Spontaneous coronary artery dissection: novel insights on diagnosis and management
Spontaneous coronary artery dissection (SCAD) remains an elusive clinical condition of unknown aetiology 8 decades after its initial pathological description (1). Coronary angiography represents the classical diagnostic tool in patients with SCAD (2-7). An intimal flap generating 2 lumens (true and false lumen) provides the angiographic hallmark of the disease (2-7). SCAD is a rare form of non-atherosclerotic coronary artery disease with a prevalence ranging from 0.1% to 1% in most angiographic series (2-7). Accordingly, limited evidence is currently available regarding its pathophysiology, prevalence, associated conditions, diagnosis, management and prognosis (2-7). A huge number of case-reports and multiple small retrospective series, usually with relatively short clinical follow-up, have been published on this clinical entity. More recently, however, larger series of patients with SCAD, prospectively studied and systematically followed, have been reported (8-14). In addition, the widespread use of new intracoronary imaging techniques in patients with ambiguous angiographic features has significantly enhanced our diagnostic accuracy (15-18). All these recent studies have generated important information leading to major advances in our understanding of this unique condition (8-18). However, altogether, the estimated total number of patients with SCAD reported to date remains less than 800 (8-14) and, therefore, we should acknowledge that the information currently available on this elusive clinical entity, still remains limited.
A young female without coronary risk factors presenting with an acute coronary syndrome constitutes the classical clinical scenario (2-7). The pathophysiology of SCAD remains poorly defined but it is widely accepted that in most patients an intimal tear constitutes the initiating event. This intimal rupture provides an “entry door” that promotes intramural bleeding with progressive separation between the true and false lumens. In other patients, however, disruption of the “vasa vasorum” leads to an intramural hematoma without a distinct intimal rupture (2-7). In any case, myocardial ischemia results from pressure-driven compression of the true lumen or from thrombotic obstruction of the true lumen (2-7). A large list of associated pathological conditions has been reported in patients with SCAD. Among them, connective tissue disorders and the peripartum status have been considered as important concomitant conditions from a pathophysiological stand point (2-7). The presence of a specific pathological substrate (namely cystic medial necrosis), able to provide a “vulnerable vessel wall”, has been also suggested (2-7).
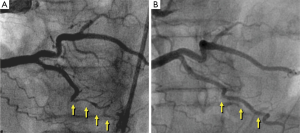
The treatment of choice for patients with SCAD in the acute phase remains unsettled (2-7). Medical management is frequently similar to that used in patients with acute coronary syndromes secondary to atherosclerotic coronary artery disease. Nevertheless, the value of early revascularization in patients with SCAD remains highly controversial as both percutaneous and surgical techniques have major limitations and may be associated with complications in these patients already presenting a disrupted and friable coronary vessel wall (2-7). Therefore, most investigators currently advocate the use of an initial conservative approach “watchful waiting strategy” for stabilized patients with SCAD (8-14). Furthermore, accumulating data suggest that spontaneous vessel healing (Figure 1) occurs in the majority of these patients during follow-up, reinforcing the idea of selecting a conservative initial strategy whenever possible (8-18).
We will present a comprehensive and systematic review of the epidemiology, etiology, clinical presentation, diagnosis and management of patients with SCAD. In addition, we will summarize the most important novel information that has advanced our understanding of this elusive and challenging clinical entity.
Associated conditions and fibromuscular dysplasia (FMD)
Many diseases have been associated with SCAD. Classical series suggested that SCAD frequently occurred in very young females without coronary risk factors (2-7). Recent series, however, suggest that patients with SCAD frequently have coronary risk factors and some of them may also have associated coronary artery disease (8). Age at presentation tend to be older in patients with associated coronary artery disease, but even in patients with “isolated” SCAD, mean age is over 50 years old and, in recent series, most women with SCAD are postmenopausal (8,11,14). Nevertheless, SCAD still occurs in up to 10% of young female presenting with an acute coronary syndrome and in 25% of those presenting with a myocardial infarction (7,13,14). Likewise, the association with the peripartum status has been consistently reported in classical series (in up to one-third of patients) but this association has been found in less than 5% of patients in recent prospective series (8,11,14). However, the subgroup of patients with postpartum SCAD appears to have a worse prognosis. Recent studies also suggest that precipitating stressors such as intense exercise (particularly isometric) or emotional stress may trigger the SCAD event (11,14).
Several angiographic markers of underlying coronary vessel wall pathology have been studied. Investigators from the Mayo Clinic specifically sought to determine the prevalence of “coronary tortuosity” in patients with SCAD (11). A total of 246 patients with SCAD (the largest series ever reported with this condition) were compared with 300 control patients (matched by age and gender). Coronary tortuosity and “severe” coronary tortuosity was significantly more prevalent among patients with SCAD than in controls. Importantly, severe coronary tortuosity was associated with a higher risk of “recurrent” SCAD. Among SCAD patients, tortuosity was more frequently found in those with hypertension and often involved the circumflex coronary artery and also non-culprit arteries. SCAD recurrences tended to occur in segments with tortuosity but also at bifurcations or at hinge points (11). Further studies are warranted to determine if coronary tortuosity indeed represents an angiographic marker of an underlying vulnerable vessel wall that might predispose to SCAD.
Canadian investigators proposed—for the first time—the association of SCAD with FMD (19,20). FMD is also a rare, non-atherosclerotic and non-inflammatory vascular disease, of unknown etiology that typically affects young females. FMD tends to involve large arteries (mainly renal, carotid, and iliac) (21) and is often detected incidentally in asymptomatic patients (21). Pathologically, FMD presents as arterial stenosis, aneurysms, dissection or thrombosis. “Medial fibroplasia” represents the most frequent underlying substrate accounting for the classical “string-of-beads” angiographic appearance (21). The group from Vancouver (19,20) reported a series of perimenopausal women with acute myocardial infarction, diffuse coronary lesions and coincidental renal FMD (19,20). Subsequently, these investigators extended their observations to 50 patients with SCAD that were systematically screened for FMD in 3 different vascular territories (iliac, renal and cerebrovascular) (13). FMD was identified in most cases and, according, these investigators proposed the possibility of a causative link. In a more recent report Saw et al. (14), evaluated baseline characteristics, predisposing conditions, and clinical outcome of 168 patients with SCAD. In this series triggers were systematically evaluated and a precipitating physical or emotional stress could be detected in 56% of patients. In addition, using a detailed screening for associated non-coronary arteriopathies, the incidence of associated FMD in this uniquely large series of patients with SCAD was 72%. At late angiographic assessment, spontaneous vessel healing was demonstrated in all patients (14).
The association of SCAD with FMD has also been confirmed by other groups. A report from the Mayo Clinic (10) confirmed that external iliac FMD was identified in half of the patients with SCAD with available ilio-femoral angiograms. A more recent report from the same institution (this time systematically using CT-angiography with screening purposes) demonstrated the presence of FMD in 2/3 of patients with SCAD (22). This group has suggested that FMD was more frequently found in patients with SCAD showing coronary tortuosity (including “corkscrew” appearance and “multi-vessel symmetric tortuosity”) (11). Altogether, currently available evidence strongly suggests that FMD is frequently found in patients with SCAD. Further studies, however, are required to confirm that the association of these infrequent—but closely related diseases—implies a clinically relevant pathophysiological link.
Diagnosis by coronary imaging
The classical diagnosis of SCAD relies on the demonstration of a radiolucent intimal “flap” on coronary angiography, frequently associated with contrast staining on the vessel wall (2-7). However, conventional angiography may lead to misdiagnosis explaining why SCAD is frequently underdiagnosed. Coronary angiography only depicts a lumenogram and fails to visualize the underlying vessel wall. Angiography is only able to unravel the lumen compromise or indirect signs of coronary wall disruption. Until very recently, the angiographic narrowing caused by an intramural hematoma impinging into the lumen was systematically misinterpreted as atherosclerotic coronary artery disease. In diffuse isolated angiographic lesions intracoronary nitroglycerin should be systematically administered to relieve overlying spasm. The accumulated experience with this disease suggests that in the appropriate clinical setting an isolated, confined, long coronary lesion in a patient with otherwise smooth normal coronary vessels, should always raise the clinical suspicious of an underlying intramural hematoma (2-7).
Recently, tomographic techniques have been able to provide novel diagnostic insights in patients with SCAD (15-18) (Figure 2). Intravascular ultrasound (IVUS) provides a clear picture of the vessel wall and of the coronary lumen. IVUS has a spatial resolution of 150 µm thus enabling an accurate visualization of the entire vessel wall. Even in large arteries the vessel wall (up to the external elastic lamina) can be fully visualized with this technique. In patients with coronary artery disease, IVUS has played a critical role to determine the extent and severity of atherosclerotic plaque burden and to guide the results of stent implantation. In patients with SCAD, ruling out atherosclerotic coronary artery disease remains of major value. In these patients, IVUS is able to depict angiographically silent disease and readily determines the presence of an intramural hematoma. In patients with a classical presentation of SCAD the dissecting membrane and the true and false lumens are clearly identified (15). Of importance, the full longitudinal and circumferential extent of an intramural hematoma is readily visualized. Thrombosis of the false lumen is nicely depicted with this technique. IVUS findings have been also used to optimize the results of stent implantation in patients with SCAD requiring coronary interventions. Of interest, IVUS provides nice pictures of the underlying substrate even in patients without anterograde coronary flow and in those with occlusive intracoronary thrombus (17).
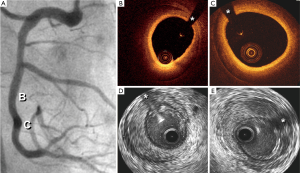
More recently, we have reported the value of optical coherence tomography (OCT) in patients with SCAD (16,17). OCT has an unsurpassed axial resolution (15 µm, 10 times superior to that of IVUS) and, therefore, provides unique and detailed insights on the underlying anatomic substrate of these patients (16). This technique readily depicts the length of the dissecting membrane, the presence of the 2 lumens, the occurrence of thrombosis in the false lumen or the presence of an intramural hematoma (16). In fact, OCT findings demonstrated that the “intimal flap” actually corresponds to an “intimo-medial” membrane (encompassing both the intima and the inner layers of the media). In addition, due to its superb near-field resolution OCT is ideally suited to accurately identify the precise location of the intimal tear and the presence of small thrombi in the true lumen. Finally, the take-off and involvement of the related side-branches can be readily analyzed (16). OCT has been also used to decide upon, plan and guide, coronary interventions (16). First, OCT is able to confirm the adequate guide-wire position within the true lumen. Stent size and length may be selected considering true vessel size and the true longitudinal extension of the disease. Interestingly, residual abluminal intramural hematoma and residual distal dissections are frequently detected with OCT after stenting. However, aggressive attempts to tackle small distal dissections are not justified, as these are benign and course with spontaneous healing. Compared with IVUS, OCT is clearly superior for the identification of the intimal rupture site and to provide a precise visualization of the intimomedial membrane (16,17). However, IVUS provides a deeper penetration on the vessel wall and, therefore, is able to fully visualize large hematomas and also penetrates across red thrombi that on OCT cause major shadowing (16,17). Finally, OCT requires a lumen free of blood and thus is not able to obtained good images in patients with severe lesions that impede adequate blood clearance. Although in patients with SCAD both techniques may provide complementary information their combined used in the clinical setting is not required for a decision-making process and only appears justified with research purposes (17).
Due to its limited resolution cardiac CT angiography is not recommended to rule out SCAD but its noninvasive nature appears very attractive to assess arterial healing, particularly in large and proximal coronary vessels (23). Likewise, cardiac magnetic resonance imaging may be able to demonstrate hyperintensity areas within the vessel wall in patients with intramural hematomas. This technique avoids additional radiation exposure but in most cases lacks the required resolution for the diagnosis of SCAD.
Clinical management and revascularization
Clinicians confronted with the acute management of patients with SCAD face a very challenging decision-making process as data on the value of different therapeutic strategies are scarce and decisions remain largely empirical (2-7). Evidence supporting the value of standard pharmacological treatment in patients with SCAD is lacking. Most investigators, however, favor the use of dual antiplatelet therapy in the acute setting although the potential value of the new P2Y12 antagonist remains unsettled. Likewise, in the early phase, heparin is usually administered but the use of IIbIIIa platelet inhibitors is often not recommended and thrombolytic therapy appears contraindicated. Beta-blockers reduce arterial shear stress and, therefore, are very attractive for the management of patients with SCAD. Alternatively, calcium-channel blockers appear indicated in patients with findings suggestive of associated coronary artery spasm (2-7).
The main controversy, however, remains the indication of coronary revascularization (8-14). Some early reports suggested the benefit of systematic revascularization when the anatomy was suitable and many case-series and small retrospective series suggested favorable clinical outcomes after percutaneous interventions or coronary surgery. A larger clinical experience with this disease and the concern of a potential problem of publication bias has led to a more critical appraisal of the results of coronary revascularization in these patients (8-14).
We recently studied a relatively large series of patients with SCAD using a systematic and prospective diagnostic and management protocol (8). A conservative initial management strategy was selected for patients presenting with SCAD. In this study coronary revascularization was only indicated for patients with ongoing or recurrent ischemia. During the study a high level of clinical suspicion was also maintained and a liberal use of intracoronary imaging (IVUS or OCT) was recommended. Finally, we also obtained a systematic late clinical follow-up and, in most patients, a late angiographic assessment was also scheduled (8). A total of 45 consecutive patients with SCAD were included in this study. Coronary risks factors were common and only 1 patient was in the peripartum period. Revascularization was only required in 1/3 of patients during admission (14 patients coronary stenting, 2 bypass surgery). Overall, the event-free survival at 3 years was 92%. Notably, at late angiographic assessment the angiographic image of SCAD spontaneously resolved or significantly improved in most patients (8). Our study, therefore, strongly suggested that in stabilized patients with SCAD a “conservative” medical management (watchful waiting strategy) provides an excellent clinical and angiographic outcome (8).
In a series of 50 patients with SCAD from the Vancouver General Hospital (13) an excellent clinical outcome was also obtained with a conservative initial management. This therapeutic strategy could be used in 80% of cases. No hospital mortality occurred and only 4.8% of patients suffer a recurrent myocardial infarction. Alternatively, in this series one-third of the patients that required revascularization had procedural failures or procedural-related complications. Actually, only one-third of all patients undergoing coronary interventions achieved a “permanent” or maintained success at long-term follow-up (14).
Tweet et al. reported findings from 87 consecutive patients with SCAD studied at the Mayo Clinic (10). In this study most patients (2/3) underwent coronary revascularization but the results of these procedures were suboptimal. Indeed, success of percutaneous coronary interventions was only obtained in 65% of cases. Likewise, although initial surgical results were favorable eventually most grafts were occluded at late follow-up (10). More recently, these investigators performed a retrospective analysis of an even larger series of 189 patients presenting with a first episode of SCAD (12). Specifically, this study sought to compare clinical outcomes in patients treated with revascularization with those conservatively managed. Definition of revascularization success included: (I) Conventional success: <30% residual stenosis after stent placement including the non-stented segment of dissection; (II) SCAD-specific definition of success: improvement in baseline coronary flow (at least ≥1 grade improvement in patients with TIMI grade 0/1 flow) or maintenance/improvement of TIMI 2/3 flow. Using this combined definition, only half of the patients treated with coronary interventions achieved procedural success. In addition, compared with the conservative therapy group, those treated with revascularization had a higher risk of requiring emergent coronary surgery (12). Finally, at 5 years, freedom from target vessel revascularization or recurrent SCAD was similar in both groups. It was concluded that coronary interventions for patients with SCAD are associated with high rates of technical failure even in those presenting with preserved vessel flow and that this strategy does not protect against subsequent target vessel revascularization or recurrent SCAD. Likewise, it was suggested that an initial strategy of conservative management, with subsequent prolonged observation, was preferable (12).
Nevertheless, in patients with clear ongoing or recurrent ischemia revascularization is clearly indicated. In this setting, coronary interventions are of value to restore coronary flow in occluded vessel in order to reduce infarct size. In patients with refractory angina but a normal coronary flow revascularization is also indicated but should be carefully planned. In this scenario, unstable patients with left main involvement and those with multiple severe and long dissections appear ideal candidates for surgical revascularization (8-14). Otherwise, coronary stenting should be considered (Figure 3). When percutaneous revascularization is indicated care should be taken to ensure optimal results and to minimize complications. During these interventions it may be challenging to advance the coronary guide-wire into the true lumen, particularly in diffuse and distal coronary segments. It should be also kept in mind that coronary stenting may lead to propagation of an intramural hematoma, compromising coronary blood flow in the main vessel and in the affected side-branches (8-14). As discussed, intracoronary imaging may be of value during these interventions (15-18). Under appreciation of the extent intramural hematoma can result in stent under sizing, with the associated long-term risk of late stent malapposition following vessel healing. Direct stenting should be considered to cover the entry door and the segments showing severe lumen compromise. Ensuring adequate coverage of the disease edges may prevent propagation of an intramural hematoma. However, a conservative stenting approach should be favoured whenever possible in an attempt to avoid a “full-metal-jacket” final result (8). Residual distal dissection should be left untreated when they do not cause significant residual stenosis and when coronary flow is normal (8). Importantly, residual distal dissections tend to heal and disappear at late follow-up (8). Disease in small distal vessel should not be treated. The use of drug-eluting stents in this setting remains controversial but most operators select these devices when long segments should be treated (8). Very recently, the use of cutting balloon to fenestrate the membrane and decompress the true lumen has been proposed (24). Likewise, the use of bioresorbable vascular scaffolds emerges as an appealing option for selected patients with SCAD as complete vessel restoration during follow-up is expected.
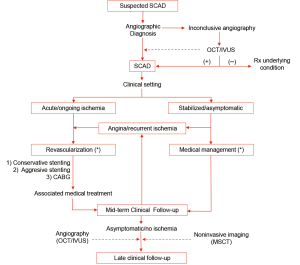
Overall, the long-term prognosis of patients who survived their initial SCAD presentation is very favorable. Nevertheless, some of these patients are still at risk for recurrences and, therefore, should be closely followed. Interestingly, recurrent SCAD events almost always affect new segments rather than initially dissected vessels. Finally, it is important to keep in mind that late vessel healing occurs in most patients. This remains a major consideration supporting the initial use of a conservative initial strategy for most of these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pretty HC. Dissecting aneurism of coronary artery in a woman aged 42. BMJ 1931;1:667.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010;96:801-8. [PubMed]
- Alfonso F. Spontaneous coronary artery dissection: new insights from the tip of the iceberg? Circulation 2012;126:667-70. [PubMed]
- Alfonso F, Bastante T, Rivero F, et al. Spontaneous coronary artery dissection. Circ J 2014;78:2099-110. [PubMed]
- Giacoppo D, Capodanno D, Dangas G, et al. Spontaneous coronary artery dissection. Int J Cardiol 2014;175:8-20. [PubMed]
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027-33. [PubMed]
- Michelis KC, Olin JW, Kadian-Dodov D, et al. Coronary artery manifestations of fibromuscular dysplasia. J Am Coll Cardiol 2014;64:1033-46. [PubMed]
- Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a "conservative" therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062-70. [PubMed]
- Tweet MS, Gulati R, Aase LA, et al. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin Proc 2011;86:845-50. [PubMed]
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [PubMed]
- Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656-62. [PubMed]
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777-86. [PubMed]
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44-52. [PubMed]
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645-55. [PubMed]
- Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002;89:466-8. [PubMed]
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59:1073-9. [PubMed]
- Paulo M, Sandoval J, Lennie V, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013;6:830-2. [PubMed]
- Saw J, Poulter R, Fung A. Intracoronary imaging of coronary fibromuscular dysplasia with OCT and IVUS. Catheter Cardiovasc Interv 2013;82:E879-83. [PubMed]
- Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv 2005;64:138-45. [PubMed]
- Saw J, Poulter R, Fung A, et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012;5:134-7. [PubMed]
- Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048-78. [PubMed]
- Liang JJ, Prasad M, Tweet MS, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr 2014;8:189-97. [PubMed]
- Das Neves BC, Núñez-Gil IJ, Alfonso F, et al. Evolutive recanalization of spontaneous coronary artery dissection: insights from a multimodality imaging approach. Circulation 2014;129:719-20. [PubMed]
- Yumoto K, Sasaki H, Aoki H, et al. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv 2014;7:817-9. [PubMed]


