Non-identical yet similar: presentation of coronary artery disease in dizygotic twins
Introduction
The development of coronary artery disease (CAD) is strongly influenced by genetic and environmental factors (1,2). The genetic influence on CAD is presumably mediated by its influences on risk factors such as hypertension, dyslipidemia, diabetes, and obesity. Further, the environment-related factors like physical activity, diet, smoking, and alcohol consumption are also considered to be affected by genetic factors (3). Hence, there is a considerable debate regarding the role of genetic and environmental factors in the pathogenesis of CAD (2). It is considered that the genetic influences are of greater significance than the environmental factors as some populations are more susceptible to CAD than others even when they share similar environmental exposures (2). Since twin pairs are exposed to similar prenatal and postnatal environmental factors, a particular role of genetic vs. environmental factors can be evaluated by investigating the twins (i.e., by comparing the genetic variability of quantitative traits, cardiac anatomy, and coronary lesions) (4). The exact incidence of CAD among twins is undetermined due to limited data (2). A study involving 10,502 Swedish twins estimated that the relative hazard of dying due to CAD in the next 10 years is 2.8 for dizygotic male twin and 8.1 for monozygotic male twin, if the co-twin had died of premature CAD (5). Currently, very few case reports with angiographic comparison between twin pairs are documented (2,4,6-10). Some of these demonstrated concordant characteristics between twin pair (2,4,6-9), while some did not (6,7,10). Further, such reports with angiographic comparisons are still rarer for dizygotic twins than that in monozygotic twins (7). Our case provides an example of both concordant and discordant characteristics in a dizygotic twin pair presenting with different type of cardiac event and different risk factor profile. Both underwent percutaneous coronary intervention simultaneously.
Case report
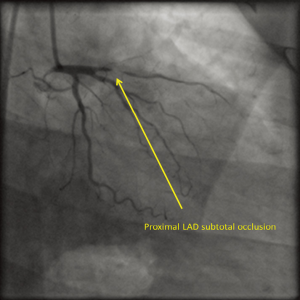
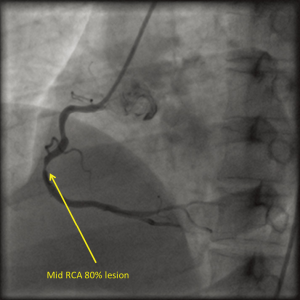
Twin A was a 40-year-old man (weight: 70 kg, height: 154 cm) working as a lawyer. He was diagnosed, elsewhere, with acute anterior-wall myocardial infarction (AWMI) and was thrombolysed with tenecteplase. Subsequently, he was referred to our institute for further management. Physical examination revealed clear chest and normal heart sounds. He was not having any particular coronary risk factors such as obesity, diabetes mellitus, hypertension, dyslipidemia, or smoking. However, his mother was a known case of CAD and had undergone coronary artery bypass grafting (CABG). Coronary angiography revealed right dominant circulation and double-vessel disease with subtotal occlusion (Type C) in proximal part of the left anterior descending artery [(LAD); Figure 1] and 80% discrete lesion (Type A) in mid portion of the right coronary artery [(RCA); Figure 2]. The left circumflex artery (LCX) was normal. The patient was treated successfully with deployment of everolimus-eluting stents in proximal LAD (2.75 mm × 23 mm) and mid RCA (3.0 mm × 18 mm) lesions.
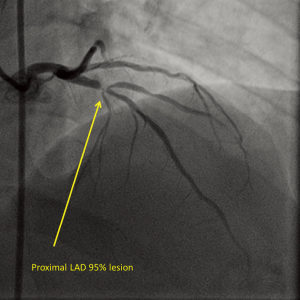
Twin B, the dizygotic twin brother of twin A, was a 40-year-old man (weight: 78 kg, height: 162 cm) working as an agriculturist. His brother persuaded him to undergo cardiac evaluation as he was experiencing chest pain upon exertion for last 3 months and was taking medication for effort angina (Class III). He was a known case of diabetes mellitus and dyslipidemia, while other coronary risk factors such as obesity, smoking, and hypertension were absent. We suggested twin B to undergo coronary angiography, which revealed right dominant circulation and a single-vessel disease with 95% lesion (Type C) in proximal LAD (Figure 3). The RCA and LCX were free of disease. Subsequently, an everolimus-eluting stent (3.0 mm × 28 mm) was successfully implanted in the LAD.
Discussion
Twins have been the interest of research since antiquity (11). Twins’ studies, in general, compare the concordance of a trait or a disease between monozygotic twin pairs (genetically identical; resulting from the division of a single fertilized egg) and/or dizygotic twin pairs (genetically non-identical; resulting from the separate fertilization of two eggs; sharing about 50% of their genome). Even in the field of cardiovascular research, the classical twin design has provided an invaluable tool to evaluate the genetic basis of CAD (11). Although family history is the most direct indication of genetic susceptibility to CAD (associated with 2-3-fold increased risk), twins’ studies are considered to be more powerful because of certain unique characteristic of twins, such as their matching age, similar prenatal environment, and similar exposure to a range of environmental variables (e.g., lifestyle, diet, physical activity, family environment) as compared to the general population or even family studies. These variables, both measured and unmeasured, do contribute to the expression of CAD (11). Therefore, it becomes much easier to attribute phenotypic differences between twins to genetic rather than to environmental factors (11). An Indian study has estimated that the twining rate is 11.70 per 1,000 live births, with 3.67 per million for monozygotic twins and 8.03 per million for dizygotic twins (12).
The first scientific cardiac studies involving twins began with analyses of electrocardiograms, which revealed certain similarities in their electrocardiographic tracings. Subsequent studies during the late 20th century focused on CAD with considerable attention and debate about the role of genetic vs. environmental factors in the pathogenesis of this condition (2). In the last two decades, various twin studies have also established the genetic link for number of CAD risk factors, including lipid levels (13,14), blood pressure (14), smoking (15), body mass index (BMI) (16), physical activity (17), C-reactive protein (18), plasma homocysteine (19) and diabetes (20). Despite such advances, little is known about angiographic expression of CAD in twin pairs. In this context, our case provides important insights by comparing the CAD lesions in a dizygotic twin pair who had different risk factor profile.
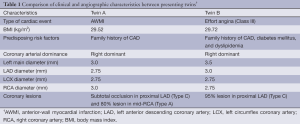
The twins in our study are of interest for several reasons: (I) dizygotic nature; (II) simultaneous development of coronary insufficiency; (III) diagnosis of CAD at relatively young age (early forties); (IV) difference in the type of cardiac event (AWMI in twin A and effort angina in twin B); (V) a strong family history of CAD on the mother’s side; (VI) difference in predisposing cardiovascular risk factor profile (i.e., diabetes and hyperlipidemia in twin B); (VII) similar environmental risk factors (i.e., similar diet pattern and absence of smoking or drinking) but difference in occupational factor; (VIII) side-by-side comparison of angiograms revealing concordant coronary arterial anatomy (Table 1); (IX) close similarity in location and severity of lesion in LAD; (X) no other lesion in the twin with predisposing risk factors presenting with effort angina, while a lesion in RCA in the twin without predisposing risk factors and presenting with AWMI; and (XI) simultaneous and successful implantation of DES in both twins. To the best of our knowledge, this is the first report from India that documents CAD in dizygotic twins. Although this report consist a single dizygotic pair, the circumstances described here favors the theory of combined influences of genetic and environmental factors leading to premature atherosclerosis.
Full table
Earlier, Frings et al. (7) analyzed three dizygotic twin-pairs and reported that the dominance pattern of coronary blood supply were concordant with inter-twin variability in the diameters of coronary arteries, while the rate of concordance in location of coronary lesions was 54%. They opined that the concordance in location of coronary lesions is not determined strictly by shared genes, and environmental factors may have a secondary role. Similar findings and opinions are shared by various case reports involving twin pairs with CAD (2,4,6,9).
Although the literature for CAD in twins is limited to case reports, several characteristics can be drawn: (I) onset of CAD at an early age; (II) onset of symptoms occurring within a short time span between the twins; (III) similar electrocardiograms (rest and stress); (IV) similar coronary pathology; and (V) similar metabolic and biochemical profiles (2). Clearly, genetic factors are found to be more responsible for the development of CAD—age at the onset of cardiac event, type of the cardiac event, and risk factor profile—in any given person. Additional risk factors, such as occupation, diet, and lifestyle may be responsible for the differences in the severity of disease between twins. We also opine that the location and severity of coronary lesions could be identical in non-identical twins with CAD as demonstrated in LAD lesions of the twins reported in this case.
Conclusions
In conclusion, we present a case of simultaneous presentation of CAD in dizygotic twin pair with similarities and differences in certain characteristics. Here, we support the need of aggressive medical surveillance in asymptomatic twin, regardless of young age, whose co-twin has documented CAD. We believe that the occurrence of CAD might be predetermined genetically, while the location of CAD lesion and its severity might be subjected to the modification of environmental factors among dizygotic twins. The challenge to dissect out the complexity of the genetic-environment relationships and individual variability should be investigated in future studies with larger number of discordant twin pairs.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:143-52. [PubMed]
- Samuels LE, Samuels FS, Thomas MP, et al. Coronary artery disease in identical twins. Ann Thorac Surg 1999;68:594-600. [PubMed]
- de Lange M, Snieder H, Ariëns RA, et al. The genetics of haemostasis: a twin study. Lancet 2001;357:101-5. [PubMed]
- Gullu AU, Kizilay M, Ates M, et al. The comparison of angiographic lesions and clinical outcomes in identical twins. Interact Cardiovasc Thorac Surg 2007;6:575-6. [PubMed]
- Marenberg ME, Risch N, Berkman LF, et al. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med 1994;330:1041-6. [PubMed]
- Holmes DR Jr, Kennel AJ, Smith HC, et al. Coronary artery disease in twins. Br Heart J 1981;45:193-7. [PubMed]
- Frings AM, Mayer B, Böcker W, et al. Comparative coronary anatomy in six twin pairs with coronary artery disease. Heart 2000;83:47-50. [PubMed]
- Herrington DM, Pearson TA. Clinical and angiographic similarities in twins with coronary artery disease. Am J Cardiol 1987;59:366-7. [PubMed]
- Turley AJ, Chen V, Hall JA. Simultaneous presentation of coronary heart disease in identical twins. Postgrad Med J 2008;84:100-2. [PubMed]
- Russo P, Steffenino G, Dellavalle A, et al. Concomitant myocardial infarction in identical twins with similar coronary lesions. G Ital Cardiol 1995;25:341-3. [PubMed]
- Mangino M, Spector T. Understanding coronary artery disease using twin studies. Heart 2013;99:373-5. [PubMed]
- Sharma K. The twinning rates and epidemiological characteristics of births in southeast Uttar Pradesh, India. Acta Genet Med Gemellol (Roma) 1997;46:47-56. [PubMed]
- Evans A, Van Baal GC, McCarron P, et al. The genetics of coronary heart disease: the contribution of twin studies. Twin Res 2003;6:432-41. [PubMed]
- Snieder H, van Doornen LJ, Boomsma DI. The age dependency of gene expression for plasma lipids, lipoproteins, and apolipoproteins. Am J Hum Genet 1997;60:638-50. [PubMed]
- Koopmans JR, Slutske WS, Heath AC, et al. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet 1999;29:383-93. [PubMed]
- Lajunen HR, Kaprio J, Keski-Rahkonen A, et al. Genetic and environmental effects on body mass index during adolescence: a prospective study among Finnish twins. Int J Obes (Lond) 2009;33:559-67. [PubMed]
- Eriksson M, Rasmussen F, Tynelius P. Genetic factors in physical activity and the equal environment assumption--the Swedish young male twins study. Behav Genet 2006;36:238-47. [PubMed]
- MacGregor AJ, Gallimore JR, Spector TD, et al. Genetic effects on baseline values of C-reactive protein and serum amyloid a protein: a comparison of monozygotic and dizygotic twins. Clin Chem 2004;50:130-4. [PubMed]
- Siva A, De Lange M, Clayton D, et al. The heritability of plasma homocysteine, and the influence of genetic variation in the homocysteine methylation pathway. QJM 2007;100:495-9. [PubMed]
- Kaprio J, Tuomilehto J, Koskenvuo M, et al. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia 1992;35:1060-7. [PubMed]


