Boosting autophagy in the diabetic heart: a translational perspective
Introduction
Despite the great advances in both primary and secondary prevention, cardiovascular diseases still represent the main cause of death in Western countries (1). Metabolic derangements such as diabetes, obesity, and dyslipidemia are critical factors that directly promote the development of coronary artery disease, stroke, and heart failure and dramatically affect the prognosis of subjects affected by cardiovascular diseases (1).
These metabolic abnormalities are frequently associated in the context of metabolic syndrome. Metabolic syndrome has a significant impact on the general population with a prevalence ranging from 25% to 35% (1,2). Metabolic syndrome and diabetes promote atherosclerotic plaque formation and rupture; they promote changes in cardiac geometry and functional abnormalities such as cardiac hypertrophy and diastolic dysfunction; they increase the cardiac susceptibility to ischemia and maladaptive remodeling; they are independently associated with the incidence of heart failure (1,2). At the molecular level, metabolic syndrome and diabetes induce oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis (3). These abnormalities are responsible for endothelial dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis and dysfunction associated with metabolic syndrome and diabetes (3). This evidence strongly indicates that, aside from the battle to improve primary prevention critical for the reduction of metabolic syndrome and diabetes incidence, which unfortunately in many cases is lost, it is crucial to develop new therapeutic strategies aimed at reducing the cellular abnormalities induced by these morbid conditions. However, this can be possible only if we better elucidate the mechanisms through which obesity, dyslipidemia, and hyperglycemia induce cellular abnormalities and dysfunction.
Accumulating lines of evidence indicate that obesity, diabetes, and metabolic syndrome are associated with a significant impairment of autophagy in multiple organs (4-13). Autophagy is an evolutionarily conserved cellular mechanism for degradation of old damaged proteins and organelles through their sequestration by double membrane vesicles called autophagosomes that subsequently deliver their content to lysosomes for final digestion (14). Autophagy is a beneficial mechanism for cellular homeostasis by guaranteeing the normal turnover of mitochondria and protein and by providing new substrates for protein synthesis and energy production (14,15). Autophagy is also involved in the physiological regulation of metabolic processes (4-11,14,15). In both genetic and dietary models of obesity, autophagy is inhibited in the liver thereby promoting ER stress and insulin resistance (4,6). Autophagy defects in pancreatic β-cells contribute to the transition from obesity to diabetes (7,16). Autophagy is also reduced in the mediobasal hypothalamus of obese animals (9). Impairment of hypothalamic autophagy was found to promote increased food intake, to reduce energy expenditure and to exacerbate the progression of obesity and whole-body insulin resistance (9). Autophagy is also impaired in macrophages during obesity, leading to an increased immune response and tissue inflammation (8). Autophagy was also found to be inhibited in the adipose tissue by obesity, although other works conflicted with this finding by demonstrating that autophagy is activated in the adipose tissue in the presence of metabolic abnormalities, where it favors adipogenesis (11). In addition, proteinuria-induced autophagy is impaired in the kidneys of obese animals thereby worsening proximal tubule damage (10).
Multiple studies in recent years have investigated whether obesity, diabetes, and metabolic syndrome affect autophagy in the heart both in unstressed and stressed conditions (12,13,17-32). Although it is still debated whether these conditions affect more the autophagosome formation with respect to the autophagic flux or vice versa (18,19), the most relevant information emerging from the majority of these studies is that cardiac autophagy is inhibited by metabolic derangements (12,13,17,18-29). Autophagy defects are associated with cardiac abnormalities and increased susceptibility to cardiac stress in the presence of obesity and reactivation of autophagy appears to be cardioprotective (12,13,19,23,28). This evidence opens up new scenarios where autophagy reactivation may represent a potential future therapeutic intervention to reduce cardiac abnormalities in patients with metabolic abnormalities.
All these aspects will be discussed in our review article together with the potential ways to reactivate autophagy in the context of obesity, metabolic syndrome, and diabetes.
The impact of obesity and diabetes on cardiac autophagy
In recent years, numerous studies have clarified the role of autophagy in the heart (17,33-35). Autophagy is required for the maintenance of cardiac function and structure at baseline (17,33-35). In addition, autophagy is progressively inhibited during aging, and such decline of autophagy is paralleled by the development of aging-induced cardiac abnormalities (17,33-35). Autophagy also plays a critical role in the regulation of cardiomyocyte survival and death during stress (17,33-35). Although high activation of autophagy is detrimental in specific cardiac stress conditions, autophagy is usually an adaptive mechanism regulating the cardiac response to stress. Autophagy is rapidly activated during myocardial ischemia and starvation where it reduces infarct size by limiting the ATP reduction and ER stress and by favoring mitochondrial turnover (13,36). A similar protective effect is observed in response to chronic myocardial infarction and pressure overload, where autophagy offsets cardiac remodeling and dysfunction in response to these stresses (37,38). Autophagy is also a protective mechanism in cardiac diseases caused by the accumulation of misfolded proteins, where autophagy limits ER stress (39). Overall, this evidence strongly indicates that autophagy is a critical protective mechanism during cardiac stress. Therefore, defects of this pro-survival mechanism would affect both cardiac homeostasis and response to stress. This was confirmed by a recent study that demonstrated that autophagy activation is inversely correlated with mortality in patients undergoing cardiac surgery (40).
Obesity, diabetes, and metabolic syndrome are morbid conditions associated with an increased incidence of cardiovascular diseases (1-3). They favor the development of endothelial dysfunction and atherosclerosis, thereby leading to myocardial infarction and stroke. In addition, these conditions directly affect cardiac geometry and function, leading to cardiac hypertrophy and fibrosis, cardiac dysfunction, and heart failure (1-3,41,42). All these abnormalities are associated with oxidative stress, mitochondrial dysfunction, and ER stress (43). In addition, obesity and metabolic syndrome increase the susceptibility to myocardial ischemia, thereby suggesting a defect in the cardiomyocyte’s ability to respond to energy stress (44-46). In recent years, numerous studies investigated whether all these defects induced by metabolic derangements are associated with abnormalities of cardiac autophagy (12,13,17-32). These studies employed different dietary and genetic models of obesity, metabolic syndrome, and both type I and type II diabetes. Although autophagosome accumulation was studied through different markers in these studies, in most of the cases the ability of autophagosomes to properly fuse with lysosomes (autophagic flux) was not investigated and this aspect led to some differences in the interpretation of the results. In fact, autophagosome accumulation may be a consequence either of an increased formation or a reduced clearance, and this aspect can be clarified only by specifically assessing the flux (47). The most important and relevant information that can be extrapolated from all these studies is that autophagy is clearly inhibited in the heart in the context of obesity and diabetes (12,13,17-29). Metabolic abnormalities can affect both the formation of autophagosomes and flux depending upon the animal model, the duration of the disease, and experimental conditions.
Cardiac autophagy is impaired in animals with diet-induced insulin resistance, metabolic syndrome, and type II diabetes. We found that high-fat diet (HFD)-induced obesity and metabolic syndrome impair cardiac autophagy at baseline and after myocardial ischemia, as indicated by a reduced number of autophagosomes with and without lysosomal inhibitors (GFP-LC3 puncta) (13). Autophagy inhibition by obesity increased the susceptibility to myocardial ischemia. Consistent with our results, He et al. showed inhibition of autophagy by HFD, and data from Mentzer’s group also confirmed a reduction in autophagosome number (mCherry-LC3 puncta) in the hearts of mice fed a similar type of HFD (60% of calories from fat), thus indicating reduced autophagosome formation (23,47). Recent studies also showed reduced LC3-II levels and increased p62 accumulation in mice with HFD-induced obesity, again indicating reduced autophagosome formation (18,21,48,49). In these studies autophagy inhibition by HFD was exacerbated by adiponectin deletion, whereas it was rescued by macrophage migration inhibitory factor (MIF) knockout and catalase overexpression. This data was also confirmed in large animals. Ossabaw pigs with metabolic syndrome induced by an atherogenic diet also displayed reduced cardiac autophagy, as indicated by reduced levels of LC3-II and Beclin-1, which paralleled the development of cardiac abnormalities and dysfunction (24). Similarly, hypercholesterolemia in Yucatan pigs fed high cholesterol diet for 4 weeks inhibited LC3-II/I ratio and cardiac tolerance to myocardial ischemia (25).
Interestingly, HFD was also shown to inhibit autophagy by affecting the fusion of autophagosomes with lysosomes. In mice fed with HFD (45% of calories from fat) for 12 weeks and fasted for 6 hours before all the analyses, LC3-II and p62 levels in the heart were found to be increased with respect to controls (22). Interestingly, LC3-II and p62 levels increased after lysosome inhibition in mice fed with control diet, whereas they did not further increase in HFD mice. These results indicate a defect in autophagic flux and confirm the study by Xu et al. demonstrating an impairment of autophagic flux in HFD mice, which can be rescued by Akt2 gene deletion (19). In addition, this evidence corroborates the study by Mellor et al., which for the first time reported an accumulation of LC3-II and p62 in the hearts of mice with type II diabetes induced by high-fructose diet (30).
Of note, two recent studies from Ren’s group showed that under a similar regimen of HFD, cardiac LC3-II levels can be either decreased or increased as a consequence of autophagy inhibition (18,19). We speculate that these works may suggest that HFD affects both autophagosome formation and flux at the same time. Whether a reduction of autophagosome formation or an impairment of flux is the most prominent defect of cardiac autophagy induced by HFD may depend on the duration and type of the diet, the severity of obesity and associated metabolic abnormalities, gender and age, degree of cardiac hypertrophy and dysfunction, mouse strain, circadian factors, and different experimental conditions. Interestingly, chronic flux inhibition may transcriptionally inhibit LC3 (47), thereby suggesting a secondary inhibition of autophagosome formation and, in general, a tight relationship between autophagosome formation and clearance.
Cardiac autophagy is also impaired in mice with type I diabetes. In mice with streptozotocin-induced type I diabetes and in OVE26 diabetic mice, cardiac autophagosome production, as indicated by LC3-II levels, is significantly decreased with and without lysosome inhibitors (26-28). Autophagic flux was also found to be inhibited in mice with streptozotocin-induced diabetes, through inhibition of SIRT1 and RAB7 (29).
The main mechanism through which autophagy is impaired in the hearts of mice with obesity and diabetes is the activation of mTOR signaling, a strong negative regulator of autophagy (13,18,19,21,24,48,50). mTORC1 was found to be activated by HFD and in models of diabetes. We found a deregulated Rheb/TORC1 activation in the hearts of mice fed HFD that is responsible for autophagy suppression (13). AKT2 activation was also found to be activated by HFD and to promote mTOR activation and autophagy inhibition (19). AMPK, a negative regulator of mTOR, is suppressed in the hearts of diabetic mice (13,18,19,21,26-29,48). Of note, inhibition of AMPK was also found to inhibit autophagy in these animals through an increased interaction between Beclin-1 and BCL-2 (28), thereby confirming our previous evidence demonstrating that MST1-dependent Beclin-1-BCL-2 interaction negatively regulates autophagy and cardiomyocyte survival (37). SIRT1, a protein deacetylase known to promote autophagy, was also found to be inhibited by diabetes (24,29). Lipids can affect autophagic flux through a superoxide-dependent impairment of lysosomal acidification (22). Interestingly, Rodriguez-Navarro’s group previously showed that lipotoxicity may also directly affect chaperone-mediated autophagy, an alternative form of protein degradation (51).
Potential therapeutic efficacy of autophagy reactivation for the treatment of diabetes-induced cardiovascular abnormalities
Obesity and diabetes are associated with the development of cardiovascular abnormalities (1-3). Recent work consistently demonstrated that cardiac autophagy is inhibited in these two conditions (12,13,17-29). The most intriguing and relevant aspect of this evidence is whether reactivation of autophagy may prevent or reduce the cardiovascular abnormalities induced by metabolic derangements. Recent studies suggest that this may be true. Reactivation of autophagy in mice with type I and II diabetes improved cardiac function and reduced cardiac abnormalities (27-29), although a recent study from Liang’s group, which was conducted on mice with type I diabetes, conflicted with this notion (26). Restoration of autophagic flux by Akt2 deletion and reactivation of autophagosome formation by Mif knockout also improved cardiac function in mice fed HFD (18,19). We found that rapamycin treatment restored autophagy and improved the myocardial tolerance to ischemia in mice with HFD-induced obesity and metabolic syndrome (13). The protective effects of rapamycin were lost in the presence of autophagy disruption. This evidence, coupled with recent results obtained in other organs where autophagy reactivation reduced diabetes-induced abnormalities (4-6), supports the idea that boosting autophagy may be a therapeutic intervention in patients with diabetes.
Several approaches might be suitable for activating cardiac autophagy in patients with metabolic abnormalities (Figure 1). As mentioned before, mTORC1 signaling is activated in the heart and vasculature of mice with obesity and diabetes (13,18,19,21,24,48,50,52). When it is hyperactivated, mTORC1 signaling is detrimental by inducing cardiac hypertrophy and cellular senescence and by reducing autophagy (53). mTORC1 inhibition was shown to reduce diabetic cardiomyopathy, HFD-induced increases in infarct size after both myocardial and cerebral ischemia, and endothelial cell senescence (13,50,52). This suggests that mTOR inhibitors might be appropriate for autophagy reactivation and treatment of diabetes-induced cardiovascular abnormalities. Rapamycin is the most studied mTOR inhibitor and there is a large amount of evidence in the literature indicating its beneficial effect against chronic maladaptive cardiac remodeling, hypertrophy and acute ischemia (53). However, rapamycin might not be an ideal drug for chronic treatment, particularly in patients with diabetes, since it can disrupt the mTORC2, which promotes cardiomyocyte survival, and would aggravate the insulin resistance status (54). A recent work from Sussman’s group demonstrated that selective mTORC1 inhibition by PRAS40 reduces diabetic cardiomyopathy and improves metabolic status and insulin sensitivity in mice with HFD-induced diabetes (50). This suggests that a selective mTORC1 inhibition by PRAS40, or potentially by Astrin, an mTORC1 inhibitor (55) or by Rheb inhibition (13), could be an appropriate way to treat cardiac abnormalities induced by metabolic derangements.
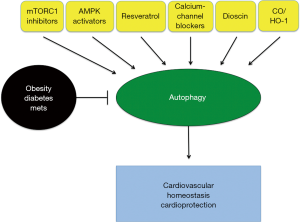
AMPK activators may also represent an alternative approach to reactivate autophagy in the diabetic heart. AMPK was found to be inhibited in the hearts of mice with both genetically and dietarily induced diabetes (13,18,19,21,26-29,48). AMPK is a positive regulator of autophagy by inhibiting the mTORC1 pathway, by directly phosphorylating ULK1, or by modulating the Beclin-1-BCL-2 interaction (28,56). In mice with type I diabetes, reactivation of AMPK by metformin rescued cardiac autophagy and diabetes-induced cardiac anomalies. The beneficial effects of metformin on cardiomyocytes exposed to hyperglycemia were lost in the presence of autophagy disruption (28). Metformin is probably the best candidate to activate autophagy in the context of diabetes, particularly of type II diabetes, since metformin can also improve insulin sensitivity and lower glycemia at the same time. Interestingly, a recent study demonstrated that catalase overexpression rescues HFD-induced suppression of autophagy by reactivating AMPK (21). These results are supported by our previous work showing that ROS oxidize and inhibit AMPK, whereas thioredoxin-1 preserves AMPK activation by reducing it (57). Notably, physiological levels of ROS are critical for autophagy activation during physiological processes, and catalase was previously shown to inhibit autophagy (58). It is possible that exaggerated and pathological levels of ROS in the hearts of mice with obesity and diabetes exert a paradoxical effect on autophagy by inhibiting it.
AMPK was previously shown to regulate SIRT1 activity (55). SIRT1 activation is also another possible approach to rescue autophagy in the presence of metabolic abnormalities (24,29). SIRT1 was found to be inhibited in the heart by both type I and type II diabetes. We previously found that SIRT1 promotes autophagy by activating the FOXO1/RAB7 pathway (59). Resveratrol, a SIRT1 activator, was previously found to reactivate autophagic flux in mice with type I diabetes and to reduce cardiac dysfunction, oxidative stress, and cardiomyocyte apoptosis (29).
Statins may also be appropriate for reducing cholesterol and increasing autophagy at the same time. In fact, previous work demonstrated that statin treatment induces mitophagy, a cargo-specific form of autophagy that specifically degrades mitochondria (60), and reduces ischemic injury (61). In addition, atorvastatin was previously found to reactivate cardiac autophagy in a pig model of metabolic syndrome (62).
A recent study demonstrated that HFD-induced obesity and diabetes lead to a cellular accumulation of calcium in the cytosol of hepatocytes, thereby leading to an impairment of autophagosome-lysosome fusion (4). Interestingly, this defect could be rescued by the calcium-channel blocker verapamil, thereby suggesting that this drug may also be suitable for autophagy reactivation in metabolic syndrome.
Dioscin was recently found to be a strong reactivator of autophagy in the liver of obese mice, thereby reducing oxidative stress and lipotoxicity (5). Soluble epoxide hydrolase inhibitor was also found to promote autophagy and inhibit ER stress in the liver of fat-1 obese mice (63).
Treatment with carbon monoxide (CO)-releasing agents can also promote autophagy in the hearts of mice fed HFD and rescue cardiac function by improving mitochondrial function (32). In fact, at low doses, CO is known to promote physiological effects and improve the metabolic status. In support of this evidence, overexpression of heme oxygenase-1, which promotes CO production, has recently been found to activate autophagy, inhibit apoptosis and oxidative stress, and rescue cardiac abnormalities and dysfunction in streptozotocin-induced diabetes (64).
Finally, physical exercise is an important therapeutic intervention to treat metabolic syndrome. A recent work from Levine’s group showed that exercise activates autophagy in the skeletal muscle of mice fed HFD by disrupting the Beclin-1-BCL-2 interaction. Exercise improved glucose tolerance in obese mice, and this effect was lost in mice with a defect in exercise-induced autophagy. This data suggests that physical exercise may be a valid option to improve the glucose tolerance of subjects with metabolic syndrome and to reactivate autophagy at the same time.
Perspectives
Numerous lines of evidence indicate that autophagy is inhibited in the presence of obesity, diabetes, and metabolic syndrome. Metabolic abnormalities appear to affect both autophagosome formation and autophagic flux. Future studies are required to understand how different metabolic abnormalities, such as obesity, hyperglycemia, and dyslipidemia impact the autophagic machinery and through which mechanisms. This would allow the development of specific strategies to reactivate autophagy in the presence of different combinations of metabolic abnormalities. Currently, the most promising therapeutic approach to rescue the autophagy suppression by metabolic abnormalities in the cardiovascular system is represented by mTORC1 inhibition. Metformin and statins are also quite appropriate because they can improve the metabolic status and increase autophagy at the same time. Ideally, molecules promoting autophagy by interfering specifically with the autophagic machinery would be the most appropriate agents to reactivate autophagy in the presence of metabolic abnormalities, so that they could be used in combination with the other antidiabetic and lipid-lowering drugs. In this regard, Levine’s group has recently developed a small peptide able to activate autophagy by interacting with the negative regulator of autophagy, GAPR-1 (65). Finally, future studies are encouraged to investigate how metabolic abnormalities specifically affect cargo-specific forms of autophagy, particularly mitophagy. The impact of metabolic derangements on mitochondrial fusion and fission also needs to be clarified since mitochondrial dynamics and autophagy affect each other, as we recently demonstrated (66).
Acknowledgements
The authors wish to thank Christopher D. Brady and Daniela Zablocki for critical reading of the manuscript and suggestions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence. SS was supported by a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association, and partially by a grant from the Italian Society of Cardiology and Italian Society of Hypertension, and is currently supported by a grant from the NJ Commission on Cancer Research.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. [PubMed]
- Eckel RH, Alberti KG, Grundy SM, et al. The metabolic syndrome. Lancet 2010;375:181-3. [PubMed]
- Stump CS, Clark SE, Sowers JR. Oxidative stress in insulin-resistant conditions: cardiovascular implications. Treat Endocrinol 2005;4:343-51. [PubMed]
- Park HW, Park H, Semple IA, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun 2014;5:4834. [PubMed]
- Liu M, Xu L, Yin L, et al. Potent effects of dioscin against obesity in mice. Sci Rep 2015;5:7973. [PubMed]
- Yang L, Li P, Fu S, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 2010;11:467-78. [PubMed]
- Lim YM, Lim H, Hur KY, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun 2014;5:4934. [PubMed]
- Liu K, Zhao E, Ilyas G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015;11:271-84. [PubMed]
- Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem 2011;286:32324-32. [PubMed]
- Yamahara K, Kume S, Koya D, et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol 2013;24:1769-81. [PubMed]
- Stienstra R, Haim Y, Riahi Y, et al. Autophagy in adipose tissue and the beta cell: implications for obesity and diabetes. Diabetologia 2014;57:1505-16. [PubMed]
- Sciarretta S, Volpe M, Sadoshima J. Is reactivation of autophagy a possible therapeutic solution for obesity and metabolic syndrome? Autophagy 2012;8:1252-4. [PubMed]
- Sciarretta S, Zhai P, Shao D, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 2012;125:1134-46. [PubMed]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27-42. [PubMed]
- Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab 2011;13:495-504. [PubMed]
- Lee MS. Role of islet β cell autophagy in the pathogenesis of diabetes. Trends Endocrinol Metab 2014;25:620-7. [PubMed]
- Sciarretta S, Yee D, Shenoy V, et al. The importance of autophagy in cardioprotection. High Blood Press Cardiovasc Prev 2014;21:21-8. [PubMed]
- Xu X, Ren J. Macrophage migration inhibitory factor (MIF) knockout preserves cardiac homeostasis through alleviating Akt-mediated myocardial autophagy suppression in high-fat diet-induced obesity. Int J Obes (Lond) 2015;39:387-96. [PubMed]
- Xu X, Hua Y, Nair S, et al. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol 2013;5:61-3. [PubMed]
- Guo R, Ren J. Deficiency in AMPK attenuates ethanol-induced cardiac contractile dysfunction through inhibition of autophagosome formation. Cardiovasc Res 2012;94:480-91. [PubMed]
- Liang L, Shou XL, Zhao HK, et al. Antioxidant catalase rescues against high fat diet-induced cardiac dysfunction via an IKKβ-AMPK-dependent regulation of autophagy. Biochim Biophys Acta 2015;1852:343-52.
- Jaishy B, Zhang Q, Chung HS, et al. Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J Lipid Res 2015;56:546-61. [PubMed]
- He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012;481:511-5. [PubMed]
- Li ZL, Woollard JR, Ebrahimi B, et al. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol 2012;32:1132-41. [PubMed]
- Glazer HP, Osipov RM, Clements RT, et al. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle 2009;8:1738-46. [PubMed]
- Xu X, Kobayashi S, Chen K, et al. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 2013;288:18077-92. [PubMed]
- Xie Z, He C, Zou MH. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 2011;7:1254-5. [PubMed]
- He C, Zhu H, Li H, et al. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013;62:1270-81. [PubMed]
- Wang B, Yang Q, Sun YY, et al. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med 2014;18:1599-611. [PubMed]
- Mellor KM, Bell JR, Young MJ, et al. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 2011;50:1035-43. [PubMed]
- Russo SB, Baicu CF, Van Laer A, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 2012;122:3919-30. [PubMed]
- Lancel S, Montaigne D, Marechal X, et al. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS One 2012;7:e41836. [PubMed]
- Sciarretta S, Zhai P, Volpe M, et al. Pharmacological modulation of autophagy during cardiac stress. J Cardiovasc Pharmacol 2012;60:235-41. [PubMed]
- Nishida K, Kyoi S, Yamaguchi O, et al. The role of autophagy in the heart. Cell Death Differ 2009;16:31-8. [PubMed]
- Ikeda Y, Sciarretta S, Nagarajan N, et al. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid Med Cell Longev 2014;2014:210934.
- Sciarretta S, Zhai P, Shao D, et al. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res 2013;113:1253-64. [PubMed]
- Maejima Y, Kyoi S, Zhai P, et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med 2013;19:1478-88. [PubMed]
- Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007;13:619-24. [PubMed]
- Bhuiyan MS, Pattison JS, Osinska H, et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest 2013;123:5284-97. [PubMed]
- Jahania SM, Sengstock D, Vaitkevicius P, et al. Activation of the homeostatic intracellular repair response during cardiac surgery. J Am Coll Surg 2013;216:719-26; discussion 726-9. [PubMed]
- Paneni F, Costantino S, Battista R, et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet 2015;8:150-8. [PubMed]
- Sciarretta S, Ferrucci A, Ciavarella GM, et al. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 2007;20:784-91. [PubMed]
- Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep 2014;16:419. [PubMed]
- Clavijo LC, Pinto TL, Kuchulakanti PK, et al. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in-hospital complications. Cardiovasc Revasc Med 2006;7:7-11. [PubMed]
- Zeller M, Steg PG, Ravisy J, et al. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med 2005;165:1192-8. [PubMed]
- Abdulla J, Køber L, Abildstrøm SZ, et al. Impact of obesity as a mortality predictor in high-risk patients with myocardial infarction or chronic heart failure: a pooled analysis of five registries. Eur Heart J 2008;29:594-601. [PubMed]
- Gottlieb RA, Andres AM, Sin J, et al. Untangling autophagy measurements: all fluxed up. Circ Res 2015;116:504-14. [PubMed]
- Guo R, Zhang Y, Turdi S, et al. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 2013;1832:1136-48.
- Wang Z, Li L, Zhao H, et al. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism 2015;64:917-25. [PubMed]
- Völkers M, Doroudgar S, Nguyen N, et al. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med 2014;6:57-65. [PubMed]
- Rodriguez-Navarro JA, Kaushik S, Koga H, et al. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc Natl Acad Sci U S A 2012;109:E705-14. [PubMed]
- Wang CY, Kim HH, Hiroi Y, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal 2009;2:ra11. [PubMed]
- Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 2014;114:549-64. [PubMed]
- Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012;335:1638-43. [PubMed]
- Thedieck K, Holzwarth B, Prentzell MT, et al. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell 2013;154:859-74. [PubMed]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016-23. [PubMed]
- Shao D, Oka S, Liu T, et al. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab 2014;19:232-45. [PubMed]
- Sciarretta S, Yee D, Ammann P, et al. Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes. Clin Sci (Lond) 2015;128:387-403. [PubMed]
- Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res 2010;107:1470-82. [PubMed]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011;12:9-14. [PubMed]
- Andres AM, Hernandez G, Lee P, et al. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal 2014;21:1960-73. [PubMed]
- Sabe AA, Elmadhun NY, Sadek AA, et al. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in Ossabaw swine with metabolic syndrome. J Thorac Cardiovasc Surg 2014;148:3172-8. [PubMed]
- López-Vicario C, Alcaraz-Quiles J, García-Alonso V, et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A 2015;112:536-41. [PubMed]
- Zhao Y, Zhang L, Qiao Y, et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One 2013;8:e75927. [PubMed]
- Shoji-Kawata S, Sumpter R, Leveno M, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 2013;494:201-6. [PubMed]
- Ikeda Y, Shirakabe A, Maejima Y, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 2015;116:264-78. [PubMed]


