DPP-4 inhibitors, heart failure and type 2 diabetes: all eyes on safety
Heart failure (HF) burden in people with type 2 diabetes (T2D)
HF is a frequent condition occurring in patients with diabetes (DM) (1,2). Diabetic men and women display a 6 to 8-fold increase in the prevalence of HF, with the highest number of cases observed in the latter group (3). Among patients with HF, 15-26% has DM, a condition which amplifies morbidity and mortality (4-6). The recent ATLAS study showed that the proportion of DM patients with HF may reach 20% (7). HF patients with concomitant DM have a further increase in morbidity and mortality due to coexistence of several mechanisms including disturbed neurohormonal axis as well as structural and functional abnormalities occurring in the diabetic myocardium (8). About one fifth of patients with chronic HF has DM, and such prevalence reaches 40% for patients with worsening HF (5). T2D individuals are at increased risk for both HF with preserved (HFPEF) and reduced (HFREF) ejection fraction (9). Notably, prospective analyses have shown that the prognosis of HFPEF is comparable to the one reported for HFREF patients, with a 50-60% mortality rate after 5 years (10). Recent data show that diabetic HFPEF patients display increased HF hospitalization or HF death as compared to non-DM subjects (30.9% vs. 19.0%, respectively), with an estimated 68% increased risk after adjusting for relevant confounders (adjusted HR, 1.68; 95% CI: 1.26-2.25, P<0.001) (11). Another important aspect to be considered is that the prognosis of DM patients with HF remains worse even though these patients are receiving care that is similar to non-DM people (5). This may be explained by the fact that in DM patients, hyperglycemia and insulin resistance may significantly amplify microvascular disease, defects of intracellular calcium handling as well as reduced myocardial lipid uptake leading to metabolic disturbances, mitochondrial insufficiency and severe myocyte dysfunction (8).
Does glycemic control reduce the risk of HF in diabetic patients?
Several studies have shown that poor glycemic control, as indicated by glycated haemoglobin (HbA1c) levels, is associated with an increased risk of HF. The UKPDS study which included patients with newly diagnosed DM, showed a significant association between long-term glycemic control and HF risk. In this study, the adjusted rate of HF was reduced to 2.3 events/100 person-years in those with HbA1c levels <6%, from 11.9 in those whose HbA1c levels were >10%, with a near linear relationship between lowering of HbA1c and risk of HF (12). Unfortunately, these data could not be confirmed by the recent randomized controlled trials ACCORD, ADVANCE and VADT which tested the effects of intensive glycemic control on micro and macrovascular outcomes (13). In these studies, the achievement of target HbA1c values (<7%) was not associated with a reduction of HF-related hospitalizations. In line with such disappointing results, a recent meta-analysis including 7 randomized controlled trials with a total of 37,229 patients showed that the risk of HF-related events did not differ significantly between intensive glycemic control and standard treatment (OR 1.20, 95% CI 0.96-1.48), tough effect estimate was highly heterogeneous (14). Indeed, among the 4 trials that had a high rate of thiazolidinediones use (i.e., PROactive, ACCORD, VADT, and RECORD), the risk of HF was elevated in individuals randomized to intensive blood glucose control. On the other hand, among the remaining 3 trials (i.e., UKPDS, ADVANCE, and VA-CSDM), the risk ratio was close to null with a wide CI, highlighting the limitedness of the available data (risk ratio, 0.96; 95% CI: 0.81-1.13) (14).
It is therefore difficult to conclude that hyperglycemia may not be relevant in HF patients and further studies are needed to clarify this important issue. In contrast with this meta-analysis, an earlier cohort study including 25,958 men and 22,900 women with T2D demonstrated that each 1% increase in HbA1c was associated with an 8% increased risk of HF (95% CI: 5-12%) (15). In this study, an HbA1c ≥10, relative to HbA1c <10 was associated with 1.56-fold (95% CI: 1.26-1.93) greater risk of HF. Similarly, in the Reykjavik Study, a linear and independent relationship between increasing fasting plasma glucose and the development of HF was observed (16). More recently, in a clinical trial cohort of 531,546 subjects at high CV risk followed-up for a mean of 2.4 years, Held et al. showed that dysglycemia was an independent predictor of hospitalization for HF regardless of DM status (17). Collectively, these data suggest that (I) glycemic burden may be important for HF occurrence and hospitalization; and (II) choice of glucose lowering drugs may affect HF risk in DM patients.
The recent case of DPP-4 inhibitors
Available anti-diabetic drugs are effective for the management of hyperglycemia, however many DM patients have cardiovascular (CV) problems and attention should be paid to the risk/benefit ratio of the different formulations (18). Thiazolidinediones, namely rosiglitazione, are associated with a number of complications including bladder cancer, bone fractures, fluid retention with increased body weight, and last but not least, risk of HF (13). Moreover, insulin may induce fluid retention although it did not affect the risk of HF in the ORIGIN trial (19).
In the attempt to explore the effects of new anti-hyperglycemic therapies, recent randomized trials unveiled an increase in the risk of HF-hospitalizations in DM patients treated with the dipeptidyl-peptidase-4 (DDP-4) inhibitors (DPP4i) as compared to placebo (20). Before moving into the interpretation of clinical findings it is appropriate to mention how DPP-4i work and what is the experimental background supporting putative CV benefits of this class of drugs.
DPP-4i block the degradation of glucagon like peptide-1 (GLP-1), gastric inhibitory peptide (GIP) and a variety of other peptides including brain natriuretic peptide. Therefore, these drugs raise GLP-1/gastric inhibitory polypeptide levels resulting in enhancement of insulinotropic effects of glucose (21). Experimental evidence supports the notion that DPP-4 are important drivers of myocardial damage (22). Indeed, mice with genetic deletion of DDP-4 display longer survival rates following myocardial infarction (MI) (23,24). This observation was explained by increased cardiac expression of phosphorylated AKT (pAKT), pGSK3beta, and atrial natriuretic peptide (ANP) in Dpp4−/− hearts. Interestingly, treatment with the DPP-4i sitagliptin was able to recapitulate these molecular changes thereby protecting against myocardial ischemic damage (23). Accordingly, sitagliptin treatment in patients with or without T2D was associated with a significant recovery of the ischemic myocardium, as assessed by dobutamine stress echocardiography (25). Taken together, these data suggest that DDP-4 inhibition may represent an attractive mechanism-based approach fostering cardioprotective pathways in the failing and ischemic myocardium (21).
Lessons from SAVOR-TIMI 53 and EXAMINE trials
Current evidence suggests that such experimental findings are somehow “lost in translation”. Indeed, recent clinical trials which were launched to test CV effects of DPP-4i not only failed to show any DDP4-related CV benefit, but were even associated with an increase in HF hospitalizations (20).
In the SAVOR-TIMI 53 trial, 16,492 T2D patients who had a history of, or were at risk for, CV events, were randomized to receive saxagliptin or placebo and followed for a median of 2.1 years (Table 1) (26). A primary end-point event occurred in 613 patients in the saxagliptin group and in 609 patients in the placebo group (7.3% and 7.2%, respectively, HR, 1.00; 95% CI: 0.89-1.12; P=0.99 for superiority; P<0.001 for noninferiority). The major secondary end point of a composite of CV death, MI, stroke, hospitalization for unstable angina (UA), coronary revascularization, or HF occurred in 1,059 patients in the saxagliptin group and in 1,034 patients in the placebo group (12.8% and 12.4%, respectively, HR, 1.02; 95% CI: 0.94-1.11; P=0.66). Unexpectedly, more patients in the saxagliptin group than in the placebo group were hospitalized for HF (3.5% vs. 2.8%; HR, 1.27; 95% CI: 1.07-1.51; P=0.007) (26). Corresponding rates at 12 months were 1.9% vs. 1.3% (HR, 1.46; 95% CI: 1.15-1.88; P=0.002), with no significant difference thereafter (time-varying interaction, P=0.017). However, these findings were not paralleled by a concomitant increase in HF-related deaths in patients taking saxagliptin (44 and 40 cases in saxagliptin and placebo, respectively) (26). Subjects at greatest risk of HF hospitalization had previous HF, an estimated glomerular filtration rate ≤60 mL/min, or elevated baseline levels of N-terminal pro B-type natriuretic peptide. There was no evidence of heterogeneity between N-terminal pro B-type natriuretic peptide and saxagliptin (P for interaction =0.46), although the absolute risk excess for HF with saxagliptin was greatest in the top pro-BNP quartile (HR, 1.31; 95% CI: 1.04-1.66; P=0.02), whereas the lowest quartile was not associated with any treatment difference (0.6% and 0.6%, HR, 0.84; 95% CI: 0.46-1.52; P=0.56) (29). Another concern was represented by differences in glycemic status between groups. Although designed as a glycemic equipoise study, fasting plasma glucose as well as HbA1c significantly differed among patients allocated to saxagliptin and placebo. In line with this data, a larger number of minor (P=0.002) and major (P=0.047) hypoglycemic episodes were recorded in the saxagliptin arm (Table 1).
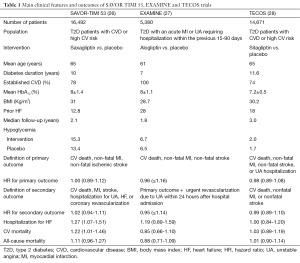
Full table
In the EXAMINE trial, 5,380 patients with T2D and acute coronary syndrome in the preceding 90 days, were randomized to treatment with the DPP-4i alogliptin or placebo (27). This double-blind, non-inferiority trial showed no differences in primary and secondary CV endpoints between alogliptin and placebo after a mean follow up of 4 years. In contrast with SAVOR-TIMI 53, this study did not show outcome differences as far as HF is concerned. There was a 7% non-significant increase in the alogliptin arm (3.1) vs. placebo group (2.9%). However, post-hoc analyses revealed that the rate of HF was increased in patients taking DPP-4i who had signs of HF (HR, 1.76; 95% CI: 1.07-2.90) (13,30). As observed in SAVOR-TIMI 53, data from EXAMINE confirmed that the risk of hospital admission for HF was highest among patients with BNP concentrations in the top quartile at baseline (30). The lack of statistical significance for HF hospitalization in EXAMINE is likely driven by a much smaller sample size as compared to SAVOR trial (5,380 vs. 16,492 patients) (31). Hence, the study might not be powered enough as far as secondary endpoint is concerned. Along this line, a recent meta-analysis including SAVOR and EXAMINE trials has confirmed a 25% increase of HF hospitalizations related to DPP-4i (32). On the whole, these data may suggest that the increased risk of HF hospitalization with DPP-4i is likely to be a concrete and previously unrecognized side effect. A putative mechanisms which may contribute to explain the increased risk of HF hospitalizations with DPP-4i is the alteration of several neurohormonal axes, namely substance P (SP) and neuropeptide Y (NP-Y) (28). A recent study conducted in patients with the metabolic syndrome, showed that during placebo and low-dose ACE inhibition (5 mg enalapril), sitagliptin lowered blood pressure (33). However, this trend was reversed in those patients taking ACE inhibitors at higher doses. These findings led the authors to postulate that in patients taking a combination of sitagliptin and high-dose ACE inhibition, high levels of SP may foster sympathetic tone activation, thereby attenuating blood pressure reduction (33). Indeed, SP and NP-Y are detrimental bioproducts of DPP-4 inhibition which may trigger sympathetic activity and, hence, HF worsening in T2D patients. Against this, the analysis of SAVOR-TIMI 53 trial showed that the risk of HF hospitalization was higher among T2D patients who were not on ACE inhibitors (HR, 1.42; 95% CI: 1.09-1.88) as compared to those who were taking this class of drugs (HR, 1.18; 95% CI: 0.94-1.48) (20). Longer duration and prospective studies are needed to prove these novel findings and effects.
All eyes on safety: the TECOS trial
Based on these safety concerns, international regulatory agencies have required that DPP-4i—as well as other new glucose lowering drugs—not only show glucose-lowering ability but also are not associated with clinically meaningful increases in rates of major adverse CV events. In this regard, the main concern is whether HF worsening is a class effect of all DPP-4i or is rather linked to specific molecules within this family. The answer to this important question came from the recent TECOS trial which was launched to assess non inferiority as well as long-term CV safety of adding sitagliptin to usual care, as compared with usual care alone, in 14,671 patients with T2D and established CVD (Table 1) (34). During a median follow-up of 3.0 years, the primary outcome occurred in 839 patients in the sitagliptin group (11.4%) and 851 patients in the placebo group (11.6%). Sitagliptin was non-inferior to placebo for the primary composite CV outcome (HR, 0.98; 95% CI: 0.88-1.09; P<0.001). In this trial, the intention to treat (ITT) analysis showed similar outcome rates as far as HF hospitalization is concerned (HR, 1.00; 95% CI: 0.83-1.20; P=0.98). In contrast with SAVOR and EXAMINE, TECOS analysis of HF-related outcomes and CV death was performed according to FDA safety standards within the ITT population. Again, this did not show any additional risk for diabetic patients receiving sitagliptin (HR, 1.02; 95% CI: 0.90-1.15; P=0.74). Moreover, the rate of all-cause mortality was comparable in the two arms. Of note, sitagliptin was not associated with a significant risk of hypoglycemia (HR, 1.12; 95% CI: 0.89-1.40) (34). The notion that sitagliptin does not adversely impact on HF hospitalizations was further strengthened by a very recent sub-analysis of TECOS, presented in London at the last Congress of the European Society of Cardiology (ESC). The initial TECOS findings, presented earlier this year at the American Diabetes Association, were adjusted to control for baseline HF. According to a press release from the ESC, new data from TECOS show unadjusted and adjusted analyses (also pre-specified) with identical results (HR, 1.00; 95% CI: 0.84-1.20; and HR, 1.02; 95% CI: 0.83-1.26) (35). The stability of these findings was confirmed across a very extensive set of complementary/sensitivity analyses—all yielding to the conclusion of no signal of any sort of HF risk with sitagliptin.
The implications of such analysis will be very important for endocrinologists, but also for cardiologists who see many patients with T2D and coronary heart disease treated with sitagliptin. Therefore, patients with T2D and CVD can safely take the anti-hyperglycemic drug sitagliptin without an increased risk of CV complications—even if they have a history of HF. These encouraging data strongly suggest that increased HF risk is not a class effect of DPP-4i. This postulate is strengthen by the results of observational studies showing a neutral effect or even a benefit of DPP-4i on HF-related outcomes (36,37). Nonetheless, further evidence is needed to draw solid conclusions on the safety of saxagliptin and alogliptin in people with T2D and CVD.
Open issues
An important clinical question to answer is how DPP4i perform when compared with other glucose-lowering agents as far as HF-related risk is concerned. A very recent retrospective study including 127,555 unmatched T2D patients extracted from a population of 18 million individuals reported data on patients who initiated treatment with DPP-4i, thiazolidinediones, or sulphonylureas alone or in combination with metformin (38). During an average 2.6-year follow-up, after adjusting for measured confounders, the use of DPP-4i was associated with a reduced risk of HF hospitalizations as compared with sulphonylureas (HR, 0.78; 95% CI: 0.62-0.97; P=0.026). Interestingly enough, DPP-4i remained associated with a lower risk of HF hospitalization even after propensity matching (HR, 0.70; 95% CI: 0.52-0.94; P=0.018). The 28-30% lower risk of HF hospitalization detected with DPP-4i may thus derive from a beneficial protective effect of DPP-4i or from a detrimental effect of sulphonylureas on HF. Indeed, sulphonylureas have been associated with an increased risk of HF as compared with metformin (39). On the whole, these results do not presume to demonstrate unequivocal benefits of DPP-4i vs. sulphonylureas, they rather provide a wide and insightful snapshot on how different anti-diabetic drugs may affect HF risk in a population-based registry. Undoubtedly, further research is warranted to further appraise the individual effect of different glucose-lowering drugs on HF-related outcomes.
Another aspect deserving attention relates to the identification of T2D patients who should receive therapy with DPP-4i in clinical practice. This is of particular importance in light of the very recent EMPA-REG trial, showing that an inhibitor of sodium-glucose cotransporter 2 (SGLT2) yielded to a 14% reduction of the primary end point of death from CV causes, non-fatal MI and non-fatal stroke (HR, 0.86; 95% CI: 0.74-0.99), a 38% reduction of CV mortality (HR, 0.62; 95% CI: 0.49-0.77) as well as a 35% reduction in HF hospitalizations (HR, 0.65; 95% CI: 0.50-0.85) in T2D patients with established CVD (40). For the first time, a DM trial clearly demonstrated that a glucose-lowering agent, namely the SGLT2 inhibitor empagliflozin, may profoundly affect the natural history of CV complications in patients with T2D. SGLT2 inhibitors reduce plasma glucose concentration by inhibiting renal glucose reabsorption and producing glucosuria (41). Given as either monotherapy or as an add-on therapy, these drugs are reported to reduce HbA1c levels in T2D patients, including those with stage 2 or 3a chronic kidney disease. However, T2D is a complex disorder involving multiple metabolic defects and its management often requires the use of combination therapies eventually yielding to an additive reduction in HbA1c levels (42). Over the last few years, several randomized trials have investigated whether the combination of DPP-4i and SGLT2 inhibitors would provide additional benefit as far as glycemic control is concerned. The rationale of using such combination is based on the notion that SGLT2-induced glycosuria causes a compensatory increase of endogenous glucagone levels which offsets their glucose-lowering effect by approximately 50%. On the other hand, DPP4i have the ability to suppress glucagone secretion thereby blunting endogenous production of the hormone. However, such synergic effects have been only partially demonstrated in T2D patients receiving a combination therapy with DPP-4i and SGLT2 inhibitors (43). In the recent study by Rosenstock et al. 534 poorly controlled, metformin-treated T2D patients were randomized to receive dapagliflozin alone, saxagliptin alone, or a combination of saxagliptin plus dapagliflozin over a period of 24 weeks (44). Saxagliptin and dapagliflozin lowered HbA1c levels by 0.88% and 1.2%, respectively, whereas their combination yielded to a 1.47% reduction, which is significantly lower than expected from this approach (43). Consistently, another recent study showed that reductions in HbA1c with the combination empagliflozin/linagliptin were superior to those with empagliflozin or linagliptin alone as add-on to metformin, even though the differences were not striking (45). Taken together, these findings suggest that, even if well tolerated, the overall additional benefit from the combination DPP-4i/SGLT2 is rather small and may raise concerns on the cost-effectiveness of such an approach. In other words, it is important to understand whether anti-hyperglycemic therapy with DPP-4i can be safely implemented in T2D patients taking empagliflozin. This concern particularly applies to those patients with HF or at high risk of developing this complication overtime.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879-84. [PubMed]
- Lüscher TF. Risk factors for and management of heart failure. Eur Heart J 2015;36:2267-9. [PubMed]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035-8. [PubMed]
- Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725-36. [PubMed]
- Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015;3:136-45. [PubMed]
- Lüscher TF. Heart failure and comorbidities: renal failure, diabetes, atrial fibrillation, and inflammation. Eur Heart J 2015;36:1415-7. [PubMed]
- Rydén L, Armstrong PW, Cleland JG, et al. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J 2000;21:1967-78. [PubMed]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213-23. [PubMed]
- MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008;29:1377-85. [PubMed]
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. [PubMed]
- Aguilar D, Deswal A, Ramasubbu K, et al. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol 2010;105:373-7. [PubMed]
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. [PubMed]
- Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015;385:2107-17. [PubMed]
- Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011;162:938-48.e2.
- Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001;103:2668-73. [PubMed]
- Thrainsdottir IS, Aspelund T, Hardarson T, et al. Glucose abnormalities and heart failure predict poor prognosis in the population-based Reykjavik Study. Eur J Cardiovasc Prev Rehabil 2005;12:465-71. [PubMed]
- Held C, Gerstein HC, Yusuf S, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007;115:1371-5. [PubMed]
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015;36:2288-96. [PubMed]
- ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319-28. [PubMed]
- Standl E, Erbach M, Schnell O. Dipeptidyl-peptidase-4 Inhibitors and Heart Failure: Class Effect, Substance-Specific Effect, or Chance Effect? Curr Treat Options Cardiovasc Med 2014;16:353. [PubMed]
- Zhong J, Goud A, Rajagopalan S. Glycemia Lowering and Risk for Heart Failure: Recent Evidence from Studies of Dipeptidyl Peptidase Inhibition. Circ Heart Fail 2015;8:819-25. [PubMed]
- Zhong J, Maiseyeu A, Davis SN, et al. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res 2015;116:1491-504. [PubMed]
- Sauvé M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 2010;59:1063-73. [PubMed]
- Connelly KA, Zhang Y, Advani A, et al. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovasc Ther 2013;31:259-67. [PubMed]
- Read PA, Khan FZ, Heck PM, et al. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging 2010;3:195-201. [PubMed]
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. [PubMed]
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. [PubMed]
- Sanon VP, Sanon S, Pham SV, et al. Play of Chance Versus Concerns Regarding Dipeptidyl Peptidase-4 Inhibitors: Heart Failure and Diabetes. Clin Diabetes 2014;32:121-6. [PubMed]
- Rajagopalan R, Rosenson RS, Fernandes AW, et al. Association between congestive heart failure and hospitalization in patients with type 2 diabetes mellitus receiving treatment with insulin or pioglitazone: a retrospective data analysis. Clin Ther 2004;26:1400-10. [PubMed]
- Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76. [PubMed]
- Standl E, Schnell O. DPP-4 inhibitors and risk of heart failure EXAMINEd. Lancet 2015;385:2022-4. [PubMed]
- Udell JA, Cavender MA, Bhatt DL, et al. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015;3:356-66. [PubMed]
- Marney A, Kunchakarra S, Byrne L, et al. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 2010;56:728-33. [PubMed]
- Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. [PubMed]
- Available online: http://www.escardio.org/The-ESC/Press-Office/Press-releases/Last-5-years/new-tecos-analysis-adds-heart-failure-data-for-sitagliptin
- Gejl M, Starup-Linde J, Scheel-Thomsen J, et al. Risk of cardiovascular disease: the effects of diabetes and anti-diabetic drugs - a nested case-control study. Int J Cardiol 2015;178:292-6. [PubMed]
- Velez M, Peterson EL, Wells K, et al. Association of antidiabetic medications targeting the glucagon-like peptide 1 pathway and heart failure events in patients with diabetes. J Card Fail 2015;21:2-8. [PubMed]
- Fadini GP, Avogaro A, Degli Esposti L, et al. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Eur Heart J 2015;36:2454-62. [PubMed]
- Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731. [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015. [Epub ahead of print]. [PubMed]
- Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015;12:78-89. [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96. [PubMed]
- Abdul-Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes? Diabetes Care 2015;38:373-5. [PubMed]
- Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015;38:376-83. [PubMed]
- DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384-93. [PubMed]


