Cardiac CT: atherosclerosis to acute coronary syndrome
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality worldwide and makes up for more than half of all cardiovascular events in men and women <75 years of age in United States (1). Atherosclerosis is a chronic disease characterised by plaque formation inside the arteries as a result of complex interaction between lipoproteins, endothelium and inflammatory cells. All the cardiovascular risk factors contribute to the pathogenesis by aggravating the underlying inflammation (2). The development and progression of atherosclerosis is likely determined, in part, by other still unidentified risk factors and genetic host susceptibility. It initially begins as fatty streak and grows over many years to become advanced atherosclerotic plaque. In majority of men and women with coronary atherosclerosis, the initial presentation is with acute myocardial infarction or sudden cardiac death and two thirds of acute coronary syndromes (ACS) are due to disruption of atherosclerotic plaque. The features of ruptured plaques on histopathology include large plaque volumes and large necrotic cores that are covered by thin fibrous cap (<65 µm), and typically infiltrated with monocytes and macrophages (3). Plaques vulnerable to rupture are termed thin cap fibroatheroma (TCFA) which share similar histopathological characteristics as ruptured plaques except that the fibrous caps are still intact. The term “vulnerable plaque” has therefore been used to describe rupture prone plaques before an event occurs (4). Studies of disrupted plaques with invasive intravascular imaging such as intravascular ultrasound (IVUS) have identified imaging characteristics of plaque vulnerability. These features include positive remodelling, large lipid core and spotty calcification (5,6). Intravascular techniques are however limited by high cost and invasive nature of the test. It is therefore desirable to have a non-invasive imaging technique which can assess plaque burden and detect vulnerable plaque. Coronary computed tomographic angiography (CCTA) has established itself as a non-invasive modality with high sensitivity and high negative predictive value for detecting coronary artery stenosis (7). In addition, CCTA permits the assessment of coronary atherosclerotic plaque morphology and composition in good agreement with IVUS (8).
Coronary plaque imaging of atherosclerotic plaques by CCTA
CCTA is performed on multidetector CT (MDCT) systems after the injection of iodine contrast media for opacification of lumen. Current generation scanners range from 64-320 detector rows with spatial resolution of approximately 230 to 625 µm and temporal resolution of approximately 75 to 175 ms. The spatial resolution of latest generation CT scanners is marginally lower than the 100 and 200 µm afforded by IVUS and invasive coronary angiography (ICA) respectively. The improved temporal resolution of new generation scanners permit scan acquisition for patients with atrial fibrillation and higher heart rates with preserved image quality. Plaques are identified on CCTA as any discernible structure outside the lumen that is either calcified or has attenuation value less than the lumen. They are usually classified into three categories: non-calcified, mixed or calcified.
Retrospectively ECG-gated 64 detector CCTA require radiation exposure of 7 to 21 millisieverts (mSv) (9). Over the years, various advances in imaging techniques have enabled moderate reduction in radiation. These techniques include using retrospective ECG gating, lower tube potential, new scanner technology including wide detector array and high pitch dual source spiral CT (10-13). In addition, radiation dose can be further reduced by using adaptive statistical iterative reconstruction (IR), which aids in reducing noise level of low-dose acquisitions (14). These dose saving techniques have been widely adopted and currently, CCTA would involve low dose radiation of 2-3 mSv. More recent studies have demonstrated image acquisition with “ultra-low” radiation dose of <1 mSv and the impact of reduced dose and IR were analyzed by subjective image quality and noise parameters. However, beyond the study of coronary lumen, the effect of very low radiation on the study of plaques, vessel wall and its application in novel CT techniques such as CT myocardial perfusion and CT fractional flow reserve is necessary. Furthermore, the incremental clinical benefit of very low vs. low dose radiation (e.g., <1 vs. 2-3 mSv) is unclear (15).
Qualitative assessment of coronary plaque and stenosis assessment—comparison with ICA
ICA is established in the assessment of coronary arteries and in the measurement of severity of luminal stenosis. There are several studies that have compared the diagnostic accuracy of CCTA to ICA, based on visual assessment using a binary approach in classifying lumen stenosis as <50% or ≥50%. The 64 detector CCTA identified significant coronary artery stenosis with a sensitivity of 85-99%, specificity of 64-90% and a negative predictive value of 83-99% when compared to ICA in three large multicentre studies (9,16,17). These patients had low to intermediate risk of CAD. In a meta-analysis comprising 7,516 patients with suspected and known CAD in 89 studies, the per-patient sensitivity and specificity for >16 slice CCTA was 98% and 89%, respectively (18). Furthermore, there was excellent interobserver and intraobserver agreement for stenosis ratings in high quality images (19,20). These studies demonstrate that among all other non-invasive imaging modalities, CCTA most closely resembles ICA in terms of providing coronary assessment. Accordingly current European and US guidelines advocate the use of CT in patients with low to intermediate risk of CAD with a class IIa and IIb indication (21,22). In addition, since 2010, the ACC/AHA appropriate use criterion has recommended that it is appropriate to use CCTA as an upfront investigation in this population (23).
Quantitative assessment of atherosclerotic plaques
Comparison with IVUS
CCTA provides vessel lumen geometry and volumetric assessment of plaque which had previously been obtained only on IVUS. Geometric parameters including minimal lumen area, minimal luminal diameter, diameter stenosis and area stenosis can be quantified using dedicated computed software (Figure 1). Furthermore, the total volume of plaque in a coronary segment, defined as the volume between the vessel lumen and outer wall, can be determined using a semi-automated method based on software detection of the lumen and outer vessel walls (Figure 1). Plaque area or burden, defined as vessel area- lumen area/vessel area, can be quantified and volume of calcified and non-calcified plaque (NCP) based on relative CT densities can also be derived.
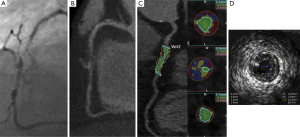
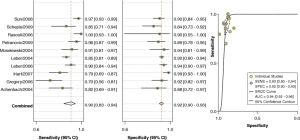
In a recent meta-analysis by Voros et al. (25), including a cohort of 946 patients from 33 studies that compared the accuracy of 64-detector CCTA to IVUS (25), CCTA was demonstrated to have an excellent sensitivity (94%) and specificity (92%) to qualitatively detect atherosclerotic plaque (Figure 2). Compared to IVUS, CCTA was found to slightly overestimate luminal area (0.46 mm2 or by 6.7%; P=0.005), while there was no significant differences in plaque area (0.09 mm2; P=0.88), volume (5.3 mm3; P=0.21) and percent area stenosis (−1.81%; P=0.12) (Figure 3). In addition, two separate studies showed that CCTA underestimated plaque volume in NCPs and overestimated plaque volume in mixed/calcified plaque (26,27). The overestimation in calcified plaque is typically related to blooming artefact.
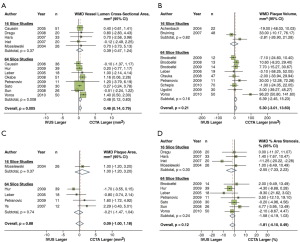
Albeit the high diagnostic accuracy of CCTA is required to qualitatively assess plaque, its accuracy and reproducibility to quantify plaque is important, as this may determine the potential use of CCTA to non-invasively monitor plaque progression. Two studies have demonstrated that the mean difference in quantifying minimal luminal diameter and minimal luminal area between two experienced observers was small (19,28). There was excellent interobserver correlation for quantification of plaque volume with r values ranging between 0.83 and 0.99, although the limits of agreement were wide ranging from 87 to 226% (29). High quality images were used in these studies and were found to significantly improve reproducibility. The reported mean interobserver difference in calcified and NCP volume was −0.74% and −0.76% with corresponding limits of agreement of ±23.5% and ±11.5%, respectively in high quality coronary CCTA datasets (19). Otsuka and colleagues demonstrated that interobserver and intraobserver reproducibility was optimised by exclusion of images with heavy calcification and image blurring (30).
Assessment of coronary arteries with high calcium score
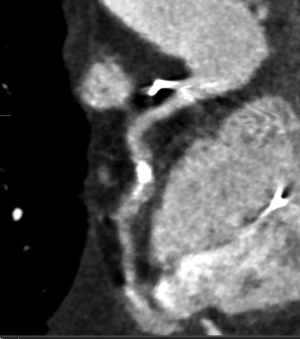
The “Achilles heel” of cardiac CT is calcification, which hampers diagnostic accuracy by causing blooming artefact (Figure 4). As a result, the specificity of CCTA in calcified vessels is significantly reduced. Recent studies have shown that a higher coronary artery calcium score (CACS ≥400 or ≥600) resulted in reduction in specificity by 35% to 48% (31,32). Therefore, current guidelines do not recommend CCTA for patients with very high CACS. These patients should instead undergo a functional assessment or ICA based on their pre-test probability of CAD. Subtraction algorithms have been developed to be applied on CT images acquired using dual energy CT scanners which may remove calcium and improve the evaluation of coronary artery segments with heavy calcification (33,34). Clinical studies examining the utility of this technique to assess CAD are currently underway.
Assessment of coronary artery bypass grafts (CABG)
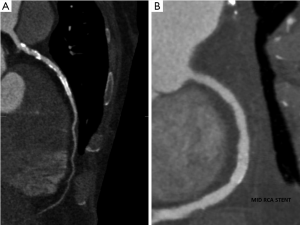
CCTA is also used to assess coronary bypass grafts with the accuracy being higher in grafts than in native vessels. This superior diagnostic accuracy may be due to larger vessel diameter of the grafts, lower propensity to develop calcified plaque and due to lower motion artefacts in the grafts (Figure 5) (35-37). In addition, grafts that cannot be detected or accessed by ICA can be visualised with CCTA. The 32 detector CCTA was used in a study to evaluate 52 patients with a mean follow up period of 9.6±7.2 years following CABG surgery (38). The diagnostic accuracy of CCTA for the detection or exclusion of significant stenosis in arterial and venous grafts on per segment analysis was 100%. The sensitivity and specificity were lower in the distal runoffs and in the native vessels. In a meta-analysis by Hamon et al. (39), the diagnostic accuracy of assessing grafts by 16 and 64 slice CCTA was exceptionally high (sensitivity, 97.6%; specificity, 96.7%; and NPV, 98.9%).
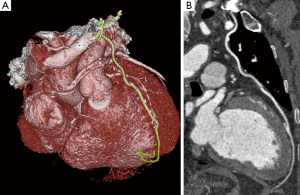
Assessment of in-stent restenosis
The evaluation of stents by CCTA is more challenging than that of native coronary arteries (40). This is predominantly attributable to beam hardening and partial volume artefact from stent struts. It has been demonstrated that a difference in CT density in Hounsfield units (HU) of ≤19% between a reference vessel (aorta) and inside the lumen of a stent, along with a mean CT density of ≥300 inside a stent correlated with a patent stent with high sensitivity and specificity (41). According to a meta-analysis, the diagnostic accuracy of 64 slice CCTA for stents was 90%, with sensitivity of 89.7%, specificity of 92.2%, positive predictive value (PPV) of 72.5%, and NPV of 97.4%. When non-assessable segments were included, the sensitivity and specificity significantly decreased to 79% and 81% respectively (42). The diagnostic accuracy is also influenced by the diameter of the stent. Overall, diagnostic accuracy is significantly higher in stents with diameter ≥3 m (Figure 6) (42-45). When CCTA was used to evaluate in stent restenosis using IVUS as reference standard, it was demonstrated that the sensitivity, specificity, PPV, NPV, and accuracy were 67%, 78%, 57%, 85%, and 75%, respectively, for stents <3.0 mm in diameter; whereas for stents ≥3.0 mm in diameter, the sensitivity, specificity, PPV, NPV, and accuracy were 89%, 100%, 100%, 97%, and 98%, respectively (46). Moreover, the diagnostic performance of CCTA has been found to be superior in stents with thinner struts (<100 µm) compared to stents with thicker struts (47,48). Due to these limitations, use of CCTA for evaluation of in stent restenosis is not considered for routine clinical use, except for the assessment of unprotected left main stent (49). High definition cardiac tomography (HDCT) equipped with superior spatial resolution of 0.23 mm compared to 0.625 mm offered by standard CCTA, may improve the accuracy of CCTA in assessing stents. HDCT improved coronary stent visualisation with an increase in luminal stent diameter from 42.3% to 54.1% and area visualisation from 25.8% to 54% when compared to standard CCTA in an ex vivo phantom model (50). In a comparison study of HDCT vs. standard definition CCTA, 25 stents were assessed in 14 patients who were examined by both the scanners on the same day. Partial volume effects were significantly reduced by HDCT resulting in an increase in percentage of stents with no or only minor artefacts, from 44% to 96% (51).
Assessment of plaque progression
While invasive imaging modalities such as IVUS and optical coherence tomography (OCT) have been used to evaluate the efficacy of various anti-atherosclerotic therapies on plaque progression, a non-invasive test would be more assessable and safe. CCTA has been touted as the non-invasive imaging modality to study the surrogate end points for cardiovascular outcomes, such as plaque progression. Several studies have explored this potential. Zeb et al. (52) studied the effect of statin on plaque progression in 100 patients using CCTA with a mean follow up of 406±92 days. Total plaque progression was significantly reduced among subjects on statin compared to those who were not (–33.3±90.5 vs. 31.0±84.5 mm3, P=0.0006). Statin use was associated with reduced progression of NCP volume (–47.7±71.9 vs. 13.8±76.6 mm3, P<0.001) and LAP volume (–12.2±19.2 vs. 5.9±23.1 mm3, P<0.0001) as quantified on CT. Similar reduction in total plaque volumes and LAP volumes were demonstrated in two other studies in patients on statin therapy. These changes were more pronounced in non-calcified and in mixed plaque (53,54). Although these studies highlight the potential use of CCTA to monitor plaque progression/regression, studies comparing CCTA to IVUS in the longitudinal assessment of plaque progression are scarce.
Assessment of the hemodynamic significance of coronary atherosclerosis
Although CCTA accurately assesses coronary plaque and stenosis, it is limited in evaluating the hemodynamic significance of coronary stenosis. As functional significance of coronary stenosis determines prognosis and the need for revascularisation, it is of relevance in the management of patients with stable CAD (55-57). Recently, three novel techniques have been demonstrated to accurately detect vessel-specific ischemia using the gold standard invasive fractional flow reserve (FFR) as a reference (55,56,58). They are (I) non-invasive FFR (CT FFR); (II) transluminal attenuation gradient (TAG); and (III) CT perfusion imaging (CTP). These techniques may broaden the use of CCTA to assess coronary ischemia in addition to anatomy (57,59,60).
CT FFR
Non-invasive FFR or CT FFR is derived by applying computational fluid dynamics. Using a supercomputer, three dimensional models throughout the cardiac cycle representing the pressure and flow along all points of the arteries are generated during rest and simulated maximal hyperaemic conditions. Blood viscosity is assumed to be constant and CT features that affect coronary flow are taken into consideration. Currently, the technique requires 5 hours of processing time and a non-invasive FFR is derived based on approximated pressure measurements (61,62). In a multicentre, prospective cohort of 159 vessels in 103 patients, Koo et al. demonstrated that CT FFR, using a threshold of ≤0.8, detected FFR-significant (≤0.8) stenoses with a sensitivity of 84% and specificity of 82% (63). Overall, there was a good correlation between CT FFR and FFR (r=0.72, P<0.001) and the area under the ROC curve (AUC) was 0.90, which was significantly higher than CCTA alone (0.70, P<0.0001). A similar improvement in ROC curve was reported when the study was repeated in a larger multicentre prospective cohort of 285 patients (64). In a recent prospective multicentre study (the NXT trial), diagnostic accuracy, sensitivity and specificity for CT FFR were 81%, 86% and 79% respectively. Again, there was an improvement in ROC curve (CCTA AUC =0.8 vs. FFR CT AUC =0.90, P=0.0008) (65).
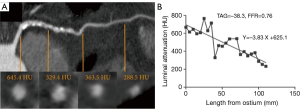
Transluminal attenuation gradient (TAG)
TAG is defined as the linear regression coefficient between intraluminal attenuation (HU) and axial distance. TAG evaluates the slope of decline in intraluminal contrast attenuation from the ostium to the distal coronary vessel (Figure 7). Choi et al. demonstrated that in a cohort of 127 patients (370 vessels) with multivessel disease, TAG performed on resting CCTA was significantly lower in occluded vessels compared to those with lesions of 0-49% stenosis on quantitative coronary angiography (QCA) (−13.46±9.59 vs. −2.37±4.67 HU/10mm, P<0.001) (66). The addition of TAG to the interpretation of CCTA improved diagnostic accuracy for anatomical stenosis severity (P=0.001), especially in vessels with calcified lesions and provided a net reclassification improvement of 0.095 (66). As the 320 detector row scanner allows isophasic, single beat imaging of the entire coronary tree, it was postulated that it would be the ideal platform for TAG assessment. The accuracy and incremental value of TAG on a 320-detector row scanner has been evaluated by Wong et al. in a cohort of 53 stable CAD patients. TAG assessed in FFR-significant vessels was significantly lower than that found in FFR non-significant vessels (−21 vs. −11 HU/10mm, P<0.001) (56). Using a retrospectively determined TAG320 cut-off of −15.1 HU/10mm, TAG320 was reported to predict FFR ≤0.8 with 77% sensitivity and 74% specificity. Importantly, the AUC for the combined use of TAG320 and CCTA was 0.88.
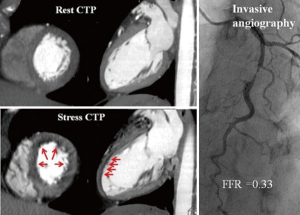
CT stress myocardial perfusion imaging
Myocardial perfusion imaging on CCTA is the acquisition of images during the first pass of iodinated contrast from the arteries into the myocardium where hypo perfusion is represented by hypo-attenuated areas (Figure 8). CTP has been evaluated in numerous single centre studies to date. The sensitivity ranges from 71% to 100% and specificity from 72% to 98% depending on the scanner type and the studied population when compared with single-photon emission computed tomography- myocardial perfusion imaging (SPECT-MPI), invasive FFR and magnetic resonance imaging (MRI) perfusion imaging. In a study by George et al., CTP had a sensitivity of 81% and a specificity of 85% when compared with SPECT-MPI (67). More recently, in the Core320 study, which is a multicentre study with a cohort of 381 patients, the combined protocol of CCTA and CTP was compared to SPECT-MPI. The sensitivity and specificity for the combined protocol was 80% and 74% respectively (68). Four studies thus far have compared CTP with FFR, all demonstrating that the use of CTP provided incremental diagnostic accuracy when added to CCTA alone, by increasing the specificity and PPV for FFR-significant stenoses (55,58,69,70). Ko et al. (69) demonstrated using a 320-detector CT, in a prospective cohort of 40 patients including 120 vessels with suspected CAD, that CCTA detected FFR-significant stenoses (≤0.8) with 95% sensitivity and 78% specificity (69). The additional use of CTP increased the per-vessel specificity to 95% while sensitivity was maintained at 87%. The combined use of CCTA and CTP was associated with an increase in the ROC AUC to 0.93 from 0.85 using CCTA alone (P=0.0003). In a study by Bettencourt et al. (55), improved diagnostic accuracy was noted for FFR <0.8, from 78% using 64 detector CCTA alone, to 85% with combined protocol, which was non inferior to the accuracy of MR perfusion imaging of 88%.
Prediction of clinical outcomes using cardiac CT
A number of studies have demonstrated that the presence and burden of calcified plaque as represented by the CACS is associated with an increased risk of future adverse cardiovascular events (71-73). A direct relationship was observed between plaque burden and the rate of plaque progression to cardiovascular outcomes in a study that used IVUS to assess coronary atherosclerotic burden (74). Similarly, plaque burden assessed by CCTA predicts prognosis. In addition, the location of the lesions, number of vessels with lesions and, the presence of normal, non-obstructive and obstructive vessels determine prognosis.
CCTA meets three fundamental criteria as a good prognostic test: (I) it identifies patients at very low risk for events; (II) it provides definition of clear gradations of risk based on test results; and (III) it enables the concentration of resources in management of patients with abnormal tests (75).
Presence of obstructive disease predicts prognosis
In a single-centre consecutive cohort of 1,127 symptomatic patients, Min et al. demonstrated that all-cause mortality was predicted by a number of angiographic features as assessed on CCTA. This included the presence of moderate (≥50%) or severe (≥70%) coronary stenosis in any coronary artery (P=0.007 and P<0.001, respectively) (Figure 9), the presence of a severe stenosis in any proximal segment of a major epicardial vessel (P=0.001) and the presence of obstructive left main or left anterior descending artery stenosis (P=0.001). It was also demonstrated that CCTA-derived Duke prognostic CAD index, clinical coronary artery plaque score including a segment stenosis score and segment involvement score, were significant predictors of all-cause mortality (P<0.001) (77).
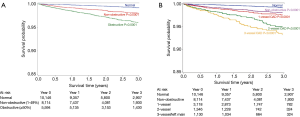
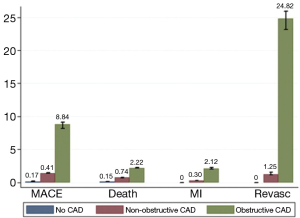
In a pooled analysis of 18 CCTA prognostic studies involving 9,592 symptomatic patients with predominantly suspected CAD (78), Hulten et al. (Figure 10) analysed the annual event rates for major cardiac events and death. The event rates in obstructive coronary disease defined as the presence of any lesion with ≥50% stenosis was 8.8% and 3.2% which was significantly higher (P<0.005) than patients with non-obstructive disease (1.4% and 0.74%) and normal coronary arteries on CCTA (0.17% and 0.15%), respectively.
In the CONFIRM Registry, a large international multicentre registry which included 24,775 patients at a mean follow up of 2.3 years, it was demonstrated that the presence of obstructive disease on CCTA not only risk stratified patients into clinically important risk tertiles (76), but was also a significant predictor of all-cause mortality and provided significant incremental value to LVEF and clinical variables with a net reclassification index of 17.8% (P<0.001) (79). Furthermore, using data from the same registry, Hadamitzky described the novel use of an optimised score which accounted for the number of proximal segments with a stenosis >50% or with mixed or calcified plaque (80). The use of this CCTA-based scoring system was demonstrated to significantly improve overall risk prediction beyond the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (ATP III) score (80).
Presence of non-obstructive disease predicts prognosis
Lin et al. (81) demonstrated that the presence of non-obstructive coronary artery stenosis (<50%) on CCTA was associated with higher mortality [hazard ratio (HR) =1.98, P=0.03] when compared with normal findings. The highest risk was among those who had non-obstructive CAD in three epicardial vessels (HR =4.75, P=0.0002) and ≥5 coronary segments (HR =5.12, P=0.0002) in a cohort of 2,583 symptomatic patients with mean follow up of 3.1 years. Importantly the higher mortality for non-obstructive disease was observed even in patients with low 10-year Framingham risk (3.4%, P<0.0001), as well as those with no traditional, medically treatable cardiovascular risk factors including diabetes mellitus, hypertension and dyslipidemia (5.7%, P<0.0001).
Presence of normal findings predicts prognosis
Lastly, normal findings on CCTA were found to be associated with very low event rates (76-79). This is comparable to the background event rate among healthy low-risk individuals (<1%) (82) and is similar to that reported in patients with normal stress testing evaluated on echocardiography or nuclear myocardial perfusion imaging (83).
In a more recent study that assessed the prognostic value of CCTA in 218 patients with a median follow up period of 6.9 years, the outcome was progressively worse in patients with nonobstructive and obstructive disease. Patients with normal coronary arteries on CCTA had excellent prognosis (84).
CT plaque quantification predicts prognosis
Versteylen et al. demonstrated that the use of a semi-automated plaque quantification algorithm may provide additional prognostic value in prediction of future ACS beyond conventional CT assessment of stenosis alone and Framingham risk scores (85). At a mean 26±10 months, patients with ACS were found to have higher total plaque volume (94 vs. 29 mm3, P<0.001), NCP volume (28 vs. 4 mm3, P<0.001) and plaque burden (57% vs. 36%, P<0.01).
CCTA—ACS
Predicting ACS
There is growing evidence to suggest that the susceptibility of an individual to develop an acute coronary event is determined by plaque morphology rather than the extent of luminal stenosis. In the COURAGE study (86), where patients who received optimal medical management, the consistent predictor of death, myocardial infarction and non-ST segment elevation ACS was the anatomic burden and not the ischemic burden. The occurrence of major adverse clinical outcomes may be because of the disruption rather than by the ischemia-producing nature of obstructive plaques. In this context, the prognostic significance of CCTA assumes importance as it has the advantage of being a noninvasive test that can be used to assess individual plaque characteristics apart from the assessment of luminal stenosis.
CT plaque characteristics predict future ACS
Two-thirds of ACS is caused by atherosclerotic plaque rupture. A number of CT-based coronary plaque characteristics of a “vulnerable plaque” associated with culprit lesions for future ACS have been identified in retrospective observational studies (Figure 11).
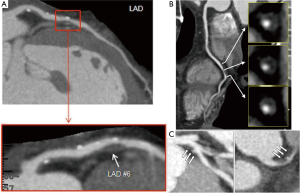
CCTA features of LAP, positive remodelling and spotty calcification predict ACS
Motoyama et al. compared CCTA features of 38 patients with ACS to 33 patients with stable angina (90). Plaques in ACS group had higher frequency of NCP <30 HU (79% vs. 9%, P<0.0001), spotty calcification (63% vs. 21%, P=0.0005) and positive remodelling (87% vs. 12%, P<0.0001). Presence of all three features showed a high PPV, and their absence demonstrated a high negative predictive value for future ACS. Furthermore, mean plaque volumes were higher in ACS group (192.8±114.9 vs. 103.8±51.8 mm3, P=0.001) in a stable angina group in another study (91). It has been shown in pathological studies, that the size of necrotic core in TCFAs ranges from 1.6 to 1.7 mm2 with a length of 8 mm (range, 2-17 mm), and in ruptured plaques ranges from 2.2 to 3.8 mm2 with a length of 9 mm (range, 2.5-22 mm) (92,93). Imazeki and colleagues demonstrated that remodelling detected on CCTA correlated with IVUS and remodelling index (RI) was significantly larger in patients with ACS (1.19±0.18) than in those with stable angina (0.89±0.10, P<0.0001) (94,95). Pathological studies have established a relationship between positive vessel remodelling and plaque vulnerability, showing an increase in inflammatory marker concentrations, larger lipid cores, paucity of smooth muscle cells, and medial thinning in positively remodelled vessels (95). In a large prospective study with a cohort of 1,059 patients, Motoyama et al. demonstrated that the CT angiographic features of LAP and positive remodelling were associated with subsequent development of acute coronary events (87). ACS developed in 10 (22%) of 45 patients who had these two CT features against 4 (0.5%) in 820 patients who did not have LAP or positive remodelling. None of the 167 patients with normal CCTA sustained acute events (P<0.001). Both features were independent predictors of acute coronary events [HR =23, 95% confidence interval (CI): 7-75, P<0.001]. Importantly, the plaques associated with early ACS when compared with late ACS had larger LAP volume.
CCTA features of Napkin ring sign, plaque ulceration and intraplaque dye penetration predict ACS
Vulnerable or rupture prone atherosclerotic plaques have histologically been described as TCFA, distinguished by a large necrotic core with an overlying thin intact fibrous cap, macrophage infiltration and often increased number of intraplaque vasa vasorum (92). Pathological studies have demonstrated the thickness of the fibrous cap thickness is the most important plaque characteristic to identify plaque vulnerability, followed by the magnitude of macrophage inflammation and the size of necrotic core (96). Kashiwagi and colleagues proposed that the presence of a ring-like attenuation in a CT angiographic cross section may be a surrogate marker of TCFAs after comparing OCT and CCTA findings (88). In a total of 100 patients with ACS, coronary lesions were divided into TCFA and non-TCFA group based on OCT findings. CCTA-verified positive remodelling was observed more frequently in the TCFA (75%) than in the non-TCFA group (30%, P<0.001). The TCFA group also demonstrated LAP more frequently; CT attenuation value in the TCFA group (35±32 HU) was significantly lower than the non-TCFA group (62±34 HU, P<0.001). Notably a ring-like attenuation in the TCFA group was found to be 11-fold more frequent than in the non-TCFA group (44% vs. 4%, P<0.001). The sensitivity, specificity, PPV, and negative predictive value of ring-like enhancement for detecting TCFA are 44%, 96%, 79%, and 85%, respectively (42). In another study by Tanaka and colleagues, 67 patients with de novo angina were divided into a plaque rupture group (n=27) and a non-rupture group (n=40) based on the IVUS. The 64-slice CCTA revealed that the prevalence of an ulcer-like enhancement space (37% vs. 5%, P<0.01), a ring-like sign (41% vs. 18%, P=0.04), in the plaque rupture group was higher than those in the non-rupture group (97). It is postulated that the ring like enhancement represents highly active vasa vasorum neovascularisation. Vasa vasorum density is seen as surrogate marker of plaque vulnerability as it strongly correlated with macrophage infiltration in atherosclerotic plaque (98). In addition, it has also been postulated that the ring-like enhancement may be due to large central lipid core surrounded by fibrous plaque tissue or intra mural thrombus (99).
Lastly, CT feature of plaque disruption including plaque ulceration and intraplaque dye penetration in patients with unstable angina, have been described to be modestly sensitive (53-81%) and highly specific (82-95%) for plaque erosion and/or intraplaque haemorrhage as demonstrated on invasive angiography. Madder et al. demonstrated that in 294 plaques with >25% stenosis on CCTA, 37% had features of disruption, including 27% with intraplaque dye penetration and 18% with ulceration. In addition, when compared with non-disrupted lesions, they were more voluminous (313±356 vs. 18±93 mm3, P<0.0001) and were more often complex by ICA (57.8% vs. 8.1%, P<0.0001) (89).
Use of CCTA in the assessment of acute chest pain in Emergency Departments (EDs)
Acute chest pain is one of the leading symptoms in ED all over the world. Exclusion of ACS is difficult when patients with typical chest pain have normal initial ECG and biomarkers (100). The standard evaluation of these patients includes hospital admission to undergo serial ECG’s, troponin assessment with or without additional stress testing and only a minority of such patients are eventually diagnosed with cardiac ischemia (101). Despite this expensive and comprehensive approach, 2% to 5% of ACS is still missed (102). The high sensitivity and negative predictive value of CCTA for the detection of coronary stenosis allows safe rule-out of CAD, especially in population with low to intermediate risk.
Use of CCTA in ED
CCTA has been studied in comparison with ICA for detection of ACS in several observational cohort studies. The Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography (ROMICAT) study, found that CCTA had excellent sensitivity and negative predictive value to rule out ACS in 368 subjects with inconclusive clinical evaluation of chest pain in ED (103). Hollander et al. (104) demonstrated that 84% of 568 patients with potential ACS and low Thrombolysis In Myocardial Infarction (TIMI) risk score, were discharged after CCTA. Compared to standard evaluation, when CCTA was included in triaging patients who presented with chest pain to ED, there was a reduction in the length of stay in hospital and increased discharge rates from ED without hospitalisation (105,106). These studies demonstrate that CCTA is an efficient and safe tool to assess patients with chest pain, particularly those with low to intermediate likelihood of CAD. In a meta-analysis of 64-detector CCTA, which included 1,559 patients presenting with chest pain, CCTA had a 99.3% negative predictive value in excluding major adverse cardiac events (MACE) for 30 days after initial symptom presentation in 85.2% of the study population (107). The ROMICAT and CT-STAT studies showed that the absence of significant coronary atherosclerosis is associated with the absence or a minimum number of MACE at 6 months of follow-up (103,108). Nasis et al. followed up 506 patients without plaque, patients with nonobstructive plaque and at most mild to moderate stenosis (<40% luminal narrowing). These patients were discharged from ED without further investigation and some of them, after a single troponin measurement. At median 47.4-month follow-up there were no major adverse events (0% for all; 95% CI: 0-0.7%) (109).
Better risk stratification
Among low to intermediate risk patients who presented with chest pain to the ED, CCTA was found to be a better predictor of CAD and cardiovascular outcomes than the use of TIMI score, global registry of acute coronary events (GRACE) score and risk assessment by traditional risk factors. In a study involving 250 patients evaluated by CCTA, correlation with CAD was poor for the TIMI (r=0.12) and GRACE (r=0.09-0.23) scores. Only older age and male sex were significant independent predictors of CAD while CCTA identified severe CAD in all subjects with adverse outcomes (110). In another study involving 93 patients, CCTA accurately identified ten patients (8.13%) with obstructive CAD requiring myocardial revascularization; all had a low TIMI score [0-2] and eight had a low GRACE score (111). Similarly, Linde et al. evaluated the clinical impact of CCTA on 600 patients who were randomised to a CCTA-guided strategy (299 patients) or standard care (301 patients). Referral rate for ICA was 17% with CCTA vs. 12% with standard care (P=0.1). ICA confirmed significant coronary artery stenoses in 12% vs. 4% (P=0.001), and 10% vs. 4% were subsequently revascularised (P=0.005). The PPV for the detection of significant stenoses was 71% with CCTA vs. 36% with standard care (P=0.001) (112).
Costs and resource utilization
Poon et al. demonstrated that the routine use of CCTA in ED evaluation of chest pain may reduce healthcare resource utilization. The overall admission rate was lower with CCTA (14% vs. 40%; P<0.001). Standard evaluation was associated with a 5.5-fold greater risk for admission [odds ratio (OR): 5.53; P<0.001], 1.6 times longer expected ED length of stay (OR: 1.55; P<0.001), 5 times greater likelihood of returning to ED with chest pain within 30 days (OR: 5.06; P=0.022) and a 7-fold greater likelihood of ICA without revascularization (OR: 7.17; P<0.001). There were no differences in the rates of death and acute myocardial infarction within 30 days of the index visit between the two groups (113). CCTA was found to be cost- effective compared to myocardial perfusion imaging (114). In a comprehensive cost-effectiveness model by Ladapo et al. (115), the increased overall cost of CCTA due to detection of CAD was partially offset by reduction of event rates by 3% and by lower costs of care for myocardial infarction and stroke.
Conclusions
Coronary CT angiography provides robust non-invasive, qualitative and quantitative assessment of coronary atherosclerosis with high reproducibility and accuracy. The constant advances in scanner technology; image acquisition and reconstruction techniques have expanded the use of CCTA. In addition to the anatomical information, it is now possible to assess the hemodynamic significance of atherosclerotic plaque and to predict clinical outcomes based on plaque burden and plaque morphology.
Acknowledgements
Dr. Ravi Kiran Munnur is a recipient of Cardiac Society of Australia and New Zealand (CSANZ) scholarship.
Dr. Brian Ko and Dr. Dennis Wong are funded by the National Heart Foundation of Australia and Robertson Family Scholarship.
Disclosure: The authors declare no conflict of interest.
References
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:143-52. [PubMed]
- Mallika V, Goswami B, Rajappa M. Atherosclerosis pathophysiology and the role of novel risk factors: a clinicobiochemical perspective. Angiology 2007;58:513-22. [PubMed]
- Narula J, Finn AV, Demaria AN. Picking plaques that pop. J Am Coll Cardiol 2005;45:1970-3. [PubMed]
- Muller JE, Abela GS, Nesto RW, et al. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J Am Coll Cardiol 1994;23:809-13. [PubMed]
- Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 2004;110:3424-9. [PubMed]
- Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101:598-603. [PubMed]
- Raff GL, Gallagher MJ, O'Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 2005;46:552-7. [PubMed]
- Pundziute G, Schuijf JD, Jukema JW, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J 2008;29:2373-81. [PubMed]
- Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. [PubMed]
- Bischoff B, Hein F, Meyer T, et al. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging 2009;2:940-6. [PubMed]
- Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500-7. [PubMed]
- Chen MY, Shanbhag SM, Arai AE. Submillisievert median radiation dose for coronary angiography with a second-generation 320-detector row CT scanner in 107 consecutive patients. Radiology 2013;267:76-85. [PubMed]
- Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340-6. [PubMed]
- Leipsic J, Labounty TM, Heilbron B, et al. Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am J Roentgenol 2010;195:655-60. [PubMed]
- Schoenhagen P, Baker ME. Our preoccupation with ultra-low dose radiation exposure. Low contrast resolution and cardiovascular CT imaging. J Cardiovasc Comput Tomogr 2014;8:426-8. [PubMed]
- Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135-44. [PubMed]
- Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724-32. [PubMed]
- Schuetz GM, Zacharopoulou NM, Schlattmann P, et al. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med 2010;152:167-77. [PubMed]
- Rinehart S, Vazquez G, Qian Z, et al. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr 2011;5:35-43. [PubMed]
- Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging 2009;2:1262-70. [PubMed]
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44-164. [PubMed]
- Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [PubMed]
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr 2010;4:407,e1-407,33.
- Fischer C, Hulten E, Belur P, et al. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 2013;7:256-66. [PubMed]
- Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4:537-48. [PubMed]
- Papadopoulou SL, Neefjes LA, Schaap M, et al. Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis 2011;219:163-70. [PubMed]
- Voros S, Rinehart S, Qian Z, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv 2011;4:198-208. [PubMed]
- Nakazato R, Shalev A, Doh JH, et al. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol 2013;62:460-7. [PubMed]
- Cheng VY, Nakazato R, Dey D, et al. Reproducibility of coronary artery plaque volume and composition quantification by 64-detector row coronary computed tomographic angiography: an intraobserver, interobserver, and interscan variability study. J Cardiovasc Comput Tomogr 2009;3:312-20. [PubMed]
- Otsuka M, Bruining N, Van Pelt NC, et al. Quantification of coronary plaque by 64-slice computed tomography: a comparison with quantitative intracoronary ultrasound. Invest Radiol 2008;43:314-21. [PubMed]
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012;59:379-87. [PubMed]
- Abdulla J, Pedersen KS, Budoff M, et al. Influence of coronary calcification on the diagnostic accuracy of 64-slice computed tomography coronary angiography: a systematic review and meta-analysis. Int J Cardiovasc Imaging 2012;28:943-53. [PubMed]
- Yoshioka K, Tanaka R, Muranaka K. Subtraction coronary CT angiography for calcified lesions. Cardiol Clin 2012;30:93-102. [PubMed]
- Yamada M, Jinzaki M, Imai Y, et al. Evaluation of severely calcified coronary artery using fast-switching dual-kVp 64-slice computed tomography. Circ J 2011;75:472-3. [PubMed]
- Chiurlia E, Menozzi M, Ratti C, et al. Follow-up of coronary artery bypass graft patency by multislice computed tomography. Am J Cardiol 2005;95:1094-7. [PubMed]
- Jabara R, Chronos N, Klein L, et al. Comparison of multidetector 64-slice computed tomographic angiography to coronary angiography to assess the patency of coronary artery bypass grafts. Am J Cardiol 2007;99:1529-34. [PubMed]
- Pache G, Saueressig U, Frydrychowicz A, et al. Initial experience with 64-slice cardiac CT: non-invasive visualization of coronary artery bypass grafts. Eur Heart J 2006;27:976-80. [PubMed]
- Weustink AC, Nieman K, Pugliese F, et al. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting: comparison with invasive coronary angiography. JACC Cardiovasc Imaging 2009;2:816-24. [PubMed]
- Hamon M, Lepage O, Malagutti P, et al. Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology 2008;247:679-86. [PubMed]
- Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Catheter Cardiovasc Interv 2010;76:E1-42. [PubMed]
- Abdelkarim MJ, Ahmadi N, Gopal A, et al. Noninvasive quantitative evaluation of coronary artery stent patency using 64-row multidetector computed tomography. J Cardiovasc Comput Tomogr 2010;4:29-37. [PubMed]
- Andreini D, Pontone G, Mushtaq S, et al. Multidetector computed tomography coronary angiography for the assessment of coronary in-stent restenosis. Am J Cardiol 2010;105:645-55. [PubMed]
- Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J 2006;27:2567-72. [PubMed]
- Carbone I, Francone M, Algeri E, et al. Non-invasive evaluation of coronary artery stent patency with retrospectively ECG-gated 64-slice CT angiography. Eur Radiol 2008;18:234-43. [PubMed]
- de Graaf FR, Schuijf JD, van Velzen JE, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis. Invest Radiol 2010;45:331-40. [PubMed]
- Andreini D, Pontone G, Bartorelli AL, et al. Comparison of feasibility and diagnostic accuracy of 64-slice multidetector computed tomographic coronary angiography versus invasive coronary angiography versus intravascular ultrasound for evaluation of in-stent restenosis. Am J Cardiol 2009;103:1349-58. [PubMed]
- Andreini D, Pontone G, Mushtaq S, et al. Coronary in-stent restenosis: assessment with CT coronary angiography. Radiology 2012;265:410-7. [PubMed]
- Sun Z, Almutairi AM. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: a meta-analysis. Eur J Radiol 2010;73:266-73. [PubMed]
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W, Kolh P, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. [PubMed]
- Min JK, Swaminathan RV, Vass M, et al. High-definition multidetector computed tomography for evaluation of coronary artery stents: comparison to standard-definition 64-detector row computed tomography. J Cardiovasc Comput Tomogr 2009;3:246-51. [PubMed]
- Fuchs TA, Stehli J, Fiechter M, et al. First in vivo head-to-head comparison of high-definition versus standard-definition stent imaging with 64-slice computed tomography. Int J Cardiovasc Imaging 2013;29:1409-16. [PubMed]
- Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis 2013;231:198-204. [PubMed]
- Inoue K, Motoyama S, Sarai M, et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging 2010;3:691-8. [PubMed]
- Hamirani YS, Kadakia J, Pagali SR, et al. Assessment of progression of coronary atherosclerosis using multidetector computed tomography angiography (MDCT). Int J Cardiol 2011;149:270-4. [PubMed]
- Bettencourt N, Chiribiri A, Schuster A, et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol 2013;61:1099-107. [PubMed]
- Wong DT, Ko BS, Cameron JD, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol 2013;61:1271-9. [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. [PubMed]
- Ko BS, Cameron JD, Meredith IT, et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J 2012;33:67-77. [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. [PubMed]
- Hachamovitch R, Berman DS, Kiat H, et al. Incremental prognostic value of adenosine stress myocardial perfusion single-photon emission computed tomography and impact on subsequent management in patients with or suspected of having myocardial ischemia. Am J Cardiol 1997;80:426-33. [PubMed]
- Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233-41. [PubMed]
- Min JK, Berman DS, Budoff MJ, et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr 2011;5:301-9. [PubMed]
- Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989-97. [PubMed]
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. [PubMed]
- Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. [PubMed]
- Choi JH, Min JK, Labounty TM, et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc Imaging 2011;4:1149-57. [PubMed]
- George RT, Arbab-Zadeh A, Miller JM, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging 2009;2:174-82. [PubMed]
- Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120-30. [PubMed]
- Ko BS, Cameron JD, Leung M, et al. Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: a comparison with fractional flow reserve. JACC Cardiovasc Imaging 2012;5:1097-111. [PubMed]
- Bamberg F, Becker A, Schwarz F, et al. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 2011;260:689-98. [PubMed]
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336-45. [PubMed]
- Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291:210-5. [PubMed]
- Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010;303:1610-6. [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [PubMed]
- Hachamovitch R, Di Carli MF. Methods and limitations of assessing new noninvasive tests: Part II: Outcomes-based validation and reliability assessment of noninvasive testing. Circulation 2008;117:2793-801. [PubMed]
- Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849-60. [PubMed]
- Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161-70. [PubMed]
- Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237-47. [PubMed]
- Chow BJ, Small G, Yam Y, et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging 2011;4:463-72. [PubMed]
- Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J Am Coll Cardiol 2013;62:468-76. [PubMed]
- Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011;58:510-9. [PubMed]
- Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47. [PubMed]
- Metz LD, Beattie M, Hom R, et al. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol 2007;49:227-37. [PubMed]
- Dougoud S, Fuchs TA, Stehli J, et al. Prognostic value of coronary CT angiography on long-term follow-up of 6.9 years. Int J Cardiovasc Imaging 2014;30:969-76. [PubMed]
- Versteylen MO, Kietselaer BL, Dagnelie PC, et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol 2013;61:2296-305. [PubMed]
- Mancini GB, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv 2014;7:195-201. [PubMed]
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [PubMed]
- Kashiwagi M, Tanaka A, Kitabata H, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging 2009;2:1412-9. [PubMed]
- Madder RD, Chinnaiyan KM, Marandici AM, et al. Features of disrupted plaques by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ Cardiovasc Imaging 2011;4:105-13. [PubMed]
- Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26. [PubMed]
- Pflederer T, Marwan M, Schepis T, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis 2010;211:437-44. [PubMed]
- Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13-8. [PubMed]
- Cheruvu PK, Finn AV, Gardner C, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol 2007;50:940-9. [PubMed]
- Imazeki T, Sato Y, Inoue F, et al. Evaluation of coronary artery remodeling in patients with acute coronary syndrome and stable angina by multislice computed tomography. Circ J 2004;68:1045-50. [PubMed]
- Wu YW, Kao HL, Huang CL, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur J Nucl Med Mol Imaging 2012;39:399-407. [PubMed]
- Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol 2013;61:1041-51. [PubMed]
- Tanaka A, Shimada K, Yoshida K, et al. Non-invasive assessment of plaque rupture by 64-slice multidetector computed tomography--comparison with intravascular ultrasound. Circ J 2008;72:1276-81. [PubMed]
- Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004;110:2843-50. [PubMed]
- Zerhouni EA, Barth KH, Siegelman SS. Demonstration of venous thrombosis by computed tomography. AJR Am J Roentgenol 1980;134:753-8. [PubMed]
- Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005;294:2623-9. [PubMed]
- Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010;122:1756-76. [PubMed]
- Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163-70. [PubMed]
- Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009;53:1642-50. [PubMed]
- Hollander JE, Chang AM, Shofer FS, et al. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295-304. [PubMed]
- Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. [PubMed]
- Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393-403. [PubMed]
- Takakuwa KM, Keith SW, Estepa AT, et al. A meta-analysis of 64-section coronary CT angiography findings for predicting 30-day major adverse cardiac events in patients presenting with symptoms suggestive of acute coronary syndrome. Acad Radiol 2011;18:1522-8. [PubMed]
- Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011;58:1414-22. [PubMed]
- Nasis A, Meredith IT, Sud PS, et al. Long-term outcome after CT angiography in patients with possible acute coronary syndrome. Radiology 2014;272:674-82. [PubMed]
- Halpern EJ, Deutsch JP, Hannaway MM, et al. Cardiac risk factors and risk scores vs cardiac computed tomography angiography: a prospective cohort study for triage of ED patients with acute chest pain. Am J Emerg Med 2013;31:1479-85. [PubMed]
- Hascoët S, Bongard V, Chabbert V, et al. Early triage of emergency department patients with acute coronary syndrome: contribution of 64-slice computed tomography angiography. Arch Cardiovasc Dis 2012;105:338-46. [PubMed]
- Linde JJ, Kofoed KF, Sorgaard M, et al. Cardiac computed tomography guided treatment strategy in patients with recent acute-onset chest pain: results from the randomised, controlled trial: CArdiac cT in the treatment of acute CHest pain (CATCH). Int J Cardiol 2013;168:5257-62. [PubMed]
- Poon M, Cortegiano M, Abramowicz AJ, et al. Associations between routine coronary computed tomographic angiography and reduced unnecessary hospital admissions, length of stay, recidivism rates, and invasive coronary angiography in the emergency department triage of chest pain. J Am Coll Cardiol 2013;62:543-52. [PubMed]
- Min JK, Shaw LJ, Berman DS, et al. Costs and clinical outcomes in individuals without known coronary artery disease undergoing coronary computed tomographic angiography from an analysis of Medicare category III transaction codes. Am J Cardiol 2008;102:672-8. [PubMed]
- Ladapo JA, Jaffer FA, Hoffmann U, et al. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol 2009;54:2409-22. [PubMed]


