Current diagnostic and treatment strategies for Lutembacher syndrome: the pivotal role of echocardiography
Introduction
Cardiovascular disease (CVD) is a leading cause of death and a major public health challenge worldwide (1). The proportion of CVD due to congenital heart diseases is increasing as exemplified by the increasing demand for cardiac interventions both invasive and non-invasive to treat both acquired and congenital CVD (2). This has major public health and financial implications. The situation is likely worse in low- and middle-income countries (LMIC) and those in sub-Saharan Africa in particular owing to the high endemicity of rheumatic heart disease (3). In some rare instances, congenital and acquired heart diseases co-existing.
Lutembacher syndrome (LS) is a rare clinical condition characterized by the presence of a congenital, secundum-type atrial septal defect (ASD) and an acquired mitral stenosis (MS) (commonly of rheumatic origin) (4). LS has been reported to be generally associated with long-term unfavorable natural course (5). The ASD in LS can also be iatrogenic, secondary to trans-septal puncture during mitral valvuloplasty for acquired MS. Generally, ASD in LS should have a diameter greater than 1.5 cm which later causes left to right shunt, progressive pulmonary hypertension (PH) and development of Eisenmenger syndrome (4). Another form described in the literature is the ‘reverse LS’; with instead a predominant pulmonary-to-systemic or right-to-left shunting of blood in the context of an ASD and severe tricuspid stenosis (6). Some authors have argued that LS could involve any mitral valve lesion (stenosis, insufficiency or mixed). The definition of LS has undergone several changes and adaptations with time but, currently any combination of ASD (congenital or iatrogenic) and MS (congenital or acquired) is referred to as LS (7).
There is scanty epidemiological data on LS and its prevalence is generally unknown, but current opinions suggest that it could be more common in areas of high endemicity for rheumatic fever (8). In a case series by Bashi et al. in 1987, a history of acute rheumatic fever was found in up to 40% of patients with LS in developing countries (5). Congenital MS is a rare entity, accounting for only 0.6% of cases of congenital heart diseases in autopsy studies. In patients with ASD, up to 4% have MS while the incidence of ASD in patients with MS is 0.6-0.7% (9). Nevertheless, some data suggests that the incidence of LS is 0.001 per million populations, while the proportion of iatrogenic LS stands at 11-12% (10). In a necropsy study carried out in the United States, five cases out of 25,000 autopsies conducted had a combination of MS and ASD (11). LS is common among young adults (4) and a female preponderance has also been noted (9) with the first case reported in the literature being in a 61-year-old woman (12). In this review, we focus on the role of echocardiography in the contemporary diagnostic and therapeutic modalities for LS.
Diagnosis of LS
Clinical presentation
Generally, patients with LS are known to remain asymptomatic for several years. In up to 40% of cases from developing countries, there is a history of rheumatic fever (5,13). Clinical features are usually due to ASD and variations in symptoms and signs are dependent on the size of ASD. Thus, the hemodynamic changes seen in LS result from the balance effects of the ASD and the MS, as well as compliance of the right and left ventricles which are the main determinants of the direction of blood flow (14). Commonly, patients present with fatigue, exercise intolerance and palpitations. Fatigue and exercise intolerance are due to the reduced systemic blood flow which is a result of the MS and the systemic to pulmonary shunting of blood across the ASD in diastole, thereby reducing blood flow into left ventricle (9,10). The MS and increased left atrial pressure from augmented left-to-right shunt of blood lead to atrial dilatation. This predisposes patients to atrial arrhythmias commonly atrial fibrillation (AF) and most patients present with palpitations (9).
In patients with non-restrictive ASD, the features of pulmonary congestion present late in the course of the disease. With moderate to severe MS, patients would present mainly with features of right ventricle (RV) overload and right heart failure (RHF). Patients with restrictive ASDs and moderate to severe MS, present much earlier and usually with features of pulmonary congestion from MS (5). Thus, factors which influence the natural history as well as haemodynamic features of LS include: pulmonary vascular resistance, RV compliance, severity of MS and the size of the ASD (5,14). Due to the non-specific features at the early stage of the disease, patients almost always present to hospital in advanced states (15).
Physical examination
Physical examination of patients with LS reveals signs in relation with the co-contributing lesions (i.e., ASD and MS):
-
Arterial pulses are generally of small volume with regular rhythm due to the decreased left ventricular stroke volume (SV). In those patient who already have AF, the pulse is irregular (15);
-
Jugular venous pulse (JVP): in non-restrictive ASD, both atria function as a single chamber, hence, height and contour of left atrial pressure is transmitted to right atrium and internal jugular vein. Thus, patients with LS have elevated JVP (even in the absence of RHF) as well as elevated jugular venous a-wave in absence of PH (9);
-
The precordium: a left parasternal lift is observed due to the transmitted RV and pulmonary impulses commonly in patients with a non-restrictive ASD. The left ventricle impulse is usually not appreciated due to reduced filling from MS (16). The diastolic thrill from MS is unusual due to the comparatively low mitral valve flow velocity (17);
-
Heart auscultation: a loud first heart sound is heard as well as early-to-mid diastolic murmur from the MS and also presence of pulmonary hypertension [leading to increased right atrial (RA) pressure and consequently left atrial pressure] causing increased trans-mitral pressure gradient (16). Unfortunately, these are sometimes attenuated by the ASD causing decompression of left atrium as well as further dilation of the RV in pulmonary hypertension which decreases trans-mitral pressure gradient (9,16,17). Splitting of second heart sound (S2) also occurs for the following reasons; late closure of the pulmonic component of S2 (from increased right heart flow of ASD) and early closure of the aortic component of S2 (from decreased LV and aortic flow as a result of MS and ASD) (16,17). However, when a non-restrictive ASD occurs with a mild MS, the auscultatory features resemble those of an ASD. Right ventricular third and fourth heart sounds may be audible at the left sternal border and marked on inspiration (9);
-
Murmurs and additional heart sounds: systolic murmurs (SM) may also be observed in LS (13,15) and occur due to the following reasons: (i) increased flow at pulmonic valve would lead to a systolic murmur at the upper left parasternal area from ASD; (ii) holosystolic murmur at left parasternal area from tricuspid regurgitation (TR) which may be misinterpreted as due to mitral regurgitation (MR). To differentiate, the murmur usually increases with inspiration (Carvallo sign); and (iii) Systolic murmur of TR at lower left sternal border due to RV dilation and consequent displacement of tricuspid valve (9,16). Mid diastolic murmurs may arise due to: (i) increased flow across tricuspid valve from ASD or associated tricuspid stenosis (at left lower sternal border); (ii) MS (best appreciated with the bell of stethoscope, patient coughing briskly and in left lateral position) (13,15-17). Continuous murmurs may also be appreciated in LS in which there is a restrictive ASD and severe MS usually heard at the lower right sternal area. This occurs due to the continuous shunting of blood across the small ASD (13,14);
-
Other features of overt RHF (pedal edema, hepatomegaly and ascites) are frequently noticed on presentation (15).
Investigations
Chest radiography
LS patients with a small or restrictive ASD have X-ray features consistent with MS. This is usually marked by significant left atrial enlargement and pulmonary venous congestion (9,10). Patients with a non-restrictive ASD and MS have prominent enlarged pulmonary artery and blood flow (pulmonary plethora) (9,16). Due to decompression of the left atrium via the large ASD, there is only mild enlargement of the left atrium. Other features are RA enlargement and RV enlargement are seen when there is already significant RV dilation from pulmonary hypertension (10,13). In more advanced disease, patients with LS may also develop mitral valve calcification (9).
Electrocardiography (ECG)
ECG features in LS vary depending on whether it’s a restrictive or non-restrictive ASD. With a restrictive ASD, the ECG features are consistent with those of MS whereas in case of non-restrictive, features of ASD prevail. Overall, ECG abnormalities in patients with LS range from rhythm to P-wave and QRS morphology changes and finally axial abnormalities:
-
With respect to rhythm, the common features identified are AF (15) and in some cases a sinus rhythm may be found (13);
-
For P-wave morphology, a tall, broad and or bifid wave form may be seen in lead II and a deep negative force in lead V1 suggesting bi-auricular abnormalities (13,17). Isolated left atrial abnormalities may also be observed in restrictive ASD with severe MS (13);
-
Regarding QRS morphology changes, the following have been reported: complete or incomplete right bundle branch block (11,13,16), right ventricular hypertrophy (8,9). A right axis deviation is also observed in some cases (13,16).
Doppler echocardiography
Doppler echocardiography is the gold standard technique to establish the diagnosis of LS. It is non-invasive, available in several clinical settings, and accurate for diagnosing both ASD and MS. Also, it is important in the assessment of degree of valve regurgitation and flow, volume/pressure changes (13). At varying stages of LS, the 2-dimensional (2D) transthoracic echocardiography (TTE) determines the following; Left atrial enlargement, enlargement of right side cavities, ASD, pulmonary artery enlargement and mitral valve stenosis. Color flow and Doppler imaging are also important in confirming and assessing the severity of ASD, mitral valve stenosis and regurgitation as well as TR and pulmonary pressure changes (18).
Assessment of MS
Echocardiography serves to confirm the diagnoses of MS, assess the severity, and characterize the valve anatomy. The following methods have been proposed; diastolic pressure gradient (DPG) method, pressure half-time method, continuity equation method, planimetry method and the proximal isovelocity surface area method (18).
The diastolic pressure gradient is estimated from the transmitral velocity flow curve using the simplified Bernoulli equation (ΔP=4v2) and has been shown to have a good correlation with measurements from trans septal catheterization, thus a reliable estimation (19). DPG is preferably estimated using continuous wave Doppler (CWD) owing to maximal recording of velocities. However, with pulse-wave Doppler, the sample volume should be placed at or just after the leaflet tips (17). In cases of severe valvular and subvalvular apparatus deformity, color Doppler is very useful to identify eccentric diastolic mitral jets. Whilst Doppler reliably assesses mitral gradient, this method has shortcomings [dependence on cardiac output, associated MR, heart rate which influence transmitral flow and also the mitral valve area (MVA)] which limit its efficacy (20).
The pressure half-time (T1/2) method measures the interval in milliseconds between the maximum mitral gradient in early diastole and the time point where the gradient is half the maximum initial value. A reduction in diastolic transmitral blood flow velocity is inversely proportional to valve area (cm2), and hence MVA is determined via the formula: MVA =220/T1/2 (21). T1/2 is then obtained by tracing the deceleration slope of the E-wave on Doppler spectral display of transmitral flow and the valve area is then automatically calculated by in-built software in most recent echo machines. In the presence of bimodal deceleration slope in early diastole, the slope is preferably traced at mid-diastole (22). This method is widely used for simplicity but is limited by the fact that it overestimates MVA (thus underestimating severity of MS), and also the presence of factors like left ventricular diastolic function, severe aortic regurgitation (AR), and degenerative calcific MS, which influence the relationship between T1/2 and MVA (8,23-26).
The continuity equation method states that the filling volume of diastolic mitral flow is equal to aortic SV as exemplified in the following formula for MVA; MVA = π(D2/4)(VTIAortic/VTIMitral), where D is the diameter of the left ventricular outflow tract (LVOT) and velocity time integral (VTI) is in cm (27). Some reports have suggested a good correlation with 2D TTE direct valve area measurement (28), though its accuracy and reproducibility is weak due to cummulative errors from the different measurements required to obtain MVA (18).
The proximal isovelocity surface area method is based on the hemispherical shape of the convergence of diastolic mitral flow on the atrial side of the mitral valve seen on color Doppler. Here, the MVA is estimated by dividing the obtained mitral volume flow by the maximum velocity of diastolic mitral flow on CWD (18). Here, MVA = π(r2)(Valiasing)∙Peak Vmitral−1∙α/180°, where r is the radius of convergence hemisphere (cm), Valiasing is the aliasing velocity (cm/s), Peak Vmitral is the peak CWD velocity of the mitral inflow (cm/s) and α is the opening angle of mitral leaflets relative to flow direction. This method is however technically demanding due to the multiple measurements required (29).
Planimetry method involves a direct measurement of MVA using 2D echocardiography. It is obtained by tracing the mitral orifice and opened commissures (where applicable) on parasternal short-axis view. The measurement plane is also expected to be perpendicular to the mitral orifice with an elliptical shape (28). The recommended time of the cardiac cycle to perform planimetry is mid-diastole and is best done using the cineloop mode on a frozen image. In patients with AF and those with incomplete commissural fusion (moderate MS or after commissurotomy), several measurements are required since valve area may be influenced by changes related to flow conditions (30,31). The only limitation here even for experienced sonographers reported in about 5% of situations, is in the event of a poor acoustic window or severe valve structural malformation (especially from calcification) wherein, its estimation of valve area is relatively less accurate (32). Whilst this exists, recent advances with 3D echo and 3D-guided biplane imaging helps in optimizing the position of measurement plane (improving reproducibility) and enhances accuracy of planimetry conducted even by less experienced sonographers (33,34).
Reports have suggested the superiority of 3D TTE over 2D echography in accurate planimetric assessment of MVA and in distinguishing calcification from subvalvular apparatus involvement (35). In a study comparing four echo methods in determining MVA, planimetry in practice was demonstrated to correlate best with true anatomical valve area (28). Planimetry is thus considered the best method for determination of MVA in the assessment of MS (36,37).
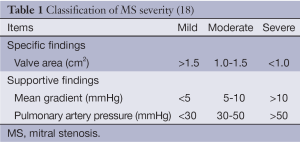
The normal mitral valve has an area of 4-6 cm2 and a funnel shaped orifice favouring RV filling with no DPG. With the onset of MS, there is reduction of MVA and progressive increase in DPG (8). Figure 1 demonstrates the severity of MS using MVA (16) and Table 1 shows the classification of MS severity (18). Other parameters factored in the evaluation of MS include mitral valve resistance, systolic pulmonary artery pressure which reflects more the consequences of MS and not severity, though is pivotal in clinical decision making (18).
Transeosophageal echocardiography (TEE) is also helpful especially when TTE is of poor quality, for detection of associated lesions (left atrial thrombosis before balloon mitral commissurotomy or following a thromboembolic event) (38) and some authors suggest considerable accuracy of TEE for assessing valve morphology and degree of commissural fusion though this needs to be supported by more evidence (28,36). Stress or Exercise echocardiography monitors gradient and pulmonary pressures during increasing workload. It is however important in patients with symptoms discordant with severity of MS (36,37).
Assessment of ASD
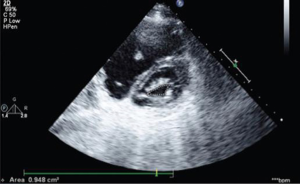
The 2D TTE (Figure 2) is the preferred imaging technique for ASD (38). The more flexible and compliant nature of the RV over the left one favors shunting from left to right leading to RA and subsequent right ventricular dilatation. These are commonly the frequent features identified in patients with a previously unidentified ASD (as is the case in LS). TTE also characterizes the hemodynamic importance of the defect (8,13,39). In the event of severe RV volume overload, a paradoxical anterior septal motion may be noted. Color flow Doppler however demonstrates the shunt across the defect (18) while the gradient across the ASD may provide additional Doppler estimation of left atrial pressure (major determinant of clinical features) in a patient with LS (8). Secundum-type ASD require the following precise evaluation prior to percutaneous intervention with TEE: rim size and quality, sizing, exploration of septum morphology and confirmation of normal pulmonary circulation (18).
Doppler echocardiographic assessment in LS
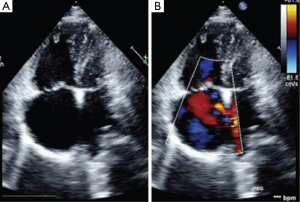
In the evaluation of MS as seen above, plannimetry by 2D-echo is the best method for MVA estimation as exemplified by studies. This is important as MS severity influences the hemodynamics, clinical presentation and natural history of patients with LS. Color flow mapping demonstrates the regurgitation at the MS and also the shunt across the ASD. The size of ASD is crucial prior to therapeutic interventions as studies have shown that ASDs with diameters >38 mm are usually ineligible for percutaneous therapy but rather open heart surgery (40). Cognizant of the fact that most ASD may either be round, oval or crescentric, a single dimension of the ASD is not usually appropriate. In case of an oval ASD, the longest diameter may be well greater than the 38 mm cut-off, but the shorter diameter less, and such a patient still eligible for transcatheter therapy. Thus, where available, 3D-echo is important for LS patients in distinguishing calcification and subvalvular involvement in MS (Figure 3). It is also envisaged that with 3D echo measurements like circular index of ASD (ratio of maximal diameter to minimal diameter), indications for transcatheter therapy in LS would be reviewed (41). The role of TEE cannot be overemphasized especially in excluding other pathologies like left atrial thrombi (contraindication to percutaneous therapy).
While obvious features of MS in association with enlargement of RV with an abnormal septal motion are suggestive of pulmonary hypertension and a functional TR [group 2 pulmonary hypertension: i.e., pulmonary hypertension due to left heart disease (42)] on echo, it should be considered however that, in the absence of clinical and echocardiographic features of pulmonary hypertension, a combination of MS with right ventricular volume overload, should alert the clinician to the possibility of LS (43). Basically, 2D and Doppler echo which are relatively widely available are essential and the mainstay in establishing diagnosis of LS. Prior to percutaneous interventions however; further assessment with 3D echo and TEE will be paramount.
Cardiac catheterization
Performing right heart catheterization (RHC) in patients with LS is not routinely recommended due to its invasive nature. However, it may be crucial in evaluating the severity of ASD, measuring MVA using the Gorlin formula (though it can be questionable in case of low output or just after balloon mitral commissurotomy), when echocardiography is not conclusive or is discordant with clinical findings (36,37). RHC is useful for detecting pulmonary vascular resistance, presence of reversible pulmonary hypertension and finally evaluating the presence of coronary artery disease in high risk patients (36,37).
Other investigations
Computerized tomography (CT) scan (contrast enhanced, 3D ultrafast CT), cardiac magnetic resonance imaging (MRI) have also been used in investigating patients however, most of the necessary details they provide for LS diagnosis are obtainable by TTE (36,39). Nuclear imaging (equilibrium and first-pass radionuclide angiocardiography) has also been employed in patients with ASD and MS. However, these investigations are more expensive, require technical expertise and are also not widely available (39).
Current management of LS
Medical therapy
This involves symptomatic relief and prophylaxis for sub-acute bacterial endocarditis (SBE).
Symptomatic relief of RHF: diuretics such as furosemide are generally used to ameliorate symptoms of RHF (15).
Atrial arrhythmias: cardiac glycosides, beta blockers and calcium channel blockers may be used for rate control while drugs like amiodarone, besides rate control, also help in achieving and maintaining a normal sinus rhythm (2).
SBE prophylaxis: LS patients who have undergone repair with prosthetic device usually need antibiotic prophylaxis for the first six months following the procedure (44).
Percutaneous trans-catheter therapy
Open heart surgery had been the preferred method of treatment of patients with LS involving ASD closure and mitral commisurotomy or valve replacement (16). Recently, progress in interventional cardiology has significantly changed the treatment of LS with trans-catheter therapies (in eligible patients) with impressive success rates (16,45). The first reports of successful trans-catheter therapy was about two decades ago by Ruiz et al. (46) and later Joseph et al. (47). Since then, several successful reports have been published (10,45,48-50). Whilst several techniques have been proposed for trans-catheter therapy in LS, the most widely used today is the Inoue balloon for percutaneous balloon mitral valvuloplasty (PBMV) for mitral valvuloplasty and the Amplatzer septal occluder for percutaneous closure of ASD. The first successful combination of these techniques was reported by Chau et al. (49). In India, the Cocoon septal occluder is still very much in use with high success rates recorded (17,51).
Indications and contraindications for percutaneous therapy (16,17)
A number of situations in which percutaneous therapy can be conducted include: ASD with Qp/Qs ratio >1.5 with adequate rims, symptomatic moderate to severe MS with valve morphology favorable for PBMV, and any degree of pulmonary hypertension, with the exclusion of patients with Eisenmenger syndrome (irreversible pulmonary hypertension). However, the following clinical situations currently are contraindications to percutaneous therapy in LS: presence of left atrial thrombus, absence of adequate rims around the septal defect and presence of anomalous pulmonary drainage, grade 3 MR or higher, bicommissural calcification, and finally lack of expertise.
The technique
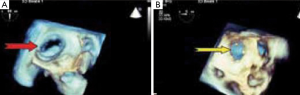
Several reports on the successful treatment of LS using the Inoue balloon (Figure 4) for mitral valvuloplasty and Amplatzer septal occluder (Figure 5) for closure of ASD, have led to their consideration as treatment of choice for LS (10). Through the femoral vein, access is made through to the right atrium, and through ASD to the left atrium, subsequently to the MS. Prior to this, anti-platelet aggregants (clopidogrel, low dose aspirin are administered) and following vascular access, 2,500-5,000 units of heparin and prophylaxis for infective endocarditis also administered (44). With the help of Mullin sheath dilator, the pig tail Inoue guide wire is inserted. Due to instability from large ASD, J-tip Amplatz extra stiff wire (ESW) is inserted through to the left ventricle. Next, Mullin sheath is then removed and Inoue balloon inserted and stabilized in the left ventricle. This is followed by inflation of balloon which dilates the stenosed valve. Upon withdrawal of the balloon, plannimetry with TTE is performed to determine the new MVA and Doppler assessment is also done for significant MR (10,51-53). Following this, the amplatzer delivery catheter is then positioned across the ASD, the left atrial disc with the centered connecting stalk is delivered. The device is then withdrawn provided that the connecting stalk is within the ASD and the left disc is adherent to atrial septum. Next, the RA disc is delivered, and the delivery wire is finally disconnected from the device, leaving the device closing the ASD. These procedures are done with fluoroscopic guidance or trans-oesophageal echo (under general anesthesia) (10,44,51). Hence in a single catheterization procedure, LS can be treated.
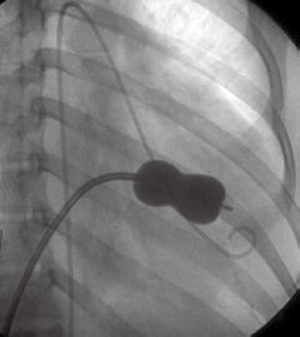
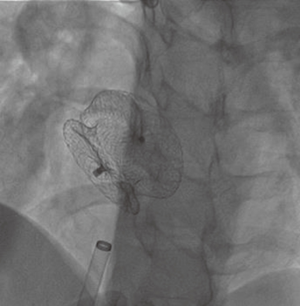
Challenges/complications of percutaneous therapy
Some practical difficulties which may be encountered are: (I) floating of Inoue catheter due to large ASD, hence the inability of delivery wire to go through stenosis to the left ventricle; (II) tendency to mitral restenosis: because of this, some authors advise to allow a lapse of a 24-48 hours after PBMV prior to ASD closure to give room for mitral valve assessment (51); (III) embolization or slippage of septal occluder probably due to large size ASD (52); (IV) persistent ASD after mitral valvuloplasty either due to presence of tail of balloon at atrial septum during inflation (increasing atrial septostomy) or inadequate deflation of balloon prior to withdrawing it (48).
Despite the above mentioned challenges associated with percutaneous therapy, current success rates of complete ASD closure with septal occluder during combined procedure stand at 93-97% (10) while success rates for PBMV with Inoue technique of 98.5% have been reported (54). Combined percutaneous therapy is documented to reduce morbidity and mortality following open heart surgery, decrease psychological trauma from thoracotomy scar and finally significantly reduces length of hospital stay following surgery (10).
Conclusions
LS remains a rare entity and echocardiography assessment is the current diagnostic modality of choice with 3D echo and TEE further helpful in excluding co-existent cardiac pathologies. Planimetry by Doppler echo remains the best method for assessing MVA. Whilst open heart surgery is frequently the treatment modality of choice in case of co-existent cardiac malformations, LS is currently an example of an exception, with recent percutaneous trans-catheter therapies, combining PBMV with Inoue balloon technique for MS and the Amplatzer Septal occluder for ASD closure. With most of these conclusions drawn essentially from case reports, we propose prospective multicenter registries to evaluate trans-catheter therapies and its long term outcome in patients with LS.
Acknowledgements
Authors’ contributions: LN Aminde conceived and drafted the manuscript. NF Takah, A Dzudie, AP Kengne contributed to the literature search and reviewed the manuscript. A Dzudie, KB Ngu, AP Kengne, K Sliwa critically reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Kengne AP, Mayosi BM. Readiness of the primary care system for non-communicable diseases in sub-Saharan Africa. Lancet Glob Health 2014;2:e247-8. [PubMed]
- Kumar S, Walters TE, Halloran K, et al. Ten-year trends in the use of catheter ablation for treatment of atrial fibrillation vs. the use of coronary intervention for the treatment of ischaemic heart disease in Australia. Europace 2013;15:1702-9. [PubMed]
- Mocumbi AO, Ferreira MB. Neglected cardiovascular diseases in Africa: challenges and opportunities. J Am Coll Cardiol 2010;55:680-7. [PubMed]
- Nagamani AC, Nagesh CM. Lutembacher Syndrome (Ch. 64). In: Vijayalakshmi IB, Syamasundar Rao P, Chugh R. eds. A Comprehensive Approach to Congenital Heart Diseases. India: Jaypee Brothers Medical Publisher, 2013:908-16.
- Bashi VV, Ravikumar E, Jairaj PS, et al. Coexistent mitral valve disease with left-to-right shunt at the atrial level: clinical profile, hemodynamics, and surgical considerations in 67 consecutive patients. Am Heart J 1987;114:1406-14. [PubMed]
- Fadel BM, Hiatt BL, Kerins DM. Isolated Rheumatic Tricuspid Stenosis with Reverse Lutembacher's Physiology. Echocardiography 1999;16:567-73. [PubMed]
- Jain VV, Gupta OP, Jain J. A rare case of situs inversus with dextrocardia, lutembacher syndrome, and pericardial effusion. Heart Views 2011;12:107-11. [PubMed]
- Budhwani N, Anis A, Nichols K, et al. Echocardiographic assessment of left and right heart hemodynamics in a patient with Lutembacher's syndrome. Heart Lung 2004;33:50-4. [PubMed]
- Perloff JK, Marelli AJ. eds. The Clinical recognition of Congenital Heart Disease. 4th ed. Philadelphia: WB Saunders, 1994:323-8.
- Cheng TO. Coexistent atrial septal defect and mitral stenosis (Lutembacher syndrome): An ideal combination for percutaneous treatment. Catheter Cardiovasc Interv 1999;48:205-6. [PubMed]
- Ali SY, Raham M, Islam M, et al. Lutembacher’s Syndrome-A case report. Faridpur Med Coll J 2011;6:59-60.
- Lutembacher R. De la stenose mitrale avec communication interauriculaire. Arch Mal Coeur 1916;9:237-60.
- Garcia JA, Krajcer Z, Pechacek LW, et al. Echocardiography in the diagnosis of Lutembacher syndrome. Cathet Cardiovasc Diagn 1978;4:283-8. [PubMed]
- Ross J Jr, Braunwald E, Mason DT. Interatrial communication and left atrial hypertension: a cause of continuous murmur. Circulation 1963;28:853-60. [PubMed]
- Aminde LN, Dzudie AT, Takah NF, et al. Occurrence of Lutembacher syndrome in a rural regional hospital: case report from Buea, Cameroon. Cardiovasc Diagn Ther 2014;4:263-6. [PubMed]
- Arora R, Patted S, Halkati P, et al. Definitive treatment of lutembacher syndrome. J Sci Soc 2014;41:215-9.
- Ledesma M, Martinez P, Cázares MA, et al. Transcatheter treatment of Lutembacher syndrome: combined balloon mitral valvuloplasty and percutaneous atrial septal defect closure. J Invasive Cardiol 2004;16:678-9. [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23. [PubMed]
- Nishimura RA, Rihal CS, Tajik AJ, et al. Accurate measurement of the transmitral gradient in patients with mitral stenosis: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol 1994;24:152-8. [PubMed]
- Rahimtoola SH, Durairaj A, Mehra A, et al. Current evaluation and management of patients with mitral stenosis. Circulation 2002;106:1183-8. [PubMed]
- Thomas JD, Weyman AE. Doppler mitral pressure half-time: a clinical tool in search of theoretical justification. J Am Coll Cardiol 1987;10:923-9. [PubMed]
- Gonzalez MA, Child JS, Krivokapich J. Comparison of two-dimensional and Doppler echocardiography and intracardiac hemodynamics for quantification of mitral stenosis. Am J Cardiol 1987;60:327-32. [PubMed]
- Thomas JD, Wilkins GT, Choong CY, et al. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation 1988;78:980-93. [PubMed]
- Flachskampf FA, Weyman AE, Guerrero JL, et al. Calculation of atrioventricular compliance from the mitral flow profile: analytic and in vitro study. J Am Coll Cardiol 1992;19:998-1004. [PubMed]
- Karp K, Teien D, Bjerle P, et al. Reassessment of valve area determinations in mitral stenosis by the pressure half-time method: impact of left ventricular stiffness and peak diastolic pressure difference. J Am Coll Cardiol 1989;13:594-9. [PubMed]
- Messika-Zeitoun D, Meizels A, Cachier A, et al. Echocardiographic evaluation of the mitral valve area before and after percutaneous mitral commissurotomy: the pressure half-time method revisited. J Am Soc Echocardiogr 2005;18:1409-14. [PubMed]
- Nakatani S, Masuyama T, Kodama K, et al. Value and limitations of Doppler echocardiography in the quantification of stenotic mitral valve area: comparison of the pressure half-time and the continuity equation methods. Circulation 1988;77:78-85. [PubMed]
- Faletra F, Pezzano A Jr, Fusco R, et al. Measurement of mitral valve area in mitral stenosis: four echocardiographic methods compared with direct measurement of anatomic orifices. J Am Coll Cardiol 1996;28:1190-7. [PubMed]
- Messika-Zeitoun D, Fung Yiu S, Cormier B, et al. Sequential assessment of mitral valve area during diastole using colour M-mode flow convergence analysis: new insights into mitral stenosis physiology. Eur Heart J 2003;24:1244-53. [PubMed]
- Iung B, Cormier B, Ducimetière P, et al. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation 1996;94:2124-30. [PubMed]
- Shaw TR, Sutaria N, Prendergast B. Clinical and haemodynamic profiles of young, middle aged, and elderly patients with mitral stenosis undergoing mitral balloon valvotomy. Heart 2003;89:1430-6. [PubMed]
- Zamorano J, Cordeiro P, Sugeng L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol 2004;43:2091-6. [PubMed]
- Sebag IA, Morgan JG, Handschumacher MD, et al. Usefulness of three-dimensionally guided assessment of mitral stenosis using matrix-array ultrasound. Am J Cardiol 2005;96:1151-6. [PubMed]
- Messika-Zeitoun D, Brochet E, Holmin C, et al. Three-dimensional evaluation of the mitral valve area and commissural opening before and after percutaneous mitral commissurotomy in patients with mitral stenosis. Eur Heart J 2007;28:72-9. [PubMed]
- Tezcan M, Isilak Z, Atalay M, et al. Echocardiographic assessment of Lutembacher syndrome. Kardiol Pol 2014;72:660. [PubMed]
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. [PubMed]
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68. [PubMed]
- Black IW, Hopkins AP, Lee LC, et al. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol 1991;18:398-404. [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [PubMed]
- Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J 2008;156:706-12. [PubMed]
- Fraisse A, Trivedi KR. Transcatheter closure of atrial septal defects: how large is too large? Cardiovasc Diagn Ther 2014;4:213-4. [PubMed]
- Dzudie A, Kengne AP, Thienemann F, et al. Predictors of hospitalisations for heart failure and mortality in patients with pulmonary hypertension associated with left heart disease: a systematic review. BMJ Open 2014;4:e004843. [PubMed]
- Forman HR, Kotler MN, Segal BL, et al. Lutembacher's syndrome: recognition by echocardiography. J Clin Ultrasound 1979;7:53-6. [PubMed]
- Bhambhani A, Somanath HS. Percutaneous treatment of Lutembacher syndrome in a case with difficult mitral valve crossing. J Invasive Cardiol 2012;24:E54-6. [PubMed]
- Aroney C, Lapanun W, Scalia G, et al. Transcatheter treatment of Lutembacher syndrome. Intern Med J 2003;33:259-60. [PubMed]
- Ruiz CE, Gamra H, Mahrer P, et al. Percutaneous closure of a secundum atrial septal defect and double balloon valvotomies of a severe mitral and aortic valve stenosis in a patient with Lutembacher's syndrome and severe pulmonary hypertension. Cathet Cardiovasc Diagn 1992;25:309-12. [PubMed]
- Joseph G, Abhaichand Rajpal K, Kumar KP. Definitive percutaneous treatment of Lutembacher’s syndrome. Catheter Cardiovasc Interv 1999;48:199-204. [PubMed]
- Shabbir M, Ahmed W, Akhtar K. Transcatheter treatment of Lutembacher's syndrome. J Coll Physicians Surg Pak 2008;18:105-6. [PubMed]
- Chau EM, Lee CH, Chow WH. Transcatheter treatment of a case of Lutembacher syndrome. Catheter Cardiovasc Interv 2000;50:68-70. [PubMed]
- Ledesma M, Martinez P, Cázares MA, et al. Transcatheter treatment of Lutembacher syndrome: combined balloon mitral valvuloplasty and percutaneous atrial septal defect closure. J Invasive Cardiol 2004;16:678-9. [PubMed]
- Goel S, Nath R, Sharma A, et al. Successful percutaneous management of Lutembacher syndrome. Indian Heart J 2014;66:355-7. [PubMed]
- Vadivelu R, Chakraborty S, Bagga S. Transcatheter therapy for Lutembacher's syndrome: The road less travelled. Ann Pediatr Cardiol 2014;7:37-40. [PubMed]
- Inoue K, Feldman T. Percutaneous transvenous mitral commissurotomy using the Inoue balloon catheter. Cathet Cardiovasc Diagn 1993;28:119-25. [PubMed]
- Wilson NJ, Smith J, Prommete B, et al. Transcatheter closure of secundum atrial septal defects with the Amplatzer septal occluder in adults and children-follow-up closure rates, degree of mitral regurgitation and evolution of arrhythmias. Heart Lung Circ 2008;17:318-24. [PubMed]