Hypertrophic cardiomyopathy: still connecting the dots between genotype and phenotype
The current era of cardiac magnetic resonance (CMR) imaging has resulted in a resurgence of interest in hypertrophic cardiomyopathy (HCM). We are now much more aware of the various phenotypic expressions of this disease, which can manifest with typical septal thickening or other variants of hypertrophy, such as apical, mid-wall or concentric. Additionally, CMR has enabled us to appreciate the wide variety of abnormalities in papillary muscle morphology, which in some cases can contribute to left ventricular outflow tract or mid-cavitary level obstruction (1). In some subjects, mitral valve elongation, myocardial crypts, prominent apical trabeculation, papillary muscle thickening (with or without apical displacement) and abnormal chordal attachments may be subtle phenotypic HCM variants, despite normal left ventricular wall thickness. These abnormalities may be symptomatic to varying degrees or remain subclinical and be noted incidentally or on HCM familial screening programs. Whether these subjects will go on to develop progressive left ventricular hypertrophy (LVH) or whether they are manifesting incomplete phenotypic penetrance of the disease process remains unclear.
CMR delayed gadolinium enhancement (DGE) imaging has provided the most dramatic evolution in our understanding of HCM in recent times. Its incremental utility over structural cine based sequences provides qualitative and quantitative fibrosisassessment. Thereby, highlighting that hemodynamic obstruction is only one facet of the disease process and that fibrosis burden is likely also central to the risk of adverse outcomes including arrhythmia and sudden cardiac death (SCD). Although correlation between fibrosis amount and adverse outcomes appears proportional, there remains ongoing controversy regarding the definition of the percentage of fibrosis. Part of the issue stems from the heterogeneity of methodology between centers in defining delayed enhancement. Some advocate that the definition of positive DGE is 6 standard deviations from that of ‘normal’ non-enhancing myocardium, whilst others use alternative numbers of standard deviations or the full width at half-maximum technique (2,3). Variations in vendor specific scanner sequences, different analysis platforms, alternative contrast agents and individual inherent patient factors, such as renal function and hemodynamic status, may also impact upon uniformity and reproducibility of results between centers. There remains no definitive cut-point of percentage DGE above which the risk of SCD is independently increased. Although, a significant burden of DGE may be persuasive in decision making regarding defibrillator implantation in borderline cases (4). T1 mapping provides an alternative method, by which to quantify myocardial fibrosis. It relies on Look-Locker based techniques to extrapolate curves of signal intensity from which the T1 time can be established and correlated with collagen volume fraction either with or without contrast. T1 mapping has proven particularly useful for quantification of diffuse fibrosis as, unlike DGE, it does not rely on “normal” myocardium as a reference point. Although perhaps less essential in HCM, where fibrosis tends to be more focal, T1 mapping has shown supportive benefit to traditional DGE based imaging (5,6).
HCM is a genetic disorder resulting from mutations in sarcomeric proteins, which manifests in characteristic cardiomyocyte disarray and varying degrees of LVH and interstitial fibrosis (7). To date, over thirty different causative mutations have been identified and are more likely to be diagnosed in familial, rather than sporadic, cases (8). Those with identifiable mutations tend to have worse outcomes (9). Some mutations have also been described as more malignant than others, resulting in disease that presents earlier, with more severe LVH (10). However, there is a paucity of conclusive data to support the relationship between genotype, phenotype and clinical outcomes in HCM. In particular, individual genetic mutations have not been linked with the various phenotypic manifestations of LVH or associated structural abnormalities. Some gene positive subjects demonstrate a normal phenotype, whilst others manifest severe disease (Figure 1). Perhaps some individuals or families have multiple as yet unidentified interacting genes or environmental factors, which in combination result in a multi-hit phenomenon and more severe disease expression? Preliminary data suggests that disease expression is more severe in subjects with multiple mutations, even if the same mutations in isolation represent a relatively benign phenotype (11). Ongoing genetic testing of probands and family members continues, although currently is not adequate to estimate outcome or guide treatment. Stratification of genetically “at risk” subjects and genomic therapy remains a pipedream, with much work still required before we can conclusively link genotype and phenotype in this condition. Currently, treatment remains targeted towards symptomatic individuals, or those asymptomatic individuals with severe LVH and family history of SCD. In particular, specific genetic mutations are not used to risk stratify or guide intervention such as defibrillator implantation.
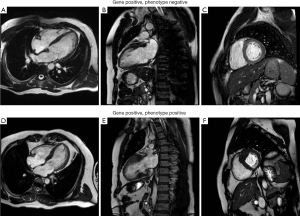
Recently published data in the European Heart Journal from Ellims et al. at the Baker IDI Heart and Diabetes Institute in Australia takes an important step in connecting the dots between genotype and phenotype in HCM (12). They recruited 139 consecutive subjects with HCM and 25 healthy controls, who all underwent echocardiography, CMR with DGE and post-contrast T1 mapping to examine both focal and diffuse myocardial fibrosis respectively. They subsequently examined the relationship between post-contrast T1 values and myocardial collagen content on tissue specimens from a subgroup of 9 subjects who underwent septal myectomy. Lastly, they performed clinical genetic screening with next-generation parallel sequencing on 56 subjects with HCM for common HCM related DNA mutations. Subjects with known identifiable pathogenic or likely pathogenic genetic mutations were then classified as gene positive.
As expected, subjects with HCM had increased left ventricular ejection fraction, LV mass and maximum LV wall thickness and worse diastolic function compared with the control group. Diagnostic quality of CMR was excellent, with 98% of DGE and 99% of T1 mapping sequences being interpretable. DGE was identified in 86% of HCM subjects, although accounted for a mean of only 4.6±6.1% of total LV mass. This seems surprisingly low and appears driven by focal DGE at the right ventricular insertion point. DGE was also unrelated to symptoms of dyspnea overall. Unfortunately, correlation of DGE with longitudinal outcome data was beyond the scope of this manuscript. In contrast, T1 values in the HCM subjects correlated both with symptoms of dyspnea and estimated left ventricular filling pressure and were notably different from the comparative T1 values in controls. This supports the premise that diffuse, underlying myocardial fibrosis occurs in HCM, in regions without obvious DGE. An inverse correlation between T1 values and body mass index is suggestive of diffuse reactive fibrosis in subjects with metabolic disease, in line with previous studies (13), however data regarding the complete metabolic profile of study subjects is not provided for verification. Interestingly, there was no correlation between T1 values and percentage or presence of DGE.
These results suggest that the presence of focal and diffuse fibrosis in HCM is unrelated and that diffuse interstitial fibrosis results in more diastolic dysfunction, manifesting as dyspnea. However, interpretation of results in this way must be cautioned, as there are significant limitations with consecutive, non-randomized subject recruitment in a study such as this, which can bias the findings. Specifically, it seems plausible that a significant proportion of subjects with familial HCM would have presented for screening purposes and were asymptomatic, whilst de novo cases without family history of HCM would most likely have presented due to symptoms. Comparative data between the symptomatic and asymptomatic subjects is not provided, but one wonders if the symptomatic subjects were also older and had more metabolic derangement and other non-HCM related comorbidities, which may have contributed to increased diffuse fibrosis, diastolic dysfunction and dyspnea in these subjects? In the subjects who underwent septal myectomy, an inverse correlation between myocardial collagen content and post-contrast T1 values was also noted (r =−0.7, P=0.03), despite no discrete DGE being identified within these basal septal regions preoperatively. These findings mirror the results of the widely referenced initial T1 mapping paper from this group in 2008, which examined the relationship between post-contrast T1 values and myocardial collagen content from post-transplant endomyocardial biopsy specimens (14). As acknowledged by the authors, pre-contrast T1 mapping to enable ECV calculation was not performed in this study and in retrospect may have been additive.
The most interesting and particularly novel aspect of the study relates to the genetic analysis. Gene mutations related to HCM were identified in 64% (n=36) of tested subjects, who demonstrated higher prevalence and proportion of DGE than gene negative subjects. Conversely, post-contrast T1 values were higher in the gene positive group, consistent with less diffuse fibrosis. The most plausible explanation for this disparity between groups is that gene positive subjects were more likely to have familial HCM (P=0.03) and may have presented for screening purposes, rather than with symptomatic obstructive disease. This is supported by greater rates of dyspnea (P=0.01) and higher peak LVOT gradients (55±49 vs. 28±41 mmHg; P=0.03) in the gene negative group, which likely prompted their investigation in the first place. The location of maximal wall thickness is not stipulated for the two groups, although they state that phenotypic differences were not related to specific gene mutations. However, one wonders if gene negative subjects had a greater predominance of basal septal hypertrophy, which predisposed to the described increased rates of LVOT obstruction and smaller end-systolic volumes. The consequentially increased left ventricular afterload in these subjects, may have predisposed them to diffuse fibrosis, via a similar mechanism to that seen in severe aortic stenosis.
There remains an ongoing need for optimization of imaging and genetic profiling techniques to connect the dots between phenotypic pathogenesis and genotypic expression in HCM. The described findings by Ellims et al. demonstrate important observations regarding the differences in the pattern of myocardial fibrosis associated with both symptoms and HCM gene mutations. Based upon their initial findings, the possible link between gene positivity and LVH location needs further exploration in a randomized study population. Long-term outcome data will also be essential to establish a connection between genotype, phenotype and prognosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- To AC, Lever HM, Desai MY. Hypertrophied papillary muscles as a masquerade of apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2012;59:1197. [PubMed]
- Spiewak M, Malek LA, Misko J, et al. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol 2010;74:e149-53. [PubMed]
- Moravsky G, Ofek E, Rakowski H, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging 2013;6:587-96. [PubMed]
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e783-831. [PubMed]
- Ellims AH, Iles LM, Ling LH, et al. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson 2012;14:76. [PubMed]
- Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012;5:726-33. [PubMed]
- Desai MY, Ommen SR, McKenna WJ, et al. Imaging phenotype versus genotype in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2011;4:156-68. [PubMed]
- Erdmann J, Daehmlow S, Wischke S, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet 2003;64:339-49. [PubMed]
- Olivotto I, Girolami F, Ackerman MJ, et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc 2008;83:630-8. [PubMed]
- Lopes LR, Rahman MS, Elliott PM. A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart 2013;99:1800-11. [PubMed]
- Blankenburg R, Hackert K, Wurster S, et al. β-Myosin heavy chain variant Val606Met causes very mild hypertrophic cardiomyopathy in mice, but exacerbates HCM phenotypes in mice carrying other HCM mutations. Circ Res 2014;115:227-37. [PubMed]
- Ellims AH, Iles LM, Ling LH, et al. A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: linking genotype with fibrotic phenotype. Eur Heart J Cardiovasc Imaging 2014;15:1108-16. [PubMed]
- Jellis C, Wright J, Kennedy D, et al. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging 2011;4:693-702. [PubMed]
- Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 2008;52:1574-80. [PubMed]


