Cardiac anomalies in a group of HIV-infected children in a pediatric hospital: an echocardiographic study in Yaounde, Cameroon
Introduction
In 2014, the World Health Organization (WHO) estimated the number of HIV-infected people worldwide to be about 34 million. Among these, 3.4 million (10%) are below the age of 15 years (1). In this group of children, cardiac abnormalities detected by echocardiography, even at the sub-clinical stage, constitute independent risk factors of early mortality. Early detection will aid the identification of affected children and give an opportunity for early intervention (2). The most frequently described abnormalities include: LV diastolic dysfunction, dilated cardiomyopathy, LV hypertrophy, LV systolic dysfunction, pulmonary hypertension, and pericardial effusion (3,4). These cardiac manifestations are not exclusively caused by the direct toxicity of the HIV on cardiac cells and others factors, such as immune reactions, nutritional deficiencies, opportunistic infections and antiretroviral (ARV) treatments are known to play a contributory role (4). In Uganda in 2003, Lubega et al. reported a prevalence of 50% for cardiac abnormalities in a population of 230 HIV-infected children (5). All the children in the study were naive to any treatment by ARV drugs. Ever since the introduction of highly active antiretroviral therapies (HAART), which are not without any cardiac risk, some studies have been carried out, with varying results (6-8). It is therefore pertinent to determine the prevalence of the cardiac abnormalities associated with HIV infection within the era of HAART.
The Mother and Child Center of the Chantal Biya Foundation is the pioneer in the management of the HIV infection in children in Cameroon, where an important cohort of children are regularly followed-up. The aim of our study was to determine the prevalence and the spectrum of the cardiac abnormalities detected by echocardiography in a group of children from this cohort, and to look for any associated clinical manifestations.
Methods
Type of study
It was a cross-sectional descriptive study, carried out over a period of 4 months (January to April 2014) during which demographic, clinical and echocardiographic data were collected from HIV-infected children followed-up at the Mother and Child healthcare Center of the Chantal Biya Foundation
Study site
The study was carried out at the out-patient clinic responsible for the follow-up of HIV-infected patients of the Mother and Child healthcare Center of the Chantal Biya Foundation, a public hospital located in the center of Yaoundé, a town with a population of about 2.5 million. The staff of the hospital include 15 pediatricians, 2 gynecologists, 20 general practitioners and 250 nurses. The activities of the center include out-patient clinics, in-patient paediatric care and antenatal services. The unit responsible for the management of HIV/AIDS was created in 2003. The medical staff of the unit is composed of two pediatricians, a public health medical practitioner, and two general practitioners. In 2014, this unit followed-up a cohort of more than 2,000 children among whom a half are on HAART. Out-patient consultations, baseline haematological and biochemical investigations (full blood count, hepatic transaminases, glycaemia, CD4 count), as well as the HAART, are free of charge.
Study population
This comprised children aged between 1 and 15 years followed-up in the unit with a confirmed diagnosis to the HIV infection and in whom, at least one parent had given an inform consent.
Data collection procedure
Data was collected by two young medical practitioners who visited the unit at least 3 days every week. Children who satisfied the inclusion criteria for the study were identified from the hospital records. The following information was collected on each patient; name, age, gender, age at diagnosis of the HIV infection, the clinical and immunological stages at diagnosis according to WHO classification (9). The results all laboratory investigations were also recorded: the hemoglobin level and the most recent CD4 count within 6 months of evaluation. The clinical examination aimed at identifying symptoms that could result from cardiovascular anomalies, including dyspnea, cough, and effort-induced tiredness. Physical signs suggestive of cardiac dysfunction were noted: cardiac murmur, arrhythmia, congestive liver, pedal edema, muffled heart sounds and digital clubbing. All has echocardiography.
Echocardiography
The transthoracic echocardiographic exploration was done with an echocardiograph Accuson Cypress SIEMENS, with two multifrequency cardiac probes: 3V2c (3.5/3.0/2.5/2.0 MHz) for adolescents and 7V3c (7.0/6.0/5.0/3.5 MHz) for younger children and infants. The measurement criteria used were based on the most recent recommendations of the American Society of Echocardiography (ASE) (10). All the procedures were performed by the same pediatric cardiologist.
The protocol included the 2D and TM modes on the longitudinal and small axis slides. The left parasternal window in the small axis slide 2D mode was utilised for the measurement of the parameters of the LV (systolic and diastolic) and the cardiac function. Pulsed and color Doppler were used for the exploration of cardiac valves, blood flux and diastolic function of the LV. For each parameter the value recorded was the average of at least two values on consecutive cardiac cycles.
The measurement of the left ventricle internal dimension at end systole (LVIDs), the left ventricular internal dimension at end diastole (LVIDd) and the wall dimensions of the LV were done on the left parasternal window—small axis slide, at the basal (transmitral) level and the median (the pillars) level. The slide that enabled to obtain the highest value was considered. The maximum LVIDd is most often at the transmitral level for the young children and at the median level for older children and adolescents (10).
The evaluation of the systolic function of the LV was done automatically by the calculation of the left ventricular shortening fraction (LVSF):
LVSF = [LVIDd – LVIDs)/LVIDd] × 100
The LV mass was determined according to the formula derived from the diameters and wall thicknesses of the LV (11):
LV mass (g) = 0.8 × {1,04 [(LVIDd + LVPWTd + IVSd)3 – (LVIDd)3]} + 0.6 g, the dimensions are expressed in cm
The estimation of the systolic pulmonary arterial pressure (PAPs) was done on Doppler mode by the measurement of the maximal speed (V) of the flux of an eventual tricuspid valve leak, from which the transvalvular systolic gradient of the tricuspid valve was automatically deducted by the computer using the Bernouilli equation. To this value was added the estimated right atrial pressure (RAP) (10):
PAPs = 4V2 + RAP (en mmHg)
The estimation of the mean pulmonary arterial pressure (PAPm) was done from the PAPs by the equation of Chemla (12):
PAPm = (0.61 × PAPs) + 2 mmHg
Definition of abnormal findings
- LV dilatation: z-score of LVIDd >2;
- LV hypertrophy: LV mass > 88 g/m2 for females and >102 g/m2 for males (10,13);
- LV systolic dysfunction: LVSF ≤25% (10);
- LV diastolic dysfunction: fraction E/A of the mitral flux <1 (abnormal relaxation) or >2 (restrictive profile with abnormal compliance);
- Dilated cardiomyopathy: LVSF <25% and z-score of LVIDd >2;
- Right ventricle dilatation: Right ventricular internal dimension at end diastole (RVIDd) >27 mm (13);
- Pulmonary hypertension: PAPs >30 mmHg or PAPm >25 mmHg;
- Pericardial effusion: presence of pericardial fluid >5 mm (14);
- Tachycardia: cardiac frequency >100 beats/minute;
- Nutritional status (15):
- Absence of malnutrition: Weight/height index > median −2 standard deviations (SD);
- Moderate malnutrition: median −3 SD < Weight/height index < median −2 SD;
- Severe malnutrition: Weight/height index < median −3 SD.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software, IBM, Chicago, USA. Descriptive statistics for continuous variables were presented as mean ± SD (since the majority of data were normally distributed). Qualitative variables were presented as frequencies. Pearson χ2 test or Fisher exact test were used when appropriate to compare qualitative variables to bring out eventual associations. Level of statistical significance was set at P<0.05.
Ethical considerations
Our study was approved by the ethical committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I. Informed consent was obtained from all caregivers. Echocardiographic examination was performed free of charge to the patients.
Results
Characteristics of the study population
A total of 100 children were included in our study with a slight predominance of the male gender, 52/48. The mean age was 7.6±3.2 years (from 1 to 15 years old) and the modal age group 6-10 years (61-120 months). The mean hemoglobin level, available in 44 patients, was 11.4±1.5 g/dL, and 25 patients on the 44 had anemia, defined as the hemoglobin level <12 g/dL (Table 1).

Full table
Concerning the clinical classification of the HIV infection, WHO stage III was the most represented (44%) followed by stage IV (33%), and only one child was at stage I at diagnosis. Most children (57%) did not present any biologically significant immune deficiency (that is, CD4 count >25%), and 7% had a severe immune deficiency (CD4 <15%). Vertical (mother-to-child) transmission was the exclusive transmission route in our sample. The mean age at diagnosis was 3.4±3.3 years. The mean time elapsed after the diagnosis was 4.25±2.67 years, and the mean time elapsed between the diagnosis and the initiation of treatment was 2 months (not shown in the table). Only 9 patients (9%) were totally naive to any ARV treatment; of the 91% on treatment, 82% were receiving a protocol with Zidovudine (AZT). The mean duration of ARV treatment was 4.1±2.4 years. One child (1%) presented criteria for severe malnutrition, and two others were moderately malnourished, while the great majority (97%) had a satisfactory nutritional status.
The prevalence of signs and symptoms which could evoke a cardiac anomaly were variable: cough was the most frequent (22%), 10% of the patients presented with dyspnea on exertion and 19% had tachycardia. We found systolic heart murmurs grade 1 to 2 on 6 in 3% of patients. Only one patient had a gallop sound on cardiac auscultation, and none presented lower limb edema.
Echocardiographic findings (Table 2)
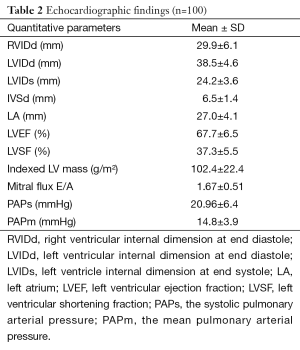
Full table
Left ventricle dilatation and systolic dysfunction
Only 1 patient (1%) had a LV dilatation (LVIDd z-score >2). This was a girl of 7 years 5 months old, at WHO clinical stage IV, whose immune status was unknown, and who had been on treatment with a protocol containing AZT for 12 months. She had anemia and a regular bradycardia (48 beats per minute). Besides, she presented other echocardiographic anomalies, notably a right ventriclar (RV) dilatation, a left atrial dilatation and a LV dilatation. No major clinical symptom at rest was reported in the patient, and she did not present any associated systolic or diastolic dysfunction.
Another patient (1%) had an isolated systolic dysfunction (LVSF <25%). It was a girl of 10 years 11 months, recently diagnosed HIV-positive (2 months ago) in the context of a multifocal tuberculosis, therefore classified WHO stage IV; she had a severe immune deficiency (CD4 <15%). The clinical signs reported by her were cough, tachycardia, hepato-splenomegaly and clinical pallor, but no dyspnea on exertion.
Therefore, no diagnosis of dilated cardiomyopathy (association of LV dilatation and systolic dysfunction) was reported in our study.
Left ventriclar hypertrophy (LVH)
Twelve patients (12%) were reported with a LVH, defined as an abnormally high LV mass index (>88 g/m2 for females, and >102 g/m2 for males). Of the 12 patients, this finding was isolated in only 1 patient. The 11 others had one or many associated abnormal findings: 2 had left atrial dilatation, 3 presented a RV dilatation, and the 6 others had both these latter 2 abnormal findings associated with the LVH.
LV Diastolic dysfunction
A diastolic dysfunction was reported in 32 participants (32%). Of these, 9 children (9%) had an Appleton type I mitral inflow (E/A <1) related to abnormal relaxation, while 23 (23%) had a restrictive profile of the mitral inflow (E/A >2) related to abnormal compliance. Twelve of the 32 participants with diastolic dysfunction (37.5%) had an associated left atrial dilatation, though not significant (P=0.24); 24 of the 32 patients (75%) also had a RV dilatation.
Pulmonary hypertension
When considering the value of the systolic PAPs, 7 participants (7%) presented with a pulmonary hypertension (PAPs >30 mmHg). But this prevalence came down to null when pulmonary hypertension was defined using PAPm calculated by the equation of Chemla, a method more precise and having better correlations with the measurement by cavity catheterization.
RV dilatation
Seventy-six (76%) participants had a right ventriclar dilatation (RVIDd >27 mm), among whom one patient presented with biventricular dilatation. Of the 76 patients with RV dilatation, 6 (7.9%) presented with a pulmonary hypertension (defined using PAPs) without any significant correlation between the two anomalies (P=0.52). Besides, the participants with the two anomalies associated were not statistically more symptomatic than those with an isolated RV dilatation, though the threshold of the symptom “cough” was close to statistical significance (P=0.066).
Pericardial effusion
This anomaly was reported in 11 patients (11%). All cases were of mild type. We did not find any significant association with the other echocardiographic findings.
Association between clinical characteristics and abnormal echocardiographic findings
Association between clinical stage and echocardiographic findings
Table 3 presents the principal echocardiographic findings according to WHO clinical stage. It seems to suggest association trends between clinical stage and RV dilatation, LVH and LV systolic dysfunction; however none of these trends was significant.
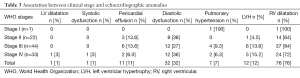
Full table
Association between the CD4 count and echocardiographic findings
We described no significant association between the CD4 count and the different abnormal echocardiographic findings (Table 4).
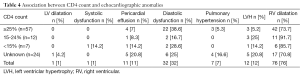
Full table
Other associations
We did not find any significant association between the echocardiographic findings on the one hand, and the presence of anemia, the nutritional status, the child’s age, and the type of ARV protocol administered on the other hand.
Discussion
The aim of our study was to describe the spectrum and prevalence of the abnormal echocardiographic findings in a group of HIV-infected children recruited consecutively, and to seek clinical characteristics eventually associated.
The different echocardiographic anomalies
The echocardiographic evaluation of the 100 participants of our study reveals a predominance of the LV diastolic dysfunction based on the study of the mitral inflow in pulsed Doppler; indeed, it was present in 32% of the participants, with 23% being a restrictive profile. Previous studies reported variable prevalences of LV diastolic dysfunction from 24% to 64% according to the technic used (7,16). Our results are close to those of Miller et al. who reported 24% in Zimbabwe using the same technic (7). The limitation to the use of the mitral inflow to evaluate diastolic dysfunction in children is linked to the fact that it depends on the loading conditions of the LV and can be modified by the heart rate which tends to be higher in children. The prognostic value of the LV diastolic dysfunction was not evaluated during the large American cohort study P2C2 (17).
The LV systolic dysfunction and LV dilatation were scarce in our study. Indeed, we reported only one case of LV systolic dysfunction (1%), and one case of LV dilatation (1%). These were female participants at WHO stage IV, with relatively late diagnosis of the infection and severe immune deficiency. Our results differ from previous studies that reported higher prevalence of LV systolic dysfunction and of LV dilatation. For example Miller et al. reported in Zimbabwe 10.9% of LV dilatation and 5.5% of LV systolic dysfunction (7); meanwhile Okoromah et al. found in a Nigerian cohort even higher prevalence, 33% of systolic dysfunction and 33.7% of dilated cardiomyopathy (6). This large divergence of findings could result from a higher mean age at diagnosis and the diagnostic and therapeutic delay in the Zimbabwean cohort. Indeed, it was a study with adolescents whose mean age was 15 years (compared to 7.6 years in our study), with a late diagnosis of the HIV infection; 71% of them had been on treatment for 20 months on average, compared to 91% on treatment for an average of 49 months in our study. This reason seems more likely when considering the fact that the two patients in our study who presented these anomalies were at WHO stage IV at diagnosis. In the Nigerian study, 83% of the patients were at the AIDS stage at diagnosis, compared to 33% only in our study (6). Once more, it seems plausible that advanced clinical stage at diagnosis tends to increase the prevalence of these anomalies of the LV. Lubega et al. in Uganda reported a prevalence of 17% of systolic dysfunction in patients at late stages who were naive to ARV treatment (5), meanwhile 91% of the participants in our study were on ARV treatment at inclusion. Besides, the mean time elapsed between the diagnosis and initiation of therapy was short in our study: 2 months. All these observations tend to suggest the critical importance of early diagnosis and treatment by HAART in HIV-infected children, which will theoretically reduce the incidence of the anomalies of the LV systolic function.
The LVH, estimated by the measurement of the LV mass indexed to body surface area, was present in 12% of our patients, with a trend of association with the clinical stage, though non-significant. Okoramah et al. in Nigeria reported a higher prevalence (17%) using the same method. We could once again attribute this divergence to the higher prevalence of AIDS stage and late diagnosis in the Nigerian cohort. LVH is well described at late stages of the HIV infection in children, and it is independently associated with a poor prognosis (2). Our figures are still largely lower than those reported by Miller et al. in Zimbabwe, 67.2%. In this study, the authors had defined LVH using the ventricular wall thickness (z-score >2); even with a stricter cut-off (z-score >3SD) the prevalence remained as high as 40%. Though this technic is validated in pediatrics, the thickness of the ventricular wall is less well correlated to the prognosis in infected children, and performs less in evaluating the existence of a LVH compared with the LV mass index used in our study (2). Besides, the absence of statistically significant association between LVH and clinical stage in our study could be a result of the actual lower prevalence of this anomaly in our cohort (due to its relative small size), thus limiting the power of the statistical tests used.
A surprising finding in our study is the prevalence of RV dilatation (76%). This anomaly was essentially isolated; indeed, only 6 patients of the 76 had a concomitant pulmonary hypertension (and even none when considering the PAPm). We observed a trend of association of this anomaly with the age (and therefore the duration of the infection), the age group >10 years having the highest prevalence, 89.5%, against 51% in the younger ones aged 0-5 years. Besides, these patients were mainly in stages III and IV (61 cases on the 76, 80%). Lubega et al. in Uganda reported a quite lower prevalence, 14% of RV dilatation in a population of children with mean age 6.8 years and all naive to any HAART (5). Miller et al. found a still lower prevalence of 30% in a population of Zimbabwean adolescents (mean age 15 years) compared to ours, despite the late diagnosis in their cohort. These Zimbabwean patients had been on treatment for mean duration of 20 months, compared to the mean 49 months in our sample. Could a direct role of the ARV molecules on the onset of RV dilatation be suggested to explain this higher prevalence in our sample? An argument against this hypothesis is the very low prevalence of concomitant LV dilatation among the patients on HAART. A more plausible explanation would be the difference in the norms used. Unlike the others indeed, we considered the right ventriclar internal dimension at end diastole (RVIDd) measured at the basal level, and a RVIDd >27 mm was considered as the cut-off for RV dilatation, based on the recommendations of the ASE published in 2010 obtained from occidental populations (10). In the absence of African norms it may be possible that this cut-off is actually overestimating the RV dilatation. This hypothesis will be comforted by the fact that we found a poor concomitant association with pulmonary hypertension, similar to an earlier report by Miller et al. (7).
Pulmonary hypertension was present in 7 (7%) of our children when defined as PAPs >30 mm. Six of the 7 had a RV dilatation. Our findings are higher than the 3.6% reported in Zimbabwe by Miller et al. (7), but much lower than the 41% described in Thailand by Pongprot et al., who evaluated children coming to consult for dyspnea (8). When a PAPm >25 mmHg was used as the cut-off definition of pulmonary hypertension, no case was reported in our sample. The equation of Chemla has been shown to be more specific to estimate the pulmonary pressure. This could be one of the reasons for the large variability of the prevalence of pulmonary hypertension in the different studies where PAPs was used.
Pericardial effusion was present in 11% of our patients. This prevalence is close to the 12.5% reported in Nigeria by Okoramah (6).
Clinical manifestations and association with echocardiographic anomalies
Our study did not find any statistically significant association between the abnormal echocardiographic findings and all the clinical variables including the symptoms; however, trends of associations can be suggested. Indeed, the two patients with LV dilatation or systolic dysfunction were females at WHO stage IV. Miller et al. reported a significant association between the female gender and the presence of a LV dilatation (7). Likewise, late stage of the disease is well described in the literature to be associated with systolic dysfunction (5,16). Cough (22%), tachycardia (19%) and dyspnea (10%) were the major clinical signs reported in our study and predominated in patients with RV dilatation, but not significantly. This finding could simply be random since the majority of our patients (76%) were found with RV dilatation (by the way poorly correlated with pulmonary hypertension), thus rendering the clinical signs less specific. Besides, patients with a LV anomaly were asymptomatic. Likewise, no patient with pericardial effusion presented muffled heart sounds on auscultation. This is in accordance with literature reports showing that the cardiac anomalies described in HIV are scarcely symptomatic or asymptomatic (5,16).
The general nutritional status was satisfactory in our study sample. Indeed, 1 patient only had a severe malnutrition and 2 had moderate malnutrition. In the studies that reported high prevalence of LV systolic dysfunction or dilatation, larger proportions of children were malnourished. For example, in the Ugandan study of Lubega et al. that described 17% of LV systolic dysfunction, there were 50% of children with stunting or growth retardation (compared to 3% in our sample) (5). Likewise in Zimbabwe, Miller et al. pointed out malnutrition as an inducing or worsening factor of the 10% of LV anomalies they found, since infected patients were more stunted compared to healthy controls (7). All these assertions emphasize the importance and the effectiveness of nutritional support put in place in the Mother and Child Healthcare center of the Chantal Biya Foundation. Malnutrition has been well described as an inducing factor of LV systolic dysfunction and other cardiac anomalies, independently of HAART (14,18). In our study the patient with severe malnutrition was found with LVH and the 2 children with moderate malnutrition had a RV dilatation in one case, and pericardial effusion in the other. Nutritional supplementation, especially with selenium is known to contribute to reverse cardiomyopathy and to restore a normal LV function in patients with malnutrition (14).
The large majority (91%) of our patients were on HAART for a mean duration of 49 months. These figures are higher than the 70% on HAART for a mean duration of 20 months in the study of Miller et al. (7). Of the 91 patients on HAART, 82 (90%) had an ARV protocol containing Zidovudine (AZT). Despite this high proportion of HAART in our sample, we did not find any significant association between the intake of ARV molecules and the presence of a cardiac anomaly. It should be noted that the design of the study was cross-sectional and not comparative, and the proportion of patients naive to HAART was low, constituting therefore a limit to evaluate the influence of HAART on the cardiac anomalies revealed by echocardiography. The low prevalence of LV systolic dysfunction in our study with the majority of patients on AZT eventually supports the fact that AZT has low risk for the heart, as previously reported by other authors (19,20).
Besides we have noted a relative late diagnosis of the HIV infection in our study (the mean age at diagnosis was 3.4 years), though vertical transmission was the exclusive transmission route, and the diagnosis is currently possible as early as 6 weeks of life by PCR or P24 antigen detection in the infant’s blood (21). Children were therefore diagnosed late when already symptomatic. This explains the high prevalence of late stages III and IV in our study. This situation corroborates the general trend in African and Thailand settings (5,7,8).
Strengths and limitations
One of the strength of this study is that it was done in our daily practice conditions. Patients were consecutively enrolled as they arrived spontaneously. Besides, the norms used were based on the most recent recommendations of ASE. The results found could hence reflect the reality in our setting.
The limits of our study include on one hand the design: cross-sectional and not longitudinal, which would have enabled us to better evaluate the influence of the different variables on the incidence of cardiac anomalies and their prognosis in terms of morbi-mortality. On the other hand, with the absence of echocardiographic references for sub-Saharan African pediatric populations, it is difficult to have a clear photograph of the spectrum of these cardiac anomalies in our context. There is thus an inherent risk of under- or over- estimating the real prevalence of the anomalies. Besides, the relative small size of our sample did not enable a sufficient statistical power to draw out eventual significant associations between clinical variables and the echocardiographic anomalies described. Moreover, we had no access to tissue Doppler imaging that could allow us to better investigate the systolic and diastolic heart function
Finally, since our study was carried out exclusively in Yaoundé, in a medical structure with enough equipment to provide nutritional support, it does not allow us to extrapolate our results to the entire Cameroonian territory, having in mind the resource-limited context of the peripheral treatment centers that yet provide care for a large majority of this vulnerable population.
Conclusions
The cardiac anomalies revealed by echocardiography are varied and highly prevalent in our study population (89% of the participants) when using norms recommended by the ASE. Less frequent are the anomalies associated with a poor prognosis: 12% of LVH and only 2% of LV systolic dysfunction or dilatation. These anomalies seem to be more prevalent in late stages of the HIV infection and remain essentially subclinical. Early diagnosis and treatment by HAART, and good nutritional status could reduce the incidence of these cardiac anomalies, especially those of the left ventricle. CD4 count is seemingly not a predictive factor. Echocardiography, a non-invasive cost-effective procedure could be a significant contribution in the early detection of many of these abnormalities.
Acknowledgements
We are grateful to all the staff of Mother and Child Center, Chantal Biya Foundation, especially those of the pediatric HIV unit for their invaluable assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: Thanks to the hospital’s Directorate, the echocardiographic examinations were free of charge.
References
- World Health Organization. Global HIV/AIDS Response, Epidemic update and health sector progress towards Universal Access, progress report 2011. Available online: who.int/iris/bitstream/10665/44787/1/9789241502986_eng.pdf
- Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: The Prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation 2000;102:1542-8. [PubMed]
- Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis 2001;1:115-24. [PubMed]
- Khunnawat C, Mukerji S, Havlichek D Jr, et al. Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol 2008;102:635-42. [PubMed]
- Lubega S, Zirembuzi GW, Lwabi P. Heart disease among children with HIV/AIDS attending the paediatric infectious disease clinic at Mulago Hospital. Afr Health Sci 2005;5:219-26. [PubMed]
- Okoromah CA, Ojo OO, Ogunkunle OO. Cardiovascular dysfunction in HIV-infected children in a sub-Saharan African country: comparative cross-sectional observational study. J Trop Pediatr 2012;58:3-11. [PubMed]
- Miller RF, Kaski JP, Hakim J, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2013;56:576-82. [PubMed]
- Pongprot Y, Sittiwangkul R, Silvilairat S, et al. Cardiac manifestations in HIV-infected Thai children. Ann Trop Paediatr 2004;24:153-9. [PubMed]
- WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children, 7 August 2006. Available online: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf
- Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465-95. [PubMed]
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55:613-8. [PubMed]
- Aduen JF, Castello R, Daniels JT, et al. Accuracy and precision of three echocardiographic methods for estimating mean pulmonary artery pressure. Chest 2011;139:347-52. [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [PubMed]
- Barbaro G. Cardiovascular manifestations of HIV infection. Circulation 2002;106:1420-5. [PubMed]
- OMS/UNICEF. Déclaration Commune. Normes de croissance OMS et identification de la malnutrtition aigüe sévère chez l'enfant. Génèse, 2009. Available online: http://www.who.int/nutrition/publications/severemalnutrition
- Singh P, Hemal A, Agarwal S, et al. Cardiac manifestations in HIV infected children. Indian J Pediatr 2015;82:230-4. [PubMed]
- Starc TJ, Lipshultz SE, Easley KA, et al. Incidence of cardiac abnormalities in children with human immunodeficiency virus infection: The prospective P2C2 HIV study. J Pediatr 2002;141:327-34. [PubMed]
- Miller TL, Orav EJ, Colan SD, et al. Nutritional status and cardiac mass and function in children infected with the human immunodeficiency virus. Am J Clin Nutr 1997;66:660-4. [PubMed]
- Miller TL, Evans SJ, Orav EJ, et al. Growth and body composition in children infected with the human immunodeficiency virus-1. Am J Clin Nutr 1993;57:588-92. [PubMed]
- Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr 1994;7:681-94. [PubMed]
- World Health Organization. Recommendation on the diagnosis of HIV infection in infants and children. 2010. Available online: http://apps.who.int/iris/bitstream/10665/44275/1/9789241599085_eng.pdf


