Understanding and treating hypertension in diabetic populations
Introduction
Diabetes mellitus is a major public health problem of epidemic proportions and its worldwide prevalence is expected to further increase in the near future (1,2). Patients with diabetes are at high risk of developing major cardiovascular complications, mostly including myocardial infarction, ischemic stroke and congestive heart failure, but also microvascular complications, such as retinopathy, nephropathy and peripheral artery disease (3). Moreover, the presence of diabetes not only increases the risk of experiencing major cardiovascular events, but it also increases the risk of developing hypertension. On the other hand, hypertension, a clinical entity in which insulin resistance is common, is strongly associated with higher risk of developing metabolic complications, including new onset diabetes, as compared to normotension, and it may precede the development of diabetes by several years.
Once established, the concomitant presence of diabetes and hypertension substantially affects cardiovascular prognosis, reduces the event-free survival, and influences the ability of achieving the recommended therapeutic targets, both in terms of fasting glucose and blood pressure (BP) levels. This detrimental association between diabetes and hypertension has also demonstrated to induce the development and promote the progression of hypertension-related organ damage at both cardiac, vascular and renal levels, thus leading to a further increased risk of developing major cardiovascular events.
Given the objective difficulties in achieving the recommended therapeutic targets in hypertensive patients with diabetes, BP goals to be achieved under pharmacological therapy have been recently revised. In the past, randomized clinical trials have, indeed, demonstrated that lowering BP levels to less than 140/90 mmHg was associated to a substantial reduced risk of developing macrovascular and microvascular complications in hypertensive patients with diabetes. Recent randomized clinical trials, however, designed to evaluate the potential benefits obtained with lowering systolic BP level below 120 mmHg, showed an increased risk of coronary events, mostly myocardial infarction, in those patients who achieved the lowest BP levels, particularly in the high-risk subsets of hypertensive populations with diabetes.
In the light of these considerations, the present article will briefly review the pathophysiological mechanisms shared by essential hypertension and diabetes mellitus, the potential sites of therapeutic interactions and the currently recommended BP targets to be achieved under pharmacological treatment in hypertensive patients with diabetes.
Pathophysiology of vascular alterations in hypertension and diabetes
Vascular remodelling, endothelial dysfunction and vascular stiffness are common features in hypertension and diabetes (Figure 1). Low-grade inflammation occurs at the vasculature level in several conditions that predispose to development and progression of cardiovascular diseases, including hypertension and diabetes (5-7). In particular, low-grade inflammation of the vascular wall participates in vascular remodelling and may contribute to the pathophysiology of hypertension (8,9). Elevated plasma levels of inflammatory mediators may be associated with increased risk of the occurrence of diabetes (10) and cardiovascular disease. Moreover, inflammation may be included in the definition of the metabolic syndrome that may in turn increase the risk of overt diabetes mellitus and cardiovascular events (11).
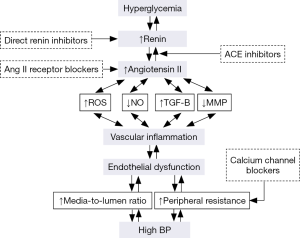
Patients with cardiovascular disease, mostly hypertension, coronary artery disease, and congestive heart failure, show increased expression and plasma concentration of different inflammatory markers and mediators, including C-reactive protein (CRP) and several adhesion molecules (selectins, ICAM-1, VCAM-1) (12,13). In particular, high levels of inflammatory mediators, particularly IL-6, ICAM-1 and CRP have been demonstrated in patients with hypertension (14), and have been associated with the risk for developing hypertension (15,16). Also, abnormal activation of the renin-angiotensin system (RAS) may play a central role in the pathophysiology and development of cardiovascular disease in these settings (17). In particular, angiotensin II induces vascular remodeling and injury by several mechanisms including vasoconstriction, cell growth, oxidative stress and inflammation by inducing cytokine release (18) and pro-inflammatory transcription factors such as nuclear factor κB (NF-κB) (19), which regulates adhesion molecules and cytokine expression in several cell types (20). These molecules induce and maintain inflammation within the vascular wall, stimulate deposition of extracellular matrix and promote hypertrophy and/or hyperplasia of vascular smooth muscle cells (21).
A large body of evidence underlines the pathophysiological role played by inflammation in the progression of cardiovascular and metabolic disease and in the triggering of cardiovascular events, as well as the need to oppose pharmacologically these mechanisms to improve cardiovascular outcomes. In the United Kingdom Prospective Diabetes Study (UKPDS), the incidence of complications of diabetes was strongly associated with elevated BP (22). Thus, lowering BP as well as therapeutic approaches to control vascular inflammation, particularly in patients with glucose intolerance or diabetes, may provide significant clinical benefits. However, two recently published post-monitoring follow-up studies of UKPDS (23,24) have shown that, although early and intensive treatment of hyperglycemia provides benefits for cardiovascular mortality that extend over time, the effects of a tight antihypertensive strategy in patients with diabetes did not seem to last during the following years (4).
Oxidative stress
Oxidative stress is characterized by increased reactive oxygen species (ROS) production. The major source of vascular ROS is NADPH oxidase, which is expressed in endothelial cells, vascular smooth muscle cells, fibroblasts and monocytes/macrophages (25,26). Angiotensin II, ET (endothelin)-1 and inflammatory mediators can increase synthesis of most subunits of NADPH oxidase and modulate basal NADPH oxidase-induced ROS production by the activation of several pathways (involving cSrc, PKC, PLA2 and PLD) (27,28).
ROS are also produced by mitochondria in the vasculature (29-35). In particular, mitochondrial p66Shc is a pivotal modulator of mitochondrial ROS through oxidation of cytochrome c (36,37), and has been considered as part of a putative transduction pathway relevant to endothelial integrity (38,39). In fact, genetic ablation of Shc in mouse has been shown to reduce the production of intracellular oxidants, to protect against age-dependent endothelial dysfunction (40), and consequently to prolong life span (36). Indeed, old mice lacking p66Shc showed increased nitric oxide (NO) bioavailability in endothelium, lower ROS levels in the absence of any difference in the expression of superoxide dismutase (SOD) (40), and no age-dependent changes in iNOS. Furthermore, p66Shc expression is increased in patients with type 2 diabetes and correlates with markers of oxidative stress such as plasma isoprostane levels (41). Genetic deletion of p66Shc protects against vascular dysfunction and oxidative stress in diabetic mice (42). Moreover, p66Shc knockdown in endothelial cells from obese mice attenuated ROS production, free fatty acids oxidation and prevented dysregulation of proinflammatory pathways. In vivo gene silencing of p66Shc may restore endothelial insulin response by affecting the IRS-1/Akt/eNOS pathway (43). Hence p66Shc may also contribute to the pathogenesis of insulin resistance.
Enhanced angiotensin II-induced ROS is involved in the mechanisms leading to vascular remodeling in part by impairing NO bioavailability and the endothelium-dependent vascular relaxation (5-9). Moreover, ROS are responsible of vascular smooth muscle cells proliferation and hypertrophy, collagen deposition and the release of pro-inflammatory cytokine and transcription factors such as NF-κB. These processes may lead to increased vascular tone and structural changes in the circulation. Inflammation contributes to vascular remodelling promoting cell growth and proliferation of vascular smooth muscle cells. Among the mechanisms that participate in the inflammatory responses in the vascular wall important role is played by the increased expression of adhesion molecules (VCAM-1, ICAM-1) on the endothelial cell membrane, the accumulation of monocyte/macrophages, and lymphocytes (44). Also, Ang II as well as increased oxidative stress are important modulator of T-cell activation and development of vascular inflammation (45,46). Innate immunity may also be involved in the mechanisms that contribute to the low-grade inflammation in hypertension. Mice deficient in vascular macrophages as well as in T and B lymphocytes did not present vascular remodelling in response to Ang II- or DOCA-salt (47). It has been shown that an imbalance between pro-inflammatory subset of T lymphocytes (Th1, Th2 and Th17) and the anti-inflammatory T regulatory (Treg) may be responsible of the inflammatory response described in cardiac and metabolic diseases (48). Treg adoptive transfer lowered BP levels and protected from vascular remodelling mice infused with either angiotensin II (49) or aldosterone (50). Therefore, reduction of Treg and T effector up-regulation are associated to increase BP and are involved in the pathogenesis of BP-induced vascular inflammation and cardiovascular remodelling.
Ang II-induced inflammation via NF-κB and AP-1 activation involves, in part, ET receptors (51). ET-1 activates NADPH oxidase, as well as other sources of ROS, (i.e., xanthine oxidase and mitochondria) to produce increased oxidant stress in vascular smooth muscle cells and blood vessels (52-54). In turn, ROS are potent stimulators of ET-1 synthesis by endothelial cells and vascular smooth muscle cells (55). ET-A receptor antagonism decreases oxidative stress, normalizes hypertrophic remodelling, decreases collagen and fibronectin deposition, and reduces adhesion molecules levels in the vasculature of aldosterone-infused rats (56). When human preproET-1 was transgenically overexpressed in the endothelium, mice exhibited endothelial dysfunction and increased activity of NADPH oxidase, leading to enhanced oxidative stress and vascular inflammation (56).
The activation of mineralocorticoid receptors may also contribute to cardiovascular dysfunction, inflammation, fibrosis and vascular damage. In particular aldosterone increases the activity of tissue angiotensin-converting enzyme (ACE) (57) and up-regulates angiotensin receptors, thus potentiating effects of the RAS. Several animal models have confirmed that aldosterone and other mineralocorticoids can cause injury of the vasculature of several organs including heart and brain, ROS formation and endothelial dysfunction (58). Aldosterone may induce endothelial dysfunction and inflammation through activation of COX-2 (cyclo-oxygenase-2) in normotensive and hypertensive rats (59).
BP reduction per se may influence inflammation, since antihypertensive drugs such as calcium channel blockers not only decreased BP in patients with hypertension, but also reduced plasma concentrations of ICAM-1, E-selectin and vWF (60). However, it has been shown that despite effective antihypertensive treatment, resistance arteries from hypertensive diabetic patients showed marked remodelling, greater than that of vessels from untreated, non diabetic, hypertensive subjects (61). Thus, considering the pro-inflammatory effects of Ang II and aldosterone, drugs that interfere with the components of RAS, such as ACE inhibitors, angiotensin II receptor blockers (ARBs) and mineralocorticoid receptor antagonists represent suitable therapeutic tools to reduce vascular inflammation and result in reduced cardiovascular events in randomized clinical trials (62-64). The addition of ARBs to antihypertensive medications resulted in an improvement in resistance artery remodelling in diabetic hypertensive patients (65). Mineralocorticoid antagonism may attenuate these deleterious effects by reducing directly the pro-inflammatory and pro-fibrotic effects of aldosterone (66,67). Moreover, eplerenone treatment was also associated with reduced stiffness, decreased collagen/elastin ratio, and a reduction in circulating inflammatory mediators in hypertensive patients (65).
Treating hypertension in diabetes
BP reduction is one of the most powerful and effective pharmacological interventions to reduce cardiovascular morbidity and mortality, particularly in the presence of diabetes mellitus. Evidence derived from randomised clinical trials have consistently demonstrated significant benefits obtained by lowering high BP levels in different clinical settings, including elderly patients with isolated systolic hypertension (68-71), essential hypertension (72-74), high cardiovascular risk profile (75-77), coronary artery disease (78,79), previous stroke (80,81), and mostly diabetes mellitus (82,83). The results of these trials have been used for supporting the evidence to define optimal BP targets to be achieved and effective therapeutic strategies to be adopted in patients with hypertension and diabetes.
Blood pressure (BP) targets in hypertension and diabetes
Previous sets of international guidelines for the clinical management of hypertension have gradually affirmed and systematically promoted the achievement of ambitious BP targets in hypertensive patients at high or very high cardiovascular risk profile, such as those with diabetes mellitus (84-88). This therapeutic strategy was summarized by the paradigm “the lower, the better”, that characterized the clinical management of hypertension and diabetes until the early 2010’s, and was predominantly applied in hypertensive patients with diabetes. The same guidelines, however, acknowledged the fact that definite evidence in favour of more ambitious BP targets (i.e., <130/80 mmHg) compared to traditional BP goals (i.e., <140/90 mmHg) was missing. In fact, a closer analysis of trials performed in patients with hypertension at different cardiovascular risk profile, including those with diabetes, demonstrated that effective BP control (defined as <140/90 mmHg in general hypertensive patients and <130/80 mmHg in hypertensive patients with diabetes mellitus or renal disease) was achieved only in a few of these trials (89,90).
In order to overcome this limitation, several randomized controlled clinical trials have been designed in the recent years. These trials were aimed to demonstrate the potential benefits derived by more intensive BP reductions in patients with hypertension and diabetes compared to those obtained by conventional BP reductions. Results from these trials, however, dramatically failed to demonstrate any potential cardiovascular (or renal) advantage, particularly for extreme BP reductions (91-93). These findings, in fact, have somehow revitalized the concept of the J-curve (94), showing an increased risk of coronary events, mostly myocardial infarction, in those patients who achieved the lowest BP levels, particularly in high-risk subsets of hypertensive populations, such as those with multiple risk factors, diabetes, chronic kidney disease (CKD) or previous history of coronary artery disease.
Quite consistently, in sub-analyses derived from relatively old randomized clinical trials, including the Systolic Hypertension in the Elderly Program (SHEP) (95), the Systolic Hypertension in Europe (Syst-Eur) (96), the International Verapamil-Trandolapril Study (INVEST) (97), the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) (98), the risk of myocardial infarction increased in high-risk patients achieving on-treatment lower systolic BP levels. A large, albeit predominant proportion of patients included in these trials were diabetics with hypertension.
More recently, several other trials have tested the hypothesis of potential benefits derived from an intensive therapy compared to a conventional therapy in terms of achieving strict or usual systolic BP control. For example, the Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD-BP) trial (91) was designed to verify the potential benefits obtained with target systolic BP levels below 120 mmHg (intensive therapy) compared to those obtained with target systolic BP levels below 140 mmHg (conventional therapy) on major cardiovascular events among high-risk patients with type 2 diabetes (91). The large and significant systolic BP fall did not result in a reduction of the incidence of the primary composite cardiovascular endpoint (non-fatal MI, non-fatal stroke and death from cardiovascular causes) (91). No benefits on major cardiovascular events, no significant changes of cardiovascular and non- cardiovascular mortality (both actually showed a slight tendency to increase), a significant reduction of fatal and non-fatal stroke (an event more directly linked to BP reduction), and a significant worsening of renal function were reported (91). Also, in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) (99) and the Treating to New Targets (TNT) (100) trials, a paradoxically increased risk of myocardial infarction (not stroke) and renal impairments were observed in those patients at very high global cardiovascular risk profile, which achieved extremely low systolic BP reductions. More recently, in the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial (101), which demonstrated that new onset of microalbuminuria can be effectively prevented in type 2 diabetic patients with high-normal BP levels by a high dose antihypertensive therapy, it has been also described a five-fold increase in the risk of developing fatal myocardial infarction in the active group as compared to placebo group, though absolute numbers of events were small.
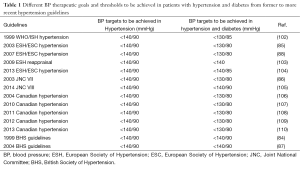
Table 1 shows the evolution of recommended BP therapeutic goals and thresholds to be achieved in patients with hypertension and diabetes from former to more recent hypertension guidelines. On the basis of the currently available clinical evidence, latest set of European guidelines recommend the BP targets of <140/90 mmHg also in hypertensive patients with diabetes. In these patients, lower diastolic BP levels (85 mmHg) might be achieved, if tolerated and not contraindicated (104).
Full table
Antihypertensive drug strategies in hypertension and diabetes
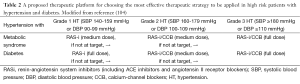
Guidelines recommend initiating drug treatment when systolic BP is more than 140 mmHg in diabetic patients (111). To achieve these goals, the pharmacological strategy may include any antihypertensive drug class, although those agents able to counteract the RAS, including ACE inhibitors and ARBs, should be preferred (111). A proposed therapeutic platform for choosing the most effective therapeutic strategy to be applied in high risk patients with hypertension and diabetes is reported in Table 2 (112).
Full table
Conclusions
Despite the impressive improvement in the clinical management of hypertension and diabetes, the achievement of effective control of therapeutic targets in this high risk population remain a major clinical challenge. Clinical studies continuously reported a very low rate of control in both hypertensive and diabetic patients, independently by age and clinical setting. In addition, recently available evidence has questioned the traditional therapeutic interventions proposed in the past, which recommended rigorous and unconditioned therapeutic targets. On the basis of these findings, more prudent targets have been proposed for both hypertension and diabetes, especially when these conditions are associated with other relevant comorbidities. A further improvement should be the adoption of different therapeutic targets on the basis of individual age, beyond global cardiovascular risk stratification. Future studies will help to answer these questions and provide useful evidence for improving the clinical management of hypertension and diabetes in the clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 2011;377:568-77. [PubMed]
- Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31-40. [PubMed]
- Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 1979;2:120-6. [PubMed]
- Volpe M, Cosentino F, Tocci G, et al. Antihypertensive therapy in diabetes: the legacy effect and RAAS blockade. Curr Hypertens Rep 2011;13:318-24. [PubMed]
- European Stroke Organisation, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851-906. [PubMed]
- Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol 2008;41:575-80. [PubMed]
- Cremer A, Butlin M, Codjo L, et al. Determination of central blood pressure by a noninvasive method (brachial BP and QKD interval). J Hypertens 2012;30:1533-9. [PubMed]
- Tsai WC, Lee KT, Wu MT, et al. Significant correlation of P-wave parameters with left atrial volume index and left ventricular diastolic function. Am J Med Sci 2013;346:45-51. [PubMed]
- Parati G, Omboni S, Palatini P, et al. Italian society of hypertension guidelines for conventional and automated blood pressure measurement in the office, at home and over 24 hours. High Blood Press Cardiovasc Prev 2008;15:283-310. [PubMed]
- Ferrucci A, Pignatelli G, Sciarretta S, et al. Hypertension in premenopausal women: is there any difference? High Blood Press Cardiovasc Prev 2014;21:195-9. [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. [PubMed]
- Tocci G, Ferrucci A, Guida P, et al. An analysis of the management of cardiovascular risk factors in routine clinical practice in Italy: an overview of the main findings of the EFFECTUS study. High Blood Press Cardiovasc Prev 2011;18:19-30. [PubMed]
- Volpe M, Tocci G. Redefining blood pressure targets in high-risk patients?: lessons from coronary endpoints in recent randomized clinical trials. Am J Hypertens 2011;24:1060-8. [PubMed]
- Preston RA, Ledford M, Materson BJ, et al. Effects of severe, uncontrolled hypertension on endothelial activation: soluble vascular cell adhesion molecule-1, soluble intercellular adhesion molecule-1 and von Willebrand factor. J Hypertens 2002;20:871-7. [PubMed]
- Cockcroft J, Mancia G. Vascular aging: shifting the paradigm of risk assessment and reduction in hypertension. J Hypertens 2012;30:S1-2. [PubMed]
- Weber MA, Julius S, Kjeldsen SE, et al. Cardiovascular outcomes in hypertensive patients: comparing single-agent therapy with combination therapy. J Hypertens 2012;30:2213-22. [PubMed]
- Menni C, Boffi L, Cesana F, et al. Variant on chromosome 9p is associated with left ventricular mass: results from two cohorts of essential hypertensive individuals. J Hypertens 2012;30:2144-50. [PubMed]
- Cuspidi C, Rescaldani M, Sala C, et al. Prevalence of electrocardiographic left ventricular hypertrophy in human hypertension: an updated review. J Hypertens 2012;30:2066-73. [PubMed]
- Mancia G. Reporting blood pressure effects of antihypertensive treatment in scientific papers: are guidelines needed? J Hypertens 2012;30:1307-9. [PubMed]
- Pueyo ME, Gonzalez W, Nicoletti A, et al. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol 2000;20:645-51. [PubMed]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 2000;52:639-72. [PubMed]
- Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412-9. [PubMed]
- Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565-76. [PubMed]
- Holman RR, Paul SK, Bethel MA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89. [PubMed]
- Verdecchia P, Dagenais G, Healey J, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. J Hypertens 2012;30:1004-14. [PubMed]
- Mancia G, Facchetti R, Parati G, et al. Visit-to-visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: methodological aspects and effects of antihypertensive treatment. J Hypertens 2012;30:1241-51. [PubMed]
- Schmieder RE, Redon J, Grassi G, et al. ESH position paper: renal denervation - an interventional therapy of resistant hypertension. J Hypertens 2012;30:837-41. [PubMed]
- Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens 2012;30:633-46. [PubMed]
- Zanchetti A, Mancia G. Longing for clinical excellence: a critical outlook into the NICE recommendations on hypertension management--is nice always good? J Hypertens 2012;30:660-8. [PubMed]
- Reboldi G, Gentile G, Angeli F, et al. Blood pressure lowering in diabetic patients. J Hypertens 2012;30:438-9. [PubMed]
- Brandes RP, Barton M, Philippens KM, et al. Endothelial-derived superoxide anions in pig coronary arteries: evidence from lucigenin chemiluminescence and histochemical techniques. J Physiol 1997;500:331-42. [PubMed]
- Mancia G. Assessing antihypertensive treatment by real life data. J Hypertens 2012;30:46-7. [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399-410. [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292. [PubMed]
- Burns EM, Kruckeberg TW, Comerford LE, et al. Thinning of capillary walls and declining numbers of endothelial mitochondria in the cerebral cortex of the aging primate, Macaca nemestrina. J Gerontol 1979;34:642-50. [PubMed]
- Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309-13. [PubMed]
- Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005;122:221-33. [PubMed]
- Spescha RD, Glanzmann M, Simic B, et al. Adaptor protein p66(Shc) mediates hypertension-associated, cyclic stretch-dependent, endothelial damage. Hypertension 2014;64:347-53. [PubMed]
- Sun L, Xiao L, Nie J, et al. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol 2010;299:F1014-25. [PubMed]
- Francia P. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 2004;110:2889-95. [PubMed]
- Schmieder RE, Volpe M, Waeber B, et al. A guide for easy- and difficult-to-treat hypertension. Int J Cardiol 2014;172:17-22. [PubMed]
- Waeber B, Volpe M, Ruilope LM, et al. Diagnosis and treatment of resistant hypertension. Blood Press 2014;23:193-9. [PubMed]
- Paneni F, Costantino S, Cosentino F. p66(Shc)-induced redox changes drive endothelial insulin resistance. Atherosclerosis 2014;236:426-9. [PubMed]
- de Boer RA, Azizi M, Danser AJ, et al. Dual RAAS suppression: recent developments and implications in light of the ALTITUDE study. J Renin Angiotensin Aldosterone Syst 2012;13:409-12. [PubMed]
- Mahfoud F, Ukena C, Pöss J, et al. Microalbuminuria independently correlates to cardiovascular comorbidity burden in patients with hypertension. Clin Res Cardiol 2012;101:761-6. [PubMed]
- Ukena C, Mahfoud F, Kindermann M, et al. Smoking is associated with a high prevalence of microalbuminuria in hypertensive high-risk patients: data from I-SEARCH. Clin Res Cardiol 2010;99:825-32. [PubMed]
- Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449-60. [PubMed]
- Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 2010;19:181-6. [PubMed]
- Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635-701. [PubMed]
- Kasal DA, Barhoumi T, Li MW, et al. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012;59:324-30. [PubMed]
- Muller DN, Mervaala EM, Schmidt F, et al. Effect of bosentan on NF-kappaB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension 2000;36:282-90. [PubMed]
- Iglarz M, Touyz RM, Amiri F, et al. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol 2003;23:45-51. [PubMed]
- Li L, Fink GD, Watts SW, et al. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 2003;107:1053-8. [PubMed]
- Touyz RM, Yao G, Viel E, et al. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 2004;22:1141-9. [PubMed]
- Kähler J, Ewert A, Weckmüller J, et al. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 2001;38:49-57. [PubMed]
- Amiri F, Virdis A, Neves MF, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation 2004;110:2233-40. [PubMed]
- Harada E, Yoshimura M, Yasue H, et al. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation 2001;104:137-9. [PubMed]
- Schiffrin EL, Gutkowska J, Genest J. Effect of angiotensin II and deoxycorticosterone infusion on vascular angiotensin II receptors in rats. Am J Physiol 1984;246:H608-14. [PubMed]
- Blanco-Rivero J, Cachofeiro V, Lahera V, et al. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension 2005;46:107-12. [PubMed]
- Agabiti Rosei E, Morelli P, Rizzoni D. Effects of nifedipine GITS 20 mg or enalapril 20 mg on blood pressure and inflammatory markers in patients with mild-moderate hypertension. Blood Press Suppl 2005;1:14-22. [PubMed]
- Endemann DH, Pu Q, De Ciuceis C, et al. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension 2004;43:399-404. [PubMed]
- Callera G, Tostes R, Savoia C, et al. Vasoactive peptides in cardiovascular (patho)physiology. Expert Rev Cardiovasc Ther 2007;5:531-52. [PubMed]
- Tegtmeyer CJ, Elson J, Glass TA, et al. Percutaneous transluminal angioplasty: the treatment of choice for renovascular hypertension due to fibromuscular dysplasia. Radiology 1982;143:631-7. [PubMed]
- Savoia C, Volpe M. Angiotensin receptor modulation and cardiovascular remodeling. J Renin Angiotensin Aldosterone Syst 2011;12:381-4. [PubMed]
- Gosse P, Jan E, Coulon P, et al. ECG detection of left ventricular hypertrophy: the simpler, the better? J Hypertens 2012;30:990-6. [PubMed]
- Fiebeler A, Schmidt F, Müller DN, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 2001;37:787-93. [PubMed]
- Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899-911. [PubMed]
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757-64. [PubMed]
- Liu L, Wang JG, Gong L, et al. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998;16:1823-9. [PubMed]
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991;265:3255-64. [PubMed]
- Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887-98. [PubMed]
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003. [PubMed]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981-97. [PubMed]
- Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895-906. [PubMed]
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022-31. [PubMed]
- ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-59. [PubMed]
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174-83.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805-16. [PubMed]
- Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782-8. [PubMed]
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. [PubMed]
- Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008;359:1225-37. [PubMed]
- DREAM Trial Investigators, Bosch J, Yusuf S, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551-62. [PubMed]
- NAVIGATOR Study Group, McMurray JJ, Holman RR, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477-90. [PubMed]
- Ramsay LE, Williams B, Johnston GD, et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ 1999;319:630-5. [PubMed]
- European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003;21:1011-53. [PubMed]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [PubMed]
- Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634-40. [PubMed]
- Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 2007;25:1751-62. [PubMed]
- Leonetti G, Cuspidi C, Facchini M. Antihypertensive therapy in the elderly: results of large trials. Ital Heart J Suppl 2001;2:1161-9. [PubMed]
- Zanchetti A, Mancia G, Black HR, et al. Facts and fallacies of blood pressure control in recent trials: implications in the management of patients with hypertension. J Hypertens 2009;27:673-9. [PubMed]
- ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-85. [PubMed]
- Patel A; ADVANCE Collaborative Group, MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829-40. [PubMed]
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010;56:196-202. [PubMed]
- Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-4. [PubMed]
- Somes GW, Pahor M, Shorr RI, et al. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1999;159:2004-9. [PubMed]
- Fagard RH, Staessen JA, Thijs L, et al. On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med 2007;167:1884-91. [PubMed]
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884-93. [PubMed]
- Zanchetti A, Julius S, Kjeldsen S, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial. J Hypertens 2006;24:2163-8. [PubMed]
- Sleight P, Redon J, Verdecchia P, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens 2009;27:1360-9. [PubMed]
- Bangalore S, Messerli FH, Wun CC, et al. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 2010;31:2897-908. [PubMed]
- Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907-17. [PubMed]
- 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-83. [PubMed]
- Zanchetti A, Grassi G, Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens 2009;27:923-34. [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219. [PubMed]
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [PubMed]
- Khan NA, McAlister FA, Campbell NR, et al. The 2004 Canadian recommendations for the management of hypertension: Part II--Therapy. Can J Cardiol 2004;20:41-54. [PubMed]
- Hackam DG, Khan NA, Hemmelgarn BR, et al. The 2010 Canadian Hypertension Education Program recommendations for the management of hypertension: part 2 - therapy. Can J Cardiol 2010;26:249-58. [PubMed]
- Rabi DM, Daskalopoulou SS, Padwal RS, et al. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 2011;27:415-433.e1-2.
- Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 2012;28:270-87. [PubMed]
- Hackam DG, Quinn RR, Ravani P, et al. The 2013 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2013;29:528-42. [PubMed]
- Cifkova R, Erdine S, Fagard R, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens 2003;21:1779-86. [PubMed]
- Volpe M, de la Sierra A, Kreutz R, et al. ARB-based single-pill platform to guide a practical therapeutic approach to hypertensive patients. High Blood Press Cardiovasc Prev 2014;21:137-47. [PubMed]


