
Carina shift as a mechanism for side-branch compromise following main vessel intervention: insights from three-dimensional optical coherence tomography
Three-dimensional rendering of optical coherence tomography (OCT) images is a method that can be used for providing additive information for assessing the result of percutaneous coronary intervention (PCI), especially in complex lesions such as in bifurcations (1). While plaque shift has been considered the main underlying mechanism for side-branch compromise after stenting (2), new theories challenge the role of plaque shift and suggest carina shift to be a major contributor (3-6). We present a case were on-line three-dimensional OCT was used to demonstrate the role of carina shift in side-branch pinching, following main vessel intervention.
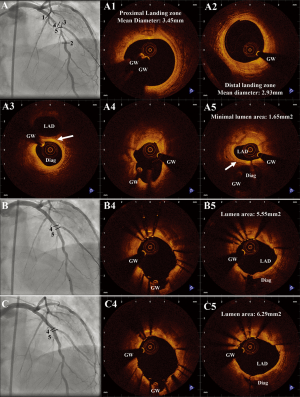
A 53-year old female underwent primary PCI of the right coronary artery. One month later, she was referred to our centre for evaluation of a lesion in the left anterior descending artery (LAD), involving the bifurcation with the first diagonal branch (Figure 1A). Both LAD and the diagonal branch were evaluated by fractional flow reserve (FFR), with a positive result (FFR = 0.75) at the LAD and a negative at the diagonal branch (FFR = 0.85). OCT evaluation using the C7 Lightlab system (St Jude/Lightlab Img, Westford, MA, USA) was performed in both vessels (Figure 1A) and revealed a heavily diseased LAD with a minimum luminal area of 1.65 mm2 and a diagonal branch with moderate plaque burden limited to the ostium. In both vessels the carina was spared of disease (Figure 1A3 and 1A5). It was decided to proceed with a provisional stenting strategy of the LAD. Stent length selection was based on the assessment of the least diseased proximal and distal ‘landing zones’ by OCT and are shown in Figures 1A1 and 1A2. A 3.0/33 mm Xience Prime™ (Abbott Vascular, Santa Clara, CA, USA) stent was implanted directly between these sites with an inflation pressure of 16 atm. OCT evaluation of the LAD after stent implantation revealed moderate underexpansion without malapposition (Figure 1B). Post-dilation was then performed using a 3.5 mm × 15 mm balloon inflated at 20 atm. The final OCT examination documented a well-expanded stent without areas of malapposition (Figure 1C). Angiography showed a good result of the LAD, but revealed a moderate pinching of the ostium of the diagonal branch with Thrombolysis In Myocardial Infarction flow grade 3 in both vessels.
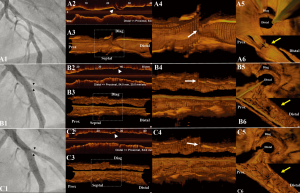
Three-dimensional models were rendered from OCT pullbacks using dedicated on-line software (QAngioOCT, Prototype Version, Medis medical imaging systems bv, Leiden, Netherlands; Videos 1,2,3,4) (7). In order to create the renderings, a DICOM file containing OCT images acquired by pullback was imported to the software and the 3-dimensional renderings were generated. By this software it is possible to generate cutaway views or fly-through views (i.e. showing the morphology of the vessel from the site of the OCT catheter) with downstream (proximal-to-distal) or upstream (distal-to-proximal) orientation. Figure 2 illustrates the involvement of carina shift as the underlying mechanism for side-branch pinching in our case. Longitudinal and 3-dimensional renderings of the OCT images clearly demonstrate a movement of the carina towards the direction of the side-branch, leading to a reduction of the area of the side-branch ostium. As demonstrated by the cross-sectional OCT images in Figure 1, there was no plaque at the carina, while after intervention there was no evidence of plaque shift (Figures 1B5,C5).
Intravascular ultrasound has been widely used for guidance in bifurcation lesions, aiding the visualization of plaque morphology at the main vessel and the side-branches and helping the selection of stent size and length as well as the selection of stenting strategy (2,3,5,6). However, due to the low spatial resolution of IVUS, all attempts for three-dimensional visualization using fusion of 3-dimensional coronary angiography and IVUS images have only focused on visualization of the luminal contour and not on the vessel morphology or the vessel-stent interaction. Furthermore, the slow pullback speed of the IVUS catheter requires the use of prospective ECG-gating or post-procedural processing. By utilizing the superior resolution of OCT, it is now possible to evaluate vessel and stent surfaces in three dimensions with a high resolution.
Plaque shift has been traditionally considered as the principal mechanism for side-branch compromise after main vessel intervention (2), however recent intravascular imaging studies have provided new insights by suggesting carina shift as a major mechanism implicated in side-branch closure (3-6). It has also been suggested that the post-stenting side-branch compromise underlying carina shift is not commonly associated with functional compromise as assessed by FFR (8), providing a plausible explanation for the poor association between angiographic and functional assessment of side-branch compromise after PCI (9). Furthermore, the presence of an ‘eyebrow’ sign (i.e. spiky carina at the longitudinal reconstruction of intravascular ultrasound images) has been associated with carina shift after main vessel stent implantation (5). Three-dimensional OCT is a new method that can assist the evaluation of bifurcation intervention, as it allows for visualization of the 3-dimensional anatomy of the region providing information on stent expansion, guidewire position, side-branch ostium, orientation of the side-branch relatively to the main vessel, and carina shape (1,10).
In our case, we used OCT in order to evaluate the preprocedural morphology of the main vessel and the side-branch, and based on our findings, opted to use a provisional stenting strategy with a relatively long stent in order to treat this diffusely diseased vessel. Post-procedural evaluation guided the use of post-dilation. Additionally, 3-dimensional OCT visualized the spiky carina morphology that was free of atheromatic disease, and illustrated how carina shift towards the side-branch led to moderate angiographic narrowing of the side-branch ostium. Our case demonstrates how OCT constitutes a valuable tool for PCI guidance and also the utility of 3-dimensional renderings for assessing the mechanism of side-branch compromise following intervention in bifurcation lesions. This might guide clinical decision making in the future with OCT three-dimensional rendering becoming available in real-time in the cathlab.
Acknowledgments
Antonios Karanasos’ work was supported by a grant from the Hellenic Heart Foundation.
Disclosures: Shengxian Tu is employed by Medis medical imaging systems bv and has a research appointment at Leiden University Medical Center. Johan H. C. Reiber is the CEO of Medis medical imaging systems bv, and has a part-time appointment at Leiden University Medical Center as Prof of Medical Imaging.
References
- Farooq V, Serruys PW, Heo JH, et al. New insights into the coronary artery bifurcation hypothesis-generating concepts utilizing 3-dimensional optical frequency domain imaging. JACC Cardiovasc Interv 2011;4:921-31. [Crossref] [PubMed]
- Maehara A, Takagi A, Okura H, et al. Longitudinal plaque redistribution during stent expansion. Am J Cardiol 2000;86:1069-72. [Crossref] [PubMed]
- Kang SJ, Mintz GS, Kim WJ, et al. Changes in left main bifurcation geometry after a single-stent crossover technique: an intravascular ultrasound study using direct imaging of both the left anterior descending and the left circumflex coronary arteries before and after intervention. Circ Cardiovasc Interv 2011;4:355-61. [Crossref] [PubMed]
- Koo BK, Waseda K, Kang HJ, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:113-9. [Crossref] [PubMed]
- Suárez de Lezo J, Medina A, Martin P, et al. Predictors of ostial side branch damage during provisional stenting of coronary bifurcation lesions not involving the side branch origin: an ultrasonographic study. EuroIntervention 2012;7:1147-54. [Crossref] [PubMed]
- Vassilev D, Gil RJ. Relative dependence of diameters of branches in coronary bifurcations after stent implantation in main vessel--importance of carina position. Kardiol Pol 2008;66:371-8; discussion 379. [PubMed]
- Tu S, Xu L, Ligthart J, et al. In vivo comparison of arterial lumen dimensions assessed by co-registered three-dimensional (3D) quantitative coronary angiography, intravascular ultrasound and optical coherence tomography. Int J Cardiovasc Imaging 2012;28:1315-27. [Crossref] [PubMed]
- Na SH, Koo BK, Kim JC, et al. Evaluation of local flow conditions in jailed side branch lesions using computational fluid dynamics. Korean Circ J 2011;41:91-6. [Crossref] [PubMed]
- Koo BK, Kang HJ, Youn TJ, et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol 2005;46:633-7. [Crossref] [PubMed]
- Okamura T, Serruys PW, Regar E. Cardiovascular flashlight. The fate of bioresorbable struts located at a side branch ostium: serial three-dimensional optical coherence tomography assessment. Eur Heart J 2010;31:2179. [Crossref] [PubMed]


