Real-time transesophageal echocardiography facilitates antegrade balloon aortic valvuloplasty
Introduction
Balloon aortic valvuloplasty (BAV) for degenerative calcific aortic stenosis (AS) is currently experiencing a resurgence, concurrent with the rapid introduction of transcatheter aortic valve replacement (TAVR). This technique is frequently employed both as an integral part of TAVR and as a bridge therapy to TAVR or surgical aortic valve replacement (1). In addition, BAV also serves as a reasonable therapeutic option primarily in patients who are initially referred for TAVR but are eventually found ineligible (2).
The immediate efficacy of BAV for high-risk patients with critical AS has been demonstrated from the standpoint of hemodynamic improvement and palliation of symptoms. However, long-term efficacy is rare, with a 1-year event-free (recurrent symptoms or death) survival rate of 30–50% (1-3). This is partly because of incomplete dilation leaving significant residual stenosis, resulting in a high restenosis rate (4). But currently, there is no consensus even on the optimal balloon size and the approach to determine it. Balloon sizing during the procedure is particularly difficult because under common fluoroscopic guidance, it is difficult to figure out the detailed response of aortic valve leaflets, commissures, or annuls that are attributed to valvuloplasty.
We report two cases where detailed information provided by real-time transesophageal echocardiography (TEE) refined the style of BAV.
Case 1
An 89-year-old woman presented with her second progressive heart failure 1 year after the first episode. Transthoracic echocardiogram demonstrated a hypertrophic left-ventricle with preserved systolic function (ejection fraction of 56%) and severe AS with a mean gradient of 69 mmHg and aortic valve area of 0.4 cm2. She lived in an assisted-care facility, and she appeared frail due to her small body habitus (weight 43 kg; height 153 cm). She had several comorbidities, including hypertension, hyperlipidemia, and type 2 diabetes (HbA1c 7.0% with oral anti-diabetic drugs). In addition, her laboratory results demonstrated moderate to severe renal insufficiency (creatinine 1.3 mg/dL and eGFR 30.1 mL/min/1.73 m2). Her predicted perioperative mortality risk following surgical aortic valve replacement was 31.4% based on the logistic EuroSCORE model. Despite aggressive medical therapy, she remained at the New York Heart Association (NHYA) functional class IV. Given her advanced age and prohibitive perioperative risk, our decision was to perform a palliative BAV. Written informed consent was obtained from her family.
Antegrade BAV (5-7) was performed in the catheterization laboratory with respirator support. A 14, 5, and 7 Fr sheath were placed in the right femoral vein, left femoral artery, and left femoral vein, respectively. The left atrium was accessed by an 8.5-Fr, 61-cm, medium curl, Agilis Nx™ sheath (St. Jude. Medical, MN, USA) from the right femoral vein using the standard transeptal puncture technique. A single-lumen 7-Fr balloon-tipped catheter (Gadelius Medical K.K, Tokyo, Japan) was advanced inside the Agilis Nx™ sheath, across the mitral valve into the left ventricle, and then looped in the left ventricular apex and directed towards the outflow tract. A 0.035-inch, 250-cm long radifocus guidewire (Terumo, Tokyo, Japan) was advanced through the inside of the balloon-tipped catheter to navigate it into the descending aorta. In the descending aorta, the radifocus wire was exchanged for a 0.032-inch, 260-cm extrastiff guidewire, and the extrastiff wire was anchored using a 10-mm GooseNeck Snare (Covidien, Plymouth, MN, USA) inserted from the left femoral artery. After removing the balloon-tipped catheter and the Agilis™ sheath, a 20-mm INOUE balloon (Toray Industries, Inc., Tokyo, Japan) was advanced over the extrastiff wire, and positioned across the aortic valve. A temporary pacemaker lead was inserted from the left femoral vein for rapid pacing (130–150 ppm) in order to stabilize the balloon position across the valve.
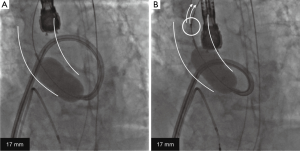
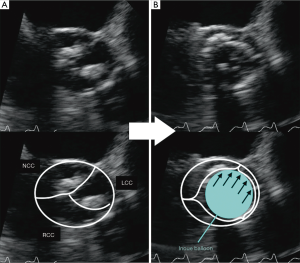
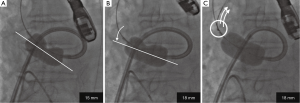
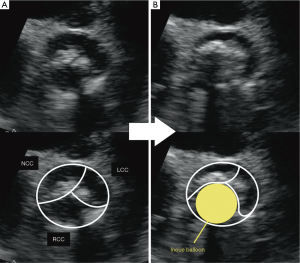
The INOUE balloon was initially inflated to 14 mm in diameter, and then incrementally increased by 1 mm. Inflations were repeated 2–4 times for each size. During the stepwise dilatation to 17 mm, fluoroscopic image demonstrated the long axis of the INOUE balloon was gradually leaning towards the greater curvature of the ascending aorta (Figure 1). Real-time TEE revealed that the asymmetric distribution of calcification precluded the circumferential-uniform expansion of the aortic valve. The non-coronary cusp (NCC) and left coronary cusp (LCC) were rigid because of marked calcification, and the less calcified right coronary cusp (RCC) was dominantly compressed (Figures 2,3) (8). To overcome the deflection toward the greater curvature, the extrastiff guidewire was snared slightly distal to the balloon’s tip, and pulled up and secured firmly (Figure 4). This intentional wire bias toward the lesser curvature of the ascending aorta provided adequate support during balloon inflation, resulting in compression of the calcified NCC and LCC as confirmed by real-time TEE (Figures 5,6) (9). The balloon size was finally increased up to 19 mm, and circumferential-uniform expansion of the balloon was achieved, which was also confirmed by real-time TEE. The mean aortic pressure gradient dropped from 68 to 23 mmHg, and the aortic valve area increased from 0.40 to 1.11 cm2.


The patient was weaned from the respirator on the day of the operation. The subsequent hospital stay was uneventful, and the patient was discharged after the cardiac rehabilitation program in our institution without dyspnea on effort.
Case 2
A 76-year-old woman with known severe AS manifested by shortness of breath (NYHA class 3) and systemic edema was referred to our center for possible valve treatment. Transthoracic echocardiogram demonstrated a severely calcified bicuspid aortic valve (the raphe was located between the NCC and LCC) with a mean gradient of 45 mmHg and aortic valve area of 0.5 cm2. Although she had several coronary risk factors, including hypertension, type 2 diabetes (HbA1c 6.6% with oral anti-diabetic drugs), and moderate renal insufficiency (creatinine 1.2 mg/dL and eGFR 33 mL/min/1.73 m2), her coronary angiogram revealed no significant stenosis. However, she was suffering from pulmonary fibrosis that required ongoing oral administration of 5 mg of prednisolone daily. In addition, her general condition was friable beyond her years and she could not move without the help of a walker. Therefore, she was considered high-risk for surgical aortic valve replacement. Given that a bicuspid aortic valve has been a contraindication to TAVR in our country, we offered her BAV as a reasonable alternative to which the patient and her family consented.
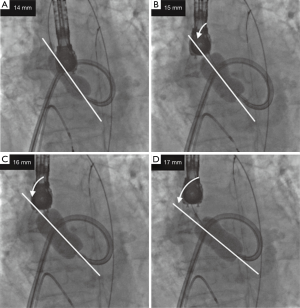
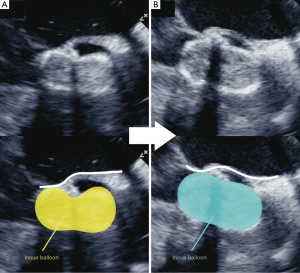
Antegrade BAV was performed in the same manner as for case 1 (Figure 7). The INOUE balloon was initially inflated to 15 mm in diameter (Figure 7A) and was incrementally increased by 1 mm. Although the balloon size was increased up to 18 mm, the dumbbell shape (waist of the balloon) of only the lesser curvature side did not disappear (Figure 7B). Real-time TEE revealed that the severely calcified raphe and fused leaflet precluded the full expansion of the balloon toward the lesser curvature (Figure 8A). To provide adequate support toward the lesser curvature of the ascending aorta, extrastiff guidewire was snared slightly distal to the balloon’s tip, and pulled up and secured firmly (Figure 7C). Real-time TEE revealed that the calcified raphe and leaflet was being compressed thoroughly up to the aortic annulus (Figures 8B,9) (10). The balloon size was finally increased to 19 mm and uniform compression of both leaflets of the bicuspid valve was achieved. Mean aortic pressure gradient dropped from 59 to 21 mmHg, and the aortic valve area increased from 0.74 to 1.04 cm2.

The patient was weaned from the respirator on the day of the operation. The subsequent hospital stay was uneventful, and the patient was discharged after the cardiac rehabilitation program in our institution, when her shortness of breath and systemic edema disappeared.
Discussion
Resurgence of BAV is being observed concurrent with the advent of TAVR. However, only a few technical improvements in the procedure have occurred since the first introduction of BAV almost two decades ago (11). Although BAV offers significant immediate hemodynamic and clinical improvement, its long-term efficacy is poor. This is mainly because of incomplete dilation leaving significant residual stenosis, resulting in a high restenosis rate (1-4). Recently, larger post-BAV valve areas have been reported to be associated with better prognosis (3). However, given that valvular response to balloon opening may vary depending on the degree or distribution of calcification, determination of whether the rigid valve is being properly dilated to the greatest possible extent is limited under fluoroscopic guidance.
Real-time TEE can provide useful information regarding whether each of the leaflets is adequately compressed during the procedure. Previous histopathological studies have reported that the major mechanisms of successful BAV for degenerative AS are cracking the calcified nodules on the leaflets and creation of cleavage planes in the stroma (2,4,12-14). In our cases, real-time TEE demonstrated that leaflets with less calcification were primarily stretched and compressed, probably because the radial balloon force was exerted more strongly on the more pliable leaflets. Intentional wire bias toward the more rigid leaflets was required in order to compress the severely calcified leaflets and achieve circumferential-uniform expansion of the aortic annulus.
Real-time TEE can also help determine the properly matched largest balloon size for a given calcified valve. Previous histopathological studies have also reported that another mechanism of successful BAV is stretching the aortic annulus at the commissure sites (wrinkling and folding cuspal margins) (2,4,12-14). Nevertheless, it is possible that oversized or off-center dilation may result in annulus disruption or leaflet avulsion with abrupt aortic regurgitation (12,14,15). In our cases, using real-time TEE guidance, we initially applied a smaller balloon size and then increased the size by 1 mm increments after each inflation step until the largest possible size was achieved. Concurrent with the intentional wire-bias technique mentioned above, this approach allowed the balloon to reach the largest possible size and achieve full expansion of the aortic annulus.
The effect of TEE-guided BAV could be maximized with the antegrade approach. First, several features of the INOUE balloon are necessary in order to fully utilize the information obtained by real-time TEE, and a large bore (14-Fr) sheath is required to use the INOUE balloon. Besides its stepwise size adjustability, its dumbbell-shaped configuration is desirable to better conform to the anatomy of the aortic valve and sinuses of Valsalva. These features may help to achieve more effective mechanical compression of the leaflets (7), possibly translating into greater increases in post-BAV valve areas than that with the retrograde approach with conventional cylindrical balloons (6). Second, anchoring the wire is necessary to bias the wire toward the appropriate direction; this intentional wire-bias technique is not possible with the retrograde approach.
The clinical implications of better BAV technique include the possibility as a standalone therapy for selected patients with severe AS. Approximately 30–50% of patients with severe AS who are initially referred for TAVR are not suitable for the procedure because of multiple comorbidities, anatomic difficulties, or hemodynamic instability (16). Given that medical management alone for those patients has a dismal prognosis, our refined BAV technique, with its safety profile, can be considered as a reasonable therapeutic option. Another implication of our refined technique is to improve the preparation (predilatation of the stenotic aortic valve) for TAVR. Current approaches for TAVR generally require BAV as predilatation for the placement of either a self-expanding or balloon-expandable prosthetic valve. Effective fragmentation of the leaflet’s calcification and full expansion of the aortic annulus by BAV prior to TAVR may favor the circular prosthetic valve deployment. Therefore, it is possible that our TEE-guided BAV approach, concurrent with the intentional wire-bias technique, may reduce the prevalence of prostheses-patient mismatch and paravalvular leakage following TAVR.
The potential for our TEE-guided antegrade BAV technique to yield larger valve areas requires further examination. It remains to be seen whether the improved acute results from our modified antegrade approach are durable and will lead to improved long-term clinical outcomes.
In summary, the present report illustrates the important role of real-time TEE in antegrade BAV with the intentional wire-bias technique. Our approach may not only facilitate optimal balloon sizing and dilatation of the aortic valve but also minimize the potential complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Ben-Dor I, Maluenda G, Dvir D, et al. Balloon aortic valvuloplasty for severe aortic stenosis as a bridge to transcatheter/surgical aortic valve replacement. Catheter Cardiovasc Interv 2013;82:632-7.
- Khawaja MZ, Sohal M, Valli H, et al. Standalone balloon aortic valvuloplasty: indications and outcomes from the UK in the transcatheter valve era. Catheter Cardiovasc Interv 2013;81:366-73. [Crossref] [PubMed]
- Ben-Dor I, Pichard AD, Satler LF, et al. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc Interv 2010;3:1150-6. [Crossref] [PubMed]
- Feldman T, Glagov S, Carroll JD. Restenosis following successful balloon valvuloplasty: bone formation in aortic valve leaflets. Cathet Cardiovasc Diagn 1993;29:1-7. [Crossref] [PubMed]
- Feldman T. Transseptal antegrade access for aortic valvuloplasty. Catheter Cardiovasc Interv 2000;50:492-4. [Crossref] [PubMed]
- Sakata Y, Syed Z, Salinger MH, et al. Percutaneous balloon aortic valvuloplasty: antegrade transseptal vs. conventional retrograde transarterial approach. Catheter Cardiovasc Interv 2005;64:314-21. [Crossref] [PubMed]
- Eisenhauer AC, Hadjipetrou P, Piemonte TC. Balloon aortic valvuloplasty revisited: the role of the inoue balloon and transseptal antegrade approach. Catheter Cardiovasc Interv 2000;50:484-91. [Crossref] [PubMed]
- Shimada Y, Ito K, Yano K. Initially, the less calcified right coronary cusp (RCC) was dominantly compressed. Asvide 2016;3:081. Available online: http://www.asvide.com/articles/834
- Shimada Y, Ito K, Yano K. By the intentional wire bias technique, the calcified non-coronary cusp (NCC) and left coronary cusp (LCC) were then compressed. Asvide 2016;3:082. Available online: http://www.asvide.com/articles/835
- Shimada Y, Ito K, Yano K. The intentional wire bias toward the lesser curvature of the ascending aorta provided adequate support during balloon inflation, resulting in compression of the calcified NCC and LCC. Asvide 2016;3:083. Available online: http://www.asvide.com/articles/838
- Feldman T. Balloon aortic valvuloplasty: still under-developed after two decades of use. Catheter Cardiovasc Interv 2013;81:374-5. [Crossref] [PubMed]
- Waller BF, McKay C, VanTassel JW, et al. Catheter balloon valvuloplasty of stenotic aortic valves. Part I: Anatomic basis and mechanisms of balloon dilation. Clin Cardiol 1991;14:836-46. [Crossref] [PubMed]
- Waller BF, Dorros G, Lewin RF, et al. Catheter balloon valvuloplasty of stenotic aortic valves--Part II: Balloon valvuloplasty during life subsequent tissue examination. Clin Cardiol 1991;14:924-30. [Crossref] [PubMed]
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987;9:655-60. [Crossref] [PubMed]
- van den Brand M, Essed CE, Di Mario C, et al. Histological changes in the aortic valve after balloon dilatation: evidence for a delayed healing process. Br Heart J 1992;67:445-9. [Crossref] [PubMed]
- Saia F, Marrozzini C, Dall'Ara G, et al. How many patients with severe symptomatic aortic stenosis excluded for cardiac surgery are eligible for transcatheter heart valve implantation? J Cardiovasc Med (Hagerstown) 2010;11:727-32. [Crossref] [PubMed]