A case of ascending aortic dissection mimicking acute myocardial infarction and complicated with pericardial tamponade
Case presentation
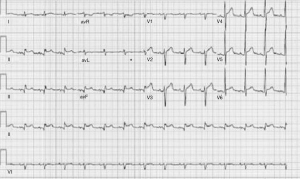
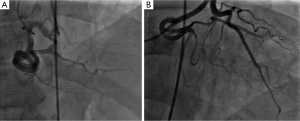
A 60-year-old male with past medical history of hypertension, presented to emergency department complaining of chest pain for 2 hours, the pain was described as tearing, substernal pain and was radiating to the neck and shoulders and associated with nausea and sweating. Initial vital signs showed Blood pressure of 120/90 mmHg in right arm, and 110/70 mmHg in left arm, Heart rate of 90 beat per minute, Respiratory rate was 18 breath per minute, temperature of 37.6 °C and Oxygen saturation of 95% breathing ambient air. Physical exam was significant for jugular venous distension and cardiac exam showed normal heart sounds, an early diastolic murmur, 3/6 in intensity, best heard on left 3rd intercostal space without radiation, and no S3 or S4 gallop. Initial lab results showed a white blood cell count of 8.1 k/µL, hemoglobin of 12.2 g/dL, platelets of 177 k/µL, creatinine of 1.19 mg/dL, AST of 30 IU/L, ALT of 29 IU/L, initial set of troponin I was 0.02 ng/mL. Twelve lead electrocardiogram (EKG) showed ST segment elevations in inferior leads (Figure 1). Emergent coronary angiography was done and revealed normal coronary arteries without evidence of dissection (Figure 2). After the catheter was pulled back across the aortic valve, an aortic root angiogram was performed and showed aortic insufficiency (grade 3), and an aortic dissection flap that started in the ascending aorta and progressed through to the descending aorta (Figure 3); this was classified as a Stanford type A (DeBakey type I).
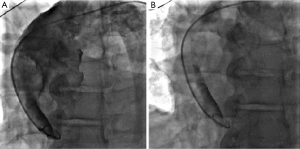
A bedside transthoracic echocardiogram was also performed after cardiac catheterization, and showed an EF of 40% with inferior wall hypokinesia, a dilated aortic root with an evidence of intimal tear in the ascending aorta and moderate aortic regurgitation. In addition, mild pericardial effusion was also noted.
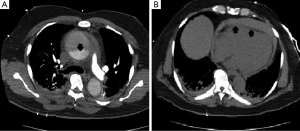
Vascular surgery team was immediately consulted, and an aortic computed tomography (CT) scan was done and revealed a Stanford type A (DeBakey type I) aortic dissection with extension to the descending aorta, and a moderate pericardial effusion (Figure 4).
After the diagnosis of complicated dissection of the ascending aorta, the decision for immediate surgical repair was made. Just before the patient was transferred to the operation room, his blood pressure dropped to 85/50 mmHg and his HR was 110 BPM. Physical exam showed distant heart sounds and jugular venous distension. At this point, it was thought that he developed cardiac tamponade and pericardiocentesis was immediately performed to improve the hemodynamics. After that, the patient blood pressure improved and he was transferred to the operation room for an emergent surgical repair. Intraoperative findings showed ruptured ascending aortic dissection and hemopericardium with blood clots. The patient underwent hemiarch ascending aortic replacement with Hemashield platinum graft after resection of the ruptured aortic dissection above the sinus of valsalva the procedure was done successfully without complications. The patient had an uncomplicated postoperative course and was discharged in a stable condition.
Case discussion
Acute aortic dissection (AD) is the most common life-threatening disorder affecting the aorta (1). The incidence of AD ranges between 5 to 30 cases per million people per year, depending on the study population (1,2). Although AD is uncommon, it frequently has fatal outcome, with an overall hospital mortality rate of 27.4%, and as high as 58% in patients with Debakey type I AD patients who didn’t receive surgical treatment (1,3). Therefore rapid diagnosis and treatment of AD is very important and can’t be overemphasized.
AD can be classified using either of two classifications based on anatomical location, Stanford and DeBakey classification. Stanford type A dissections involve the ascending aorta and type B dissections occur distal to the subclavian artery (4). Whereas in the Debakey classification, type I dissections begin in the ascending aorta and extend to the descending, type II dissections involve the ascending aorta only and type III dissections begin in the descending aorta distal to the left subclavian artery (5). Diagnostic modalities include contrast CT, transthoracic or transesophageal echocardiography, and magnetic resonance imaging (1). Contrast CT is the most commonly used modality in diagnosing AD as it is readily available in most hospitals. Proximal dissections are usually treated with emergent surgical correction, whereas distal dissections are treated preferably with medical control of blood pressure and radiographic surveillance (1-3).
The symptoms of aortic dissection may be variable and can mimic other more common conditions such as myocardial ischemia. Patients with AD often present with chest or back pain, of sudden onset and usually described as sharp or tearing pain. Also a discrepancy in the pulse or blood pressure in the upper extremities, diastolic murmur of aortic regurgitation and widening of mediastinum on chest X-ray might be noted and should raise the suspicion for AD (6). However, patients may present with symptoms related to AD complications, such as heart failure from acute aortic regurgitation, neurological deficits, syncope, or other symptoms secondary to vascular insufficiency and malperfusion syndrome. These Symptoms are often associated with occlusion of distal branch arteries by the dissection flap and include bowel, renal, cerebrovascular (6%), and myocardial (1–2%) complications (7,8).
Cardiac tamponade can rarely complicate acute proximal aortic dissection, and is one of the most common causes of death from aortic dissection. When the pressure within the aortic wall exceeds a critical limit rupture occurs, either into the pericardium with cardiac tamponade or into the pleural space or mediastinum (9). Emergency aortic repair together with intra-operative pericardial drainage is the recommended treatment approach. However, there is controversy about performing pericardiocentesis in patients who are hemodynamically unstable and while awaiting surgical repair (10,11). Pericardiocentesis is the treatment of choice for cardiac tamponade caused by various diseases, but in case of hemopericardium complicating AD drainage can aggravate the leak or rupture (11). However, according to the recent published guidelines for the diagnosis and management of patients with thoracic aortic disease, preoperative pericardiocentesis can be lifesaving when managing patients with cardiac tamponade with pulseless electrical activity or refractory hypotension complicating acute type A aortic dissection, especially when cardiac surgery is not immediately available (9,12).
One of the serious complications of type A aortic dissection is coronary malperfusion, which can be rarely complicated with acute myocardial function in 1% to 2% of the cases (13). Two pathophysiological mechanisms have been proposed to explain a branch vessel compromise associated with AD; static, where the expanding hematoma cause narrowing of the affected vessel, and dynamic; where the dissection flap can partially occlude the ostium of an artery, affecting the blood flow inside the vessel, which may lead to coronary thrombosis and consequent myocardial infarction (MI) if coronary arteries are involved (13-15). In addition, the dissection can extend directly into the coronary arteries from the aorta, and usually the right coronary artery is more often affected than the left (16).
Usually it is difficult to differentiate between myocardial ischemia and AD in patients presenting with sudden onset chest pain, also EKG may show ST segment elevation and signs of acute transmural MI when the coronary arteries are involved by the mechanisms described above. About 20% of patients with type A dissection have EKG evidence of acute ischaemia or acute MI (16). However, most of the patients will have non-specific ST-T segment changes, and only one third of the patients with AD have a normal EKG. Bedside echocardiography is a quick noninvasive method to detect wall motion that can be utilized in cases of chest pain when aortic dissection is a concern. Caution should be used in patients with STEMI and suspected AD, as treatment with thrombolysis for STEMI in non-PCI capable hospitals may have catastrophic effects in patients including rupture, expansion and uncontrolled bleeding (16-18).
American Heart Association (AHA) and American College of Cardiology (ACC) provide a guideline for the diagnosis of patients with thoracic aortic disease, using the aortic dissection detection (ADD) risk score. Patients can be divided to 3 groups: low, intermediate, and high risk based on historical and examination. The ADD risk score has a 95.7% of sensitivity, while its specificity has not been documented (19).
In cases of AD associated with MI, and coronary artery dissection or compression by the expanding hematoma; treatment with coronary artery stenting or positioning of a coronary perfusion catheter will help to maintain an adequate coronary perfusion, and reduce myocardial ischemia until surgical repair is done (20-22).
This case presentation of aortic dissection mimicking inferior MI and complicated with pericardial tamponade represents a rare and fatal combination of sequelae.
In our patient coronary angiography showed normal coronary arteries without evidence of dissection or obstruction. MI most likely resulted from functional coronary artery occlusion due to type A aortic dissection, where the dissection flap temporarily occluded the ostium of the right coronary artery and affected the blood flow to the vessel.
Few cases of aortic dissection complicated with MI or tamponade were reported before (18-23), but this combination of MI, and pericardial tamponade was rarely reported, and most of the reported cases had fatal outcomes. However, our patient had an uncomplicated postoperative course and was discharged home without complications.
In conclusion, it is necessary to have a high index of suspicion for AD in cases of chest pain. If AD is suspected in a patient with acute coronary syndrome (ACS), confirming the diagnosis with the appropriate imaging studies should be done as quickly as possible. As this may lead to the inappropriate administration of thrombolytic or anticoagulant agents resulting in catastrophic outcomes. Bedside transthoracic echocardiogram represents an easy, safe and rapid procedure to diagnose proximal aortic dissection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD), new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: a population-based study. Surgery 1982;92:1103-8. [PubMed]
- Macrina F, Puddu PE, Sciangula A, et al. Long-term mortality prediction after operations for type A ascending aortic dissection. J Cardiothorac Surg 2010;5:42. [Crossref] [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- DeBakey ME, Henly WS, Cooley DA, et al. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [Crossref] [PubMed]
- Sullivan PR, Wolfson AB, Leckey RD, et al. Diagnosis of acute thoracic aortic dissection in the emergency department. Am J Emerg Med 2000;18:46-50. [Crossref] [PubMed]
- Isselbacher EM. Diseases of the aorta. Zipes: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, 2005:1403-33. Copyright 2005 Saunders.
- Olin JW, Fuster V. Acute aortic dissection: the need for rapid, accurate, and readily available diagnostic strategies. Arterioscler Thromb Vasc Biol 2003;23:1721-3. [Crossref] [PubMed]
- Cruz I, Stuart B, Caldeira D, et al. Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. Eur Heart J Acute Cardiovasc Care 2015;4:124-8. [Crossref] [PubMed]
- Maisch B, Seferović PM, Ristić AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J 2004;25:587-610. [Crossref] [PubMed]
- Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation 1994;90:2375-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [Crossref] [PubMed]
- Creswell LL, Kouchoukos NT, Cox JL, et al. Coronary artery disease in patients with type A aortic dissection. Ann Thorac Surg 1995;59:585-90. [Crossref] [PubMed]
- Zotz R, Stern H, Mohr-Kahaly S, et al. Coronary insufficiency in type II aortic dissection. Z Kardiol 1987;76:784-6. [Crossref] [PubMed]
- Kamp TJ, Goldschmidt-Clermont PJ, Brinker JA, et al. Myocardial infarction, aortic dissection, and thrombolytic therapy. Am Heart J 1994;128:1234-7. [Crossref] [PubMed]
- Byrd BF 3rd, Panayiotou H. Proximal aortic dissection with cardiac tamponade. Long-term survival without surgery. Chest 1991;99:1047-9. [Crossref] [PubMed]
- Cook J, Aeschlimann S, Fuh A, et al. Aortic dissection presenting as concomitant stroke and STEMI. J Hum Hypertens 2007;21:818-21. [Crossref] [PubMed]
- Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation 2011;123:2213-8. [Crossref] [PubMed]
- Ohara Y, Hiasa Y, Hosokawa S. Successful treatment in a case of acute aortic dissection complicated with acute myocardial infarction due to occlusion of the left main coronary artery. J Invasive Cardiol 2003;15:660-2. [Crossref] [PubMed]
- Cardozo C, Riadh R, Mazen M. Acute myocardial infarction due to left main compression aortic dissection treated by direct stenting. J Invasive Cardiol 2004;16:89-91. [Crossref] [PubMed]
- Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emerg Trauma Shock 2011;4:273-8. [Crossref] [PubMed]
- Pólos M, Szabolcs Z, Apor A, et al. Successful surgical treatment of an acute type-A aortic dissection complicated with pericardial tamponade and ST-segment elevation. Orv Hetil 2014;155:1763-7. [Crossref] [PubMed]