Association between platelet count and the risk of bleeding among patients with nonvalvular atrial fibrillation taking dabigatran after radiofrequency ablation: a cohort study
Introduction
Atrial fibrillation (AF) remains an important risk factor for systemic embolism and stroke (1,2). Substantial evidence suggests that anticoagulation with warfarin reduces this risk by two-thirds, whereas antiplatelet therapy decreases the risk of systemic embolism and stroke by only 22% (3). There is a lot of evidence that dabigatran, which is a direct thrombin inhibitor, is convenient and safe alternatives to VKAs based on the results of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial (4). Although non-vitamin K antagonist (VKA) oral anticoagulants (OACs) (NOACs) can prevent systemic embolism and stroke, they can cause bleeding. The benefits of NOACs in treatment of nonvalvular atrial fibrillation (NVAF) are based on a balance between reducing the risk of stroke and reducing the risk of bleeding. The risk of bleeding in AF patients undergoing OAC therapy can be assessed by hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (>65 years), drugs/alcohol concomitantly (HAS-BLED) (5), older age (>75 years), reduced haemoglobin/haematocrit/ history of anaemia, bleeding history, insufficient kidney function, and treatment with antiplatelet (ORBIT) (6) and Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke (HEMORR2HAGES) scores (7). However, these scores were mainly obtained and developed mainly among patients taking VKAs. The relationship between the risk factors for bleeding and NOACs is not well understood.
Platelets play a key role by accelerating some steps in the coagulation process. A reduction in platelet count or function is a risk factor for bleeding in anticoagulated patients based on the HEMORR2HAGES scores (7). However, in this trial, all patients receiving OAC therapy were treated with warfarin or aspirin and did not consume a NOAC. To the best of our knowledge, no epidemiological trials have focused on the relationship of platelets with bleeding in NVAF patients taking dabigatran. Therefore, the goal of this paper was to investigate the relationship between platelet count and dabigatran-related bleeding in NVAF patients after catheter ablation. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-645).
Methods
Study design and population
Details of the trial design, outcome definitions and patients have been published (8). Patients were recruited from 12 study sites in China between February 2015 and December 2017. In brief, a total of 576 patients with NVAF after radiofrequency ablation received oral dabigatran (110 mg bid) treatment (9). An electronic data collection system was used for the data collection process and the data were reviewed regularly throughout the trial by an independent data and safety monitoring committee. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Second Affiliated Hospital of Nanchang University research committee (MISSION-AF, ClinicalTrials.gov Identifier: NCT02414035) and informed consent was taken from all the patients.
Study variables and definitions of terms
The primary study outcome was the first occurrence of all bleeding events during the 3-month follow-up period. Major bleeding was defined as (I) fatal bleeding; (II) a reduction in the hemoglobin concentration of at least 20 g/L with the transfusion of at least two units of blood; and (III) symptomatic bleeding in a critical area or organ. All other bleeding events were classified as minor (10,11).
The platelet counts obtained at baseline were treated as a continuous variable and measured by automatic blood analysis equipment in accordance with the standard methods that were consistent across the laboratories at the different centers. Laboratory staff members were not aware of the research protocol.
Treatment and follow-up procedure
Based on the MISSION-AF database, we analyzed the effect of platelet count on frequencies of bleeding events in NVAF patients. In present study, we only analyzed the 3-month follow-up date, because the study population included some NVAF patients with CHA2DS2-VASc scores of 0 for men and 1 for women. Anticoagulation only needs to be maintained for only 8 weeks after ablation for these patients (12). Any decisions to continue anticoagulation with dabigatran after the 3-month follow-up period were based on the guidelines (12) and at the professional physician’s discretion. In addition, the risk for bleeding events with dabigatran was highest during the first 90 days of treatment (13,14). A total of 576 NVAF patients treated with dabigatran completed the 3 months of follow-ups.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or the median (minimum, maximum). Categorical variables are expressed as numbers and frequencies. To test for differences among patients with different platelet count groups (group 1, <100×109/L; group 2, 100–200×109/L; and group 3, ≥200×109/L), we compared continuous variables using one-way analysis of variance (ANOVA). and categorical variables using the chi-squared test or Fisher’s exact test. The hazard ratio (HR) and 95% confidence interval (CI) between platelet count and bleeding events were evaluated using Cox proportional hazards models. The models were incrementally adjusted for the following potential confounders based on theoretical considerations and their availability in this study: model 1, crude model; model 2, adjusted for age, gender, smoking habits, drinking habits, BMI and the type of AF; and model 3, additionally accounted for eGFR, comorbidities (hypertension, CHD, HF, previous stroke or TIA, PAD, and a history of bleeding), medications (ACE inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs), beta-blockers, proton-pump inhibitors (PPIs), amiodarone, digoxin, antiplatelet agents, and statins). Then, we used fractional polynomial regression models to explore nonlinear associations of platelet counts and bleeding events, in which platelet counts were treated as continuous variables. If nonlinearity was detected, we first calculated the inflection point using a recursive algorithm and then constructed a two-piecewise Cox proportional hazard model on both sides of the inflection point. We determined the best-fit model based on the P values from the log likelihood ratio test. We also examined if the association between PLT count and bleeding varied by sex, age, type of AF, smoking, drinking and SBP. A sensitive analysis restricting the sample only to the patients without treatment of Antiplatelet agents was performed. All the analyses were performed with the statistical software packages in R (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A two-sided P value <0.05 was considered statistically significant for all tests.
Results
Patient characteristics
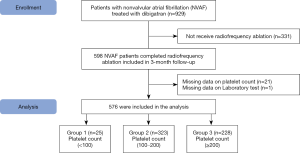
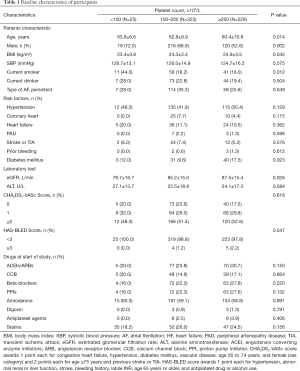
Based on the inclusion and exclusion criteria, a total of 576 patients were included for the final data analysis (mean age: 61.9±10.2 years; 61.4% were male) (Figure 1). Table 1 shows the baseline characteristics in three groups of patients with platelet counts. The patients in the group with the highest platelet count were more likely to be younger and have a higher BMI and eGFR, and this group had a higher proportion of females than the other groups. No statistically significant differences were detected in the SBP, drinking habits, CHA2DS2-VASc score, HAS-BLED score, hypertension, CHD, HF, PAD, TIA, the history of stroke, history of bleeding, ACEIs/ARBs, beta-blockers, PPIs, amiodarone, digoxin, antiplatelet agent, and statins among the different platelet count groups (P values >0.05).
Full table
Association between platelet count and the risk of bleeding
The median follow-up period was 87 days. The number of bleeding events was 50 participants (21 males and 29 females), and the incidence rate was 8.7% (50/576). The bleeding events included 33 hematuria cases, 4 gingival bleeding cases, 1 skin ecchymosis case, 5 hemoptysis cases, 6 epistaxis cases and 1 other bleeding case. Table 2 presents the relationship between platelet count and bleeding events. In the fully adjusted model (model 3), the multivariate-adjusted HRs (95% CIs) of the bleeding events associated with the middle platelet count group and the group with the highest platelet count, compared with the group with the lowest platelet count, were 0.24 (95% CI: 0.08–0.75; P=0.014) and 0.26 (95% CI: 0.08–0.86; P=0.028), respectively. Compared with the normal platelet count group (≥100×109/L), the low platelet count group (<100×109/L) was associated with a higher prevalence of bleeding (4.05 95% CI, 1.32–12.46; P=0.014). When platelet count was included in the final regression model as a categorical variable, the magnitudes of the effects on the different platelet count groups were not equal. The results suggest that the relationship between platelet count and bleeding events may be nonlinear.

Full table
Nonlinearity between platelet count and the risk of bleeding
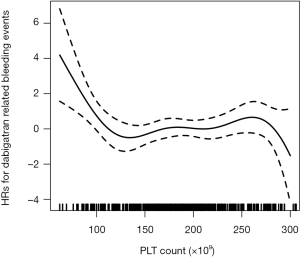
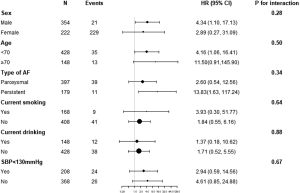
The adjusted smooth curve showed that the association between platelet count and bleeding events was nonlinear (Figure 2). There was a nonlinear relationship, with a significantly higher risk for bleeding observed in patients with lower platelet counts. We fitted the association between platelet count and bleeding events using the Cox proportional hazards regression model and two-piecewise Cox proportional hazards regression model (Table 3). The P value for the log likelihood ratio test was less than 0.05. This result indicates that the two-piecewise Cox proportional hazards regression model is more suitable for fitting the association between platelet count and bleeding events. Using a two-piecewise Cox proportional hazards regression and recursive algorithm, we calculated the inflection point to be 105×109/L. In model 3, for platelet counts <105×109/L, each 1×109 increase in platelet count was associated with an 11% decreased in the risk of bleeding events (P<0.001). For platelet counts ≥105×109/L, the relationship between platelet count and the risk of bleeding events was not significant. Details of the subgroup analyses are presented in Figure S1. The association between platelet count and bleeding events was consistent in the following subgroups: sex, age, BMI, type of AF, smoking, drinking and SBP (all P for interaction >0.05). Sensitivity analysis showed that the association between platelet count and bleeding events remained statistically significant in patients without treatment of antiplatelet agents (Table S1).

Full table
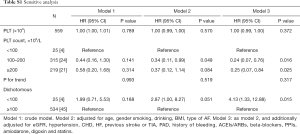
Full table
Discussion
In the present study, our purpose was to determine the relationship between platelet count and bleeding events among NVAF patients receiving radiofrequency ablation in China. A nonlinear association was found, which suggests that patients with platelet counts <105×109/L are associated with a high incidence of bleeding events. This association remained after a multivariate adjustment for other confounding factors was performed.
The association between platelet count and the risk of bleeding is likely not complex because platelets play a central role in the coagulation process. A number of previous studies have reported conflicting conclusions regarding the association between platelet count and the risk of bleeding events. A recent study showed a U-shaped association between platelet count and major bleeding events among patients with acute venous thromboembolism (VTE) receiving VKAs (15). In that study, patients were categorized into five groups (<100,000/mL, 100,000–150,000/mL, 150,000–300,000/mL, 300,000–450,000/mL, and >450,000/mL). Patients with the lowest or highest platelet counts had a remarkably increased risk of major bleeding compared with the other patients. However, patients enrolled in this study were diagnosed with acute deep vein thrombosis and pulmonary embolism. The IMPROVE study, which involved 15,156 patients, showed an increased risk of bleeding events was associated with reduced platelet count (16), and a low platelet count was defined as <50×109/L in medical patients. However, in that study, platelet count was simply categorized into two groups, so the effect of mildly reduced platelet count levels on the risk of bleeding events could not be examined. HEMORR2HAGES scores showed that a reduction in platelet count or function was a independent predictor for bleeding among anticoagulated patients (7). However, that study was mainly focused on the risk factors of bleeding events in patients taking warfarin or aspirin, and they chose 75 years as the age threshold.
The nonlinear association between platelet count and bleeding events is biologically plausible. Hemostasis can be viewed as four separate but interrelated events: compression and vasoconstriction; the formation of a platelet plug; blood coagulation; and clot retraction. In addition, platelets regulate bleeding in three stages. First, they form multicellular aggregates linked by protein strands at openings in blood vessels. The aggregates form a physical barrier that begins to limit blood loss soon after the opening occurs. Second, phospholipids on the platelet plasma membrane activate the enzyme thrombin, which initiates a cascade of events ending in clot formation. Finally, platelets possess multiple storage granules, which they discharge (secrete) to enhance coagulation. A reduction in platelet count or function can increase coagulation time, which can increase the risk of bleeding events.
The current findings suggest that a reduction in platelet count or function is linked with a high risk of bleeding. In a prospective double-blind study of 194 patients with acute VTE, patients with platelet counts <150×109 /L had an increased risk of bleeding (17). Beyth et al. (18) reported that a reduced platelet count was an independent risk factor for bleeding events in patients taking coumarins. A previous observational study also indicated that platelet count or function was associated with bleeding in AF patients taking on warfarin or aspirin (7). The existing bleeding risk scores are mainly focused on patients taking VKAs, and there are few studies examining the risk of bleeding events associated with NOCAs. In the present study, we expanded these observations in a multicenter, prospective and observational study among NVAF patients taking dabigatran and, for the first time, explored the associations of platelet count with bleeding events in NVAF patients who underwent catheter ablation.
This study has several limitations. First, the findings in this study were based on a 110 mg dabigatran dose instead of a 150 mg dabigatran dose, so the conclusions cannot be applied to patients taking 150 mg of dabigatran. Some previous studies showed that a 110 mg dose of dabigatran in Asian populations yields pharmacokinetics and clinical outcomes in Asian populations that are similar to those in Western populations taking a 150 mg dabigatran dose (19,20). Additionally, Asians have a relatively small body size and lower renal clearance, as well as genetic differences in metabolic or pharmacodynamic features, and lower doses of dabigatran may enhance the safety (21,22). Second, all patients enrolled in this study were NVAF patients who underwent catheter ablation, and additional large studies are needed to confirm our findings in all NVAF patients. In order to reduce the risk of operation, the platelet count of all patients was greater than 50×109/L, but we still observed that the risk of bleeding events of group T1 was higher than other groups. Table S2 shows that the average mean, median and range of platelet counts in all groups. Third, the sample size was small, and the mean HAS-BLED score was 0.69 in all patients; thus, the incidence of major bleeding events was very low. Therefore, all bleeding events were minor bleeding events, we can only perform a statistical analysis of the relationship between platelet count and minor bleeding. However, prior studies showed that the occurrence of minor bleeding may predict major bleeding events and may lead to a decrease in the effectiveness of OAC therapy (23). Finally, the group of patients where majority bleeding is expected (PLT counts <100×109/L) is the smallest (25 patients only out of 576), hence the conclusions can not be easily deducted.
Full table
Despite these limitations, this study may have several clinical implications. First, to our knowledge, it is the first time to assess the association of platelet count with the risk of bleeding events in NVAF patients taking dabigatran in a prospective study. We carefully controlled for confounders by using standardized clinical and laboratory procedures. These strengths enabled the researchers to investigate the association between platelet count and bleeding independent of possible confounders. Second, to avoid bias caused by differences in treatment of antiplatelet agents, we carried out a sensitivity analysis focused on the patients without treatment of antiplatelet agents, the association between platelet count and bleeding events remained statistically significant (Table S1). Third, physicians should be conscious of the significant risk for bleeding associated with reduced platelet count among NVAF patients taking dabigatran.
Conclusions
In this multicenter, prospective and observational study, we found that a reduced platelet count was independently associated with an increased risk of bleeding events in NVAF patients who underwent catheter ablation and were taking dabigatran. Our study supports a nonlinear relationship between platelet count and the risk of bleeding events. Additional randomized studies may be warranted to confirm the causality of these observational results.
Acknowledgments
Funding: This study was supported by the Major New Drug Creation Program from National Science and Technology Major Project (No. 2014ZX09303305) and the Science and Technology Planning Project of Jiangxi Province (No. 20161ACG70012).
Footnote
Reporting Checklist: The authors present the study in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-645
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-645
Peer Review File: Available at http://dx.doi.org/10.21037/cdt-20-645
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-645). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Second Affiliated Hospital of Nanchang University research committee (MISSION-AF, ClinicalTrials.gov Identifier: NCT02414035) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213-20. [Crossref] [PubMed]
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. [Crossref] [PubMed]
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Pisters R, Lane DA, Nieuwlaat R, et al. A Novel User-Friendly Score (HAS-BLED) To Assess 1-Year Risk of Major Bleeding in Patients with Atrial Fibrillation: The Euro Heart Survey. Chest 2010;138:1093-100. [Crossref] [PubMed]
- O'Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015;36:3258-64. [Crossref] [PubMed]
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713-9. [Crossref] [PubMed]
- Xiong Y, Hu L, Zhou W, et al. Association Between the Change in Total Bilirubin and Risk of Bleeding Among Patients with Nonvalvular Atrial Fibrillation Taking Dabigatran. Clin Appl Thromb Hemost 2020;26:1076029620910808. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Fries D, Giurea A, Gutl M, et al. Management of dabigatran-induced bleeding: expert statement. Wien Klin Wochenschr 2013;125:721-9. [Crossref] [PubMed]
- Kawabata M, Yokoyama Y, Sasano T, et al. Bleeding events and activated partial thromboplastin time with dabigatran in clinical practice. J Cardiol 2013;62:121-6. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996;348:423-8. [Crossref] [PubMed]
- Maura G, Blotière P, Bouillon K, et al. Comparison of the Short-Term Risk of Bleeding and Arterial Thromboembolic Events in Nonvalvular Atrial Fibrillation Patients Newly Treated With Dabigatran or Rivaroxaban Versus Vitamin K Antagonists. Circulation 2015;132:1252-60. [Crossref] [PubMed]
- Giorgi-Pierfranceschi M, Di Micco P, Cattabiani C, et al. Platelet Count and Major Bleeding in Patients Receiving Vitamin K Antagonists for Acute Venous Thromboembolism, Findings From Real World Clinical Practice. Medicine (Baltimore) 2015;94:e1915. [Crossref] [PubMed]
- Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest 2011;139:69-79. [Crossref] [PubMed]
- Nieuwenhuis HK, Albada J, Banga JD, et al. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood 1991;78:2337-43. [Crossref] [PubMed]
- Beyth RJ, Milligan PE, Gage BF. Risk factors for bleeding in patients taking coumarins. Curr Hematol Rep 2002;1:41-9. [PubMed]
- Chan YH, Kuo CT, Yeh YH, et al. Thromboembolic, Bleeding, and Mortality Risks of Rivaroxaban and Dabigatran in Asians With Nonvalvular Atrial Fibrillation. J Am Coll Cardiol 2016;68:1389-401. [Crossref] [PubMed]
- Cha MJ, Choi EK, Han KD, et al. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke 2017;48:3040-8. [Crossref] [PubMed]
- Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568-76. [Crossref] [PubMed]
- Miyamoto K, Nakasuka K, Kusano K. Effect of Renal Function on Anticoagulation Therapy in Asian Patients. Circ J 2015;79:2098-9. [Crossref] [PubMed]
- Kovacs RJ, Flaker GC, Saxonhouse SJ, et al. Practical management of anticoagulation in patients with atrial fibrillation. J Am Coll Cardiol 2015;65:1340-60. [Crossref] [PubMed]