects of miR-181c on heart failure, as well as the exact targeting mechanism and molecules. In future research, we will study the related mechanisms and the miRNA-181c targeting gene, and hope to further promote the treatment of heart failure with related drugs.
In the early screening, the abnormal down-regulation of miR-181c is expected to become a sign of early diagnosis of heart failure. As for gene therapy, the treatment of miR-181c provides a new direction for the future clinical and further treatment of heart failure. As a limitation, our current study did not carry our experiment to investigate the possible target of miRNA-181c in the inhibition of cardiomyocyte apoptosis. MiRNA-181c might target Bcl-2 to exert its biological functions, however, this needs further identification. In our future work, we will confirm the targets that miRNA-181c may bind in the cardiomyocyte apoptosis and examine their combined role in heart failure. We also plan to explore more potential miRNA targets in a more comprehensive and in-depth manner. We also look forward to continuously contributing to the treatment of heart failure.
Conclusions
We established the functional association between miR-181c and cardiomyocyte apoptosis. Our results revealed that miR-181c protected heart failure by impeding cardiomyocyte apoptosis through targeting the PI3K/Akt pathway, implying the therapeutic role of miR-181c during the exacerbation of the cardiovascular disease.
Acknowledgments
We thank Chaoyang Hospital affiliated to Capital Medical University for all the support.
Funding: This work was supported by the National Natural Science Foundation of China (Project No. 81670214, 81770253), the Beijing Natural Science Foundation (Project No. 7172080), the 1351 personnel training plan (Project No. CYMY-2017-03), the National Major Research Plan Training Program of China (Project No. 91849111), the National Natural Science Foundation of China, Youth Science Foundation Project (Project No. 81500321) and Open Foundation from Beijing Key Laboratory of Hypertension Research (Project No. 2017GXY-KFKT-01 and 2017GXY-KFKT-03).
Footnote
Reporting Checklist: The authors present the study in accordance with the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-490
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-490
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-490). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Hunan SJA Laboratory Animal Co., Ltd. (No. SJA1906055: the license number) and conducted in strict accordance with the national institutes of health guidelines for the care and use of experimental animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jhorawat R, Kumari S, Varma SC, et al. Preventive role of carvedilol in adriamycin-induced cardiomyopathy. Indian J Med Res 2016;144:725. [Crossref] [PubMed]
- Francis GS, Tang WW. Pathophysiology of congestive heart failure. Rev Cardiovasc Med 2003;4:S14-20. [PubMed]
- Chen YF, Pandey S, Day CH, et al. Synergistic effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J Cell Physiol 2018;233:3660-71. [Crossref] [PubMed]
- Saraste A, Pulkki K, Kallajoki M, et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 1999;29:380-6. [Crossref] [PubMed]
- Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000;60:1789-92. [PubMed]
- Palojoki E, Saraste A, Eriksson A, et al. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 2001;280:H2726-31. [Crossref] [PubMed]
- Li P. MicroRNAs in cardiac apoptosis. J Cardiovasc Transl Res 2010;3:219-24. [Crossref] [PubMed]
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015;12:135. [Crossref] [PubMed]
- Xu J, Tang Y, Bei Y, et al. miR-19b attenuates H2O2-induced apoptosis in rat H9C2 cardiomyocytes via targeting PTEN. Oncotarget 2016;7:10870. [Crossref] [PubMed]
- Zhou F, Fu W, Chen L. MiRNA-182 regulates the cardiomyocyte apoptosis in heart failure. Eur Rev Med Pharmacol Sci 2019;23:4917-23. [PubMed]
- Shen Y, Zhang W, Lee L, et al. Down-regulated microRNA-195-5p and up-regulated CXCR4 attenuates the heart function injury of heart failure mice via inactivating JAK/STAT pathway. Int Immunopharmacol 2020;82:106225. [Crossref] [PubMed]
- Rezaei T, Mansoori B, Hashemi ZS, et al. microRNA-181 serves as a dual-role regulator in the development of human cancers. Free Radic Biol Med 2020;152:432-54. [Crossref] [PubMed]
- Zhai F, Chen X, He Q, et al. MicroRNA-181 inhibits glioblastoma cell growth by directly targeting CCL8. Oncol Lett 2019;18:1922-30. [PubMed]
- She X, Yu Z, Cui Y, et al. miR-181 subunits enhance the chemosensitivity of temozolomide by Rap1B-mediated cytoskeleton remodeling in glioblastoma cells. Med Oncol 2014;31:892. [Crossref] [PubMed]
- Ciafre S, Galardi S, Mangiola A, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun 2005;334:1351-8. [Crossref] [PubMed]
- Banavath HN, Roman B, Mackowski N, et al. miR-181c Activates Mitochondrial Calcium Uptake by Regulating MICU 1 in the Heart. J Am Heart Assoc 2019;8:e012919. [Crossref] [PubMed]
- Constanso I, Nunez L, Hermida-Prieto M, et al. P5455 Serum microRNA-181a-5p expression pattern correlates with acute cellular rejection in heart transplantation. Eur Heart J 2019;40:ehz746.0411.
- Ren L, Zhu R, Li X. Silencing miR-181a produces neuroprotection against hippocampus neuron cell apoptosis post-status epilepticus in a rat model and in children with temporal lobe epilepsy. Genet Mol Res 2016;15:1-11. [Crossref] [PubMed]
- Vara JÁF, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004;30:193-204. [Crossref] [PubMed]
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005;4:988-1004. [Crossref] [PubMed]
- Liu AH, Cao YN, Liu HT, et al. DIDS attenuates staurosporine-induced cardiomyocyte apoptosis by PI3K/Akt signaling pathway: activation of eNOS/NO and inhibition of Bax translocation. Cell Physiol Biochem 2008;22:177-86. [Crossref] [PubMed]
- Yu W, Zha W, Ke Z, et al. Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. J Diabetes Res 2016;2016:4158591.
- Wu S, Zhu L, Yang J, et al. Hydrogen-containing saline attenuates doxorubicin-induced heart failure in rats. Pharmazie 2014;69:633-6. [PubMed]
- Kubista M, Andrade JM, Bengtsson M, et al. The real-time polymerase chain reaction. Mol Aspects Med 2006;27:95-125. [Crossref] [PubMed]
- Sheng R, Gu ZL, Xie ML, et al. Epigallocatechin gallate protects H9c2 cardiomyoblasts against hydrogen dioxides-induced apoptosis and telomere attrition. Eur J Pharmacol 2010;641:199-206. [Crossref] [PubMed]
- Park ES, Kang JC, Jang YC, et al. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J Ethnopharmacol 2014;153:552-60. [Crossref] [PubMed]
- Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci 2012;4:429. [Crossref] [PubMed]
- Xu C, Lu Y, Pan Z, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci 2007;120:3045-52. [Crossref] [PubMed]
- Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 2015;192:61-9. [Crossref] [PubMed]
- Tang Y, Zheng J, Sun Y, et al. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J 2009;50:377-87. [Crossref] [PubMed]
- Zhao Z, Du S, Shen S, et al. microRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1β. J Cell Physiol 2020;235:2710-21. [Crossref] [PubMed]
- Das S, Kohr M, Dunkerly-Eyring B, et al. Divergent Effects of miR-181 Family Members on Myocardial Function Through Protective Cytosolic and Detrimental Mitochondrial microRNA Targets. J Am Heart Assoc 2017;6:e004694. [Crossref] [PubMed]
- Lakomy R, Sana J, Hankeova S, et al. MiR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci 2011;102:2186-90. [Crossref] [PubMed]
- Schonrock N, Humphreys DT, Preiss T, et al. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci 2012;46:324-35. [Crossref] [PubMed]
- Wang H, Li J, Chi H, et al. Micro RNA-181c targets Bcl-2 and regulates mitochondrial morphology in myocardial cells. J Cell Mol Med 2015;19:2084-97. [Crossref] [PubMed]
- Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 2014;24:105-12. [Crossref] [PubMed]
- Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: size matters. Oncogene 2003;22:8983-98. [Crossref] [PubMed]
- Saiki S, Sasazawa Y, Imamichi Y, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 2011;7:176-87. [Crossref] [PubMed]
- Androulidaki A, Iliopoulos D, Arranz A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 2009;31:220-31. [Crossref] [PubMed]
- Osaki M. Oshimura Ma, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 2004;9:667-76. [Crossref] [PubMed]
- Xie D, Gore C, Zhou J, et al. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci U S A 2009;106:19878-83. [Crossref] [PubMed]
- Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122:6-7. [Crossref] [PubMed]
- Farazi TA, Spitzer JI, Morozov P, et al. miRNAs in human cancer. J Pathol 2011;223:102-15. [Crossref] [PubMed]
- Zhao L, Li Y, Song X, et al. Upregulation of miR-181c inhibits chemoresistance by targeting ST8SIA4 in chronic myelocytic leukemia. Oncotarget 2016;7:60074. [Crossref] [PubMed]
- Li Y, Wang H, Li J, et al. MiR-181c modulates the proliferation, migration, and invasion of neuroblastoma cells by targeting Smad7. Acta Biochim Biophys Sin 2014;46:48-55. [Crossref] [PubMed]
- Zhang H, Hu B, Wang Z, et al. miR-181c contributes to cisplatin resistance in non-small cell lung cancer cells by targeting Wnt inhibition factor 1. Cancer Chemother Pharmacol 2017;80:973-84. [Crossref] [PubMed]
- Smith T, Rajakaruna C, Caputo M, et al. MicroRNAs in congenital heart disease. Ann Transl Med 2015;3:333. [PubMed]
- Das S, Bedja D, Campbell N, et al. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One 2014;9:e96820. [Crossref] [PubMed]