Noninvasive imaging modalities to visualize atherosclerotic plaques
Background
Atherosclerotic cardiovascular disease is the leading causes of morbidity and mortality in the Western world (1). As the prevalence of metabolic risk factors increases in a global fashion, cardiovascular disease will be expected to further rise in the future. This underscores the development of better modality to identify atherosclerotic cardiovascular disease and develop effective therapeutic approach.
Atherosclerosis is a chronic progressive disease of the arterial wall. Its underlying pathology is characterized by a chronic inflammatory process and the influx of atherogenic lipids (2-5). Endothelial dysfunction permits subendothelial accumulation of low-density lipoprotein and the recruitment of monocytes and lymphocytes to the artery wall (6,7). This process triggers the production of various pro-inflammatory cytokines from endothelial cells (2-7). Also, highly oxidized LDL particles within vessel wall are uptaken by macrophages, leading to foam-cell formation (8). These atherosclerotic mechanisms have been considered to induce the development and propagation of atherosclerosis, leading to cardiovascular events.
Traditionally, imaging of atherosclerosis has focused on the assessment of luminal narrowing and occlusion. However, the majority of life threatening consequences of atherosclerosis including myocardial infarction result from plaque rupture and acute thrombus formation, and it is now well recognized that plaque composition is closely associated with the vulnerability of these vascular lesions (9,10). Consequently, there are emerging needs for imaging modality that enables the identification of atherosclerotic plaques with high-risk features. Particularly, non-invasive approach would be ideal to detect high-risk patients who require intensive medical therapies.
Recently, considerable advances have been made in imaging techniques for the assessment of these atherosclerotic changes. Various imaging modalities, either noninvasive or invasive, have become available to identify high-risk patients at a relatively early stage and provide the opportunity to evaluate the impact of anti-atherosclerotic medical therapies.
The current review summarizes a range of non-invasive imaging modalities to visualize plaques.
Computed tomography (CT)
There are two different types of available CT. Electron beam computed tomography (EBCT) is the non-mechanical movement of the X-ray source and multi-detector-row computed tomography (MDCT) is performed by the motion of the X-ray source and table, combined with multiple detection to acquire the data in spiral or helical fashion (11). Accumulating evidence suggests the ability of EBCT/MDCT to evaluate coronary artery calcification, atherosclerosis and fractional flow reserve (FFR).
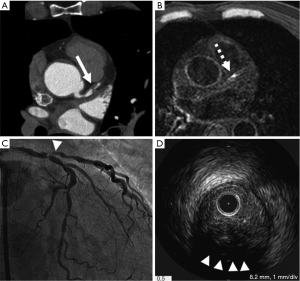
Coronary artery calcification (Figure 1)
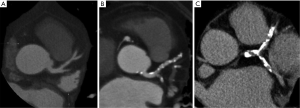
Atherosclerotic coronary calcifications are frequently observed at advanced lesions (American Heart Association plaque type Vb) (12) and at high-risk plaque as spotty or speckled pattern. Both EBCT and MDCT are able to accurately quantify coronary artery calcium (CAC). EBCT allows faster imaging by moving the X-ray source-point electronically.
Agatston et al. proposed a method to measure CAC by using CAC score (13). In addition to CAC score, a range of measures of coronary artery disease (CAD) including calcium volume score and calcium mass score has been used in other studies (14,15). However, the difference and accuracy in each CAC measure is still on debate. Currently, CAC scoring by Agatston et al. still remains the most widely used measure to evaluate the extent of CAC in both epidemiological research and clinical settings (16).
Various studies demonstrated that the amount of coronary calcium detected by CT correlates with atheroma burden of the coronary artery on histology and that CAC is an independent predictor of cardiovascular events (17-19). While the absence of CAC was associated with a very low risk of events (0.5%), in case of intermediate (CAC score 100–400) and high levels (CAC score >400) of CAC, the relative risk of cardiovascular events was 4.3 [95% confidence interval (CI), 3.1–6.1] and 7.2 (95% CI, 5.2–9.9, P<0.0001) in intermediate (CAC score 100–400) and high CAC scores (CAC score >400), respectively compared with low levels of CAC (CAC score =0) (20). In addition, CAC scores added significantly to risk prediction beyond traditional Framingham risk scores, particularly among persons considered to be at intermediate risk. According to these findings, the use of CAC quantification in intermediate risk patients is a Class IIb recommendation by the American Heart Association to improve risk assessment (20).
It was also shown that progression of CAC was associated with multiple risk factors and the increased risk for future cardiovascular events (21-23). This suggests the potential use of serial evaluation of CAC scores to assess the efficacy of novel anti-atherosclerotic therapies for the reduction of cardiovascular events. However, the benefit of slowing progression of CAC under use of anti-atherosclerotic medical therapies has not been demonstrated yet. For instance, statins did not slow the progression of CAC in any randomized controlled trials (24,25).
As such, it remains to be determined to what degree its measurement provides an incremental benefit in risk prediction with clinical use of calcium scoring and whether its use ultimately changes the treatment strategy or outcome for individual patients. The relative failure of intensive lipid lowering strategies to slow progression of coronary calcium scores also suggests that CAC measurement does not have enough ability in the management of coronary atherosclerosis as well as patients. In addition, MDCT is increasingly becoming a major CT imaging modality rather than EBCT because MDCT is capable of evaluating both coronary artery calcification and stenosis. Therefore, EBCT is less likely to play an important role in the clinical settings.
Coronary atherosclerosis
Because of high spatial resolution, MDCT is increasingly becoming a major modality to visualize coronary artery stenosis as well as atherosclerotic plaque morphology. Since the introduction of 4-slice scanners, the technique has developed rapidly and 64-slice and even 320-slice systems are currently available. These new imaging techniques have resulted in improvements in both temporal and spatial resolution, thereby enabling superior image quality (Figure 2). Large numbers of studies reported the improved accuracy in detecting significant coronary artery stenosis, although the strength of this evidence lies more in its negative predictive value rather than ability to precisely quantify the degree of lumen stenosis (26-28). Meta-analyses from 27 studies including 1,740 patients showed that sensitivity, specificity, positive predictive and negative predictive values for native coronary artery stenosis were 86%, 96%, 83%, and 96% by per-segment analysis; 97%, 91%, 93%, and 96% by per-patient analysis (29). This finding highlights the advantage of MDCT to assess coronary artery stenosis. As a consequence, the recent guidelines from the European Society of Cardiology recommends the use of MDCT in patients at low or intermediate pretest probability for CAD in order to avoid unnecessary invasive coronary angiography (30).
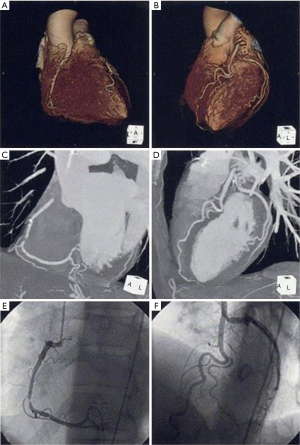
MDCT also has the potential to provide information about lesion plaque composition (Figure 3). Previous studies showed that MDCT density expressed in hounsfield units corresponds well with echogenicity and plaque composition on intravascular ultrasound (31). This ability of MDCT enables to distinguish non-calcified, mixed and calcified plaques. Recent observational study demonstrated that plaques associated with acute coronary syndrome exhibited distinct plaque features including lower density values, positive remodeling and spotty calcification (32). Additionally, the presence of positive remodeling and low-attenuation plaques on MDCT predicted future acute coronary events (33).
These observations indicate the wide availability of MDCT in the clinical setting. For instance, as MDCT is useful for screening coronary artery stenosis, unnecessary invasive coronary angiography could be avoided. High-risk features of atherosclerotic plaques on MDCT enable to apply more intensive medical therapies to prevent future events. As such, MDCT seems to be easily applicable to manage patients with suspected or established CAD.
Several important limitations of MDCT should be considered. Firstly, severe calcification limits lumen assessment due to blooming artifacts. In case of severely calcified lesions, MDCT can yield false positive results. Secondly, the technique is associated with radiation exposure, although significant dose reductions have been achieved with recent advances in scanner hardware and acquisition protocols. In addition, the resolution of MDCT to monitor plaque composition is inferior compared to invasive intravascular imaging techniques. Further improvement in plaque characterization is expected with the development of dual-energy MDCT or dedicated contrast agents.
Fractional flow reserve (FFR)
FFR is an invasive physiological index that can be measured during intracoronary administration of acetylcholine to assess the functional significance of coronary artery stenosis (34). Recently, technological innovation allows to calculate coronary flow and pressure based on anatomic MDCT image data without hyperemia, thereby measuring FFR noninvasively (35,36). Several studies demonstrated the feasibility and diagnostic performance of FFR evaluation on MDCT (FFRCT) (37-41). Recent multicenter international study investigated the ability of FFRCT for diagnosis of ischemia compared to an invasive FFR measurement in 252 stable patients with suspected or known CAD (37). In this study, diagnostic accuracy, sensitivity, specificity, positive and negative predictive values of FFRCT plus MDCT were 73% (95% CI, 67–78%), 90% (95% CI, 84–95%), 54% (95% CI, 46–83%), 67% (95% CI, 60–74%), and 84% (95% CI, 74–90%), respectively. In addition, FFRCT was associated with better evaluation of ischemia compared to CT alone (area under the receiver operating characteristic curve, 0.81; 95% CI, 0.75–0.86 vs. 0.68; 95% CI, 0.62–0.74, P<0.001) (37). Meta-analyses from 5 studies including 706 patients and 1,165 vessels showed that sensitivity and specificity were 83% (95% CI, 79–87%) and 78% (95% CI, 75–81%) by per-segment analysis; 90% (95% CI, 79–87%), and 72% (95% CI, 67–76%) by per-patient analysis, respectively (38). The area under the curve was 0.94 at the per-patient level and 0.91 at the per-vessel level (38).
These observations indicate FFRCT as a potential non-invasive toll to assess the presence of ischemia. However, several potential limitations should be considered. The diagnostic performance of FFRCT is impaired by CT imaging artifacts including misalignment, motion, beam hardening from coronary calcification, and increased image noise. Physiologic conditions may affect assumed parameters such as fluid density and viscosity on FFRCT. As viscosity is assumed from hematocrit/hemoglobin concentration, patients with severe anemia would exhibit reduced viscosity, potentially leading to the inaccurate calculation of FFRCT. Further investigation is required to elucidate the ability of FFRCT for the management of patients with CAD.
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA)
High-resolution MRI and MRA emerged as the versatile non-invasive in vivo imaging modality for coronary artery stenosis and plaque characterization.
Coronary artery stenosis
Coronary MRA assess the proximal and mid portion of coronary arteries, especially left anterior descending and right coronary artery. By contrast, the image quality of left circumflex is diminished due to an increased distance from the cardiac coil. In general, the possible imaged length for left anterior descending artery is 50 mm, for right coronary artery is 80mm and for left circumflex artery is 40 mm (42-49). Previous studies showed an excellent agreement between the proximal segments of coronary on MRA and invasive angiography (50).
As mentioned above, MDCT allows to evaluate coronary artery stenosis accurately. One recent meta-analysis showed that MDCT was more accurate than MRA for the assessment of significant coronary artery stenosis (51). However, in a recent multicenter study, whole-heart magnetic resonance (MR) at 1.5T detected significant CAD with high sensitivity (88%), moderate specificity (72%) and a negative predictive value of 88%, showing the acceptable ability of this technique to exclude the presence of significant CAD (52). Interestingly in particular, this negative predictive value is similar to that in the CORE-64 MDCT multicenter study (53). Moreover, in another study, there was no significant difference in the detection of CAD between 3T MRI and 64-slice MDCT (54). MRA might have the acceptable ability to assess coronary artery narrowing compared to MDCT.
It should be noted that MRA imaging takes more time compared to MDCT and some patients can not tolerate, whereas advantage of MRA is to visualize coronary arteries without any contrast medium. Therefore, MDCT seems to be more applicable non-invasive imaging tool in the clinical settings and MRA is good for patients with kidney disease.
Plaque composition
MRI is able to differentiate plaque components on the basis of biophysical and biochemical parameters, such as chemical composition, water content, physical state, molecular motion, or diffusion (55). Specifically, recent improvements in MR techniques such as multicontrast MR, generated by T1- and T2-weighted, proton-density-weighted, and time-of-flight imaging have been shown to help to characterize fibrocellular, lipid-rich, and calcified regions of atherosclerotic coronary plaques (56-59).
One imaging study investigated the ability of high-resolution, black-blood MR to assess coronary wall thickness (60). In 13 subjects including 8 normal subjects and 5 patients with CAD, the coronary artery wall was clearly seen in all patients and had distinct MR signal characteristics of surrounding tissue. In brief, the average coronary wall thickness for each cross-sectional image was 0.75±0.1 7 mm in the normal subject, whereas it was 4.38±0.71 mm in patients with localized atherosclerotic lesions. The difference in these wall thicknesses was statistically significant (P<0.0001).
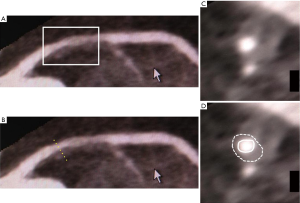
The feasibility of contrast-enhanced cardiac MRI for vessel wall imaging has been shown to characterize fibrous plaque tissue and neovascularization in patients with advanced carotid artery stenosis (61,62). In preliminary data analyzing patients with stable CAD, contrast-enhanced cardiac MRI visualized different types of plaque composition in major coronary arteries compared with MDCT (63). In addition, this imaging has been demonstrated to image inflammatory tissue signal changes in patients with carotid artery stenosis (64-66), giant cell arteritis (67) or Takayasu’s arteritis (68). In 10 patients with acute myocardial infarction, serial contrast-enhanced cardiac MRI imaging identified changes in spatial extent and intensity of coronary contrast enhancement (Figure 4) (69).
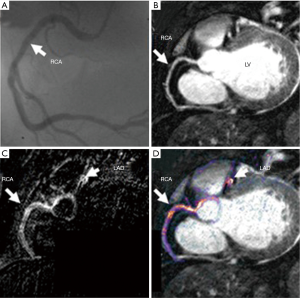
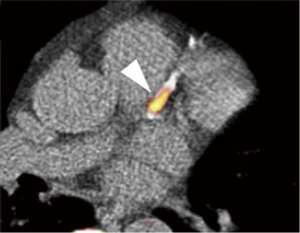
Recent studies demonstrated that the visualization of plaque instability by using non-contrast T1WI MRI imaging. Kawasaki et al. reported that the presence of coronary high-intensity plaques detected by non-contrast T1WI is associated with positive coronary remodeling, low density on MDCT, and ultrasound attenuation (Figure 5) (70). Based on this finding, Noguchi et al. investigated the predictive ability of high-intensity signals within coronary plaques on non-contrast T1-Weighted MRI in cardiovascular events in 568 patients with suspected or known CAD (71). In this study, plaque-to-myocardium signal intensity ratio >1.4 was significantly associated with an increased risk of future coronary events.
These findings suggest the promising ability of MRI in imaging coronary atherosclerosis in the clinical settings. However, most of studies were conducted in relatively small study population. In addition, MRI has several limitations such as cost, length of the examination and inability of the patient to tolerate. Further technological advances are expected to make MRI more applicable modality in the clinical settings.
Positron emission tomography (PET)
Nuclear imaging techniques such as PET can target distinct mediators and regulators involved in the cascade of atherosclerosis.
18F-FDG (fluorodeoxyglucose) PET
PET imaging with F18-FDG is currently considered to be one of the most promising imaging modalities to visualize plaque inflammation. 18F-FDG is a radioactively labeled glucose molecule that is readily consumed by the cells showing high metabolic activities (72-74). 18F-FDG was started to use for imaging of the brain and tumors (75,76). Then, its use has been extended to imaging of atherosclerotic plaque and inflammation. Its effectiveness at diseased lesions harbouring high levels of metabolic activities has been demonstrated in preclinical and clinical studies (77).
Mechanistically, the ability of cells to utilize glucose analog is a key for the adequate uptake of 18F-FDG for imaging plaque inflammation. The way that 18F-FDG enters the cells is equivalent to the way glucose does through the glucose transporter (GLUT) protein system. 18F-FDG becomes phosphorylated to 18F-FDG-6 phosphate, but it cannot be metabolized further in the glycolytic pathway. Thus, it accumulates in the cells proportionally, enabling its imaging by PET/CT.
For the analysis of signal acquired from 18F-FDG in PET/CT imaging, the standardized uptake value (SUV) has been used extensively (78-82). The SUV is calculated by dividing the decay-corrected tissue concentration (kilobecquerels per milliliter) by the injected dose of 18F-FDG per body weight (kilobecquerels per gram) (83). The target to background ratio (TBR) is another quantitative measure for the extent of plaque inflammation. The TBR is calculated by dividing the SUV of the artery of interest by that of the venous blood pool. The maximum and mean values of TBR seem to provide the most reliable results for evaluating atherosclerotic inflammation (84).
Recent study reported feasibility of FDG-PET for coronary artery inflammation imaging by using refined image acquisition methods. Rogers et al. underwent cardiac CT angiography and PET imaging with 18F-FDG after invasive angiography in 25 patients with CAD (Figure 6) (85). TBR was greater at culprit lesions in patients with acute coronary syndrome compared to those within lesions of patients with stable CAD. Additionally, TBR correlated with C-reactive protein (r=0.58, P=0.04).
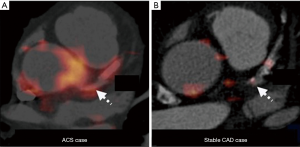
Despite its potential applicability for the evaluation of plaque inflammation, it is still difficult to image coronary artery. In particular, myocardial 18F-FDG uptake would hinder isolation of signal from the adjacent coronary artery. Coronary motion also influences quality of imaging. Imaging coronary atherosclerosis by FDG-PET still needs more technological advances.
18F-NaF (sodium fluoride) PET
Calcification is one of the important components associated with plaque stability instability. NaF is a promising PET imaging agent to visualize plaque calcification, which is a marker of active mineralization. In 269 asymptomatic cancer patients, 35% of patients had uptake of NaF within carotid arteries. In addition, NaF significantly correlated with the degree of atherosclerotic calcification on CT imaging (r=0.85, P<0.0001) and traditional cardiovascular risk factors (86). Derlin et al. retrospectively reviewed 45 studies using PET/CT in oncology patients (87). In this study, calcified lesions were associated with NaF rather than FDG (77% vs. 15%) and the overlap between NaF and FDG signals was minimal, occurring in only 6.5% of patients. In a study of 119 subjects with or without aortic valve stenosis, NaF uptake was significantly associated with prior cardiovascular events and traditional risk factors. In addition, uptake of NaF was correlated with CAC score (r=0.652, P<0.001) (Figure 7) (88). Of note, patients with high CAC scores >1,000 had no detectable NaF activity. Considering that CAC scores of >1,000 are associated with a markedly elevated cardiovascular risk, NaF might detect earlier-stage calcified lesions. Thus, NaF is a promising agent for plaque calcification and provides new insights into atheroma progression complementary to FDG-PET. On the other hand, several issues remain, including refinement of techniques to control for cardiac and respiratory motion, as well as improvement in spatial resolution. Further studies are needed to establish 18F-NaF PET-CT will provide a clinically useful technique capable of improving risk stratification, monitoring disease progression and assessing novel anti-atherosclerotic therapies. PET imaging is an expensive study with radiation exposure. Considering these advantaged and disadvantages of 18F-NaF PET imaging, we have to establish refined imaging method and conduct clinical outcome studies in large study population to elucidate the association between NaF activity within plaques and cardiovascular events.
Conclusions
Various non-invasive imaging modalities allow to evaluate both atheroma burden and composition (Table 1). This technological advance will enable to identify high-risk subjects prior to the occurrence of cardiovascular events, thereby potentially improving clinical outcomes. Although new imaging modalities will provide us a greater understanding about the mechanism of atherosclerosis, improved spatial resolution will be necessary to accurately evaluate plaque composition and assess the impact of medical therapies. In the future, more clinical trials are needed to demonstrate whether imaging assessment of atherosclerosis will improve treatment strategies and clinical outcomes. We also have to understand and investigate how these modalities can be used more effectively in the development of emerging therapies aimed to prevent cardiovascular disease.

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:e21-181. [Crossref] [PubMed]
- Fuster V. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation 1994;90:2126-46. [Crossref] [PubMed]
- Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med 1999;340:115-26. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Libby P. Inflammation in atherosclerosis. Nature 2002;420:868-74. [Crossref] [PubMed]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551-61. [Crossref] [PubMed]
- Borén J, Gustafsson M, Skålén K, et al. Role of extracellular retention of low density lipoproteins in atherosclerosis. Curr Opin Lipidol 2000;11:451-6. [Crossref] [PubMed]
- Tsimikas S, Miller YI. Oxidative modification of lipoproteins: mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des 2011;17:27-37. [Crossref] [PubMed]
- Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988;78:1157-66. [Crossref] [PubMed]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657-71. [Crossref] [PubMed]
- Budoff MJ, Achenbach S, Duerinckx A. Clinical utility of computed tomography and magnetic resonance techniques for noninvasive coronary angiography. J Am Coll Cardiol 2003;42:1867-78. [Crossref] [PubMed]
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355-74. [Crossref] [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [Crossref] [PubMed]
- Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208:807-14. [Crossref] [PubMed]
- Becker CR, Kleffel T, Crispin A, et al. Coronary artery calcium measurement: agreement of multirow detector and electron beam CT. AJR Am J Roentgenol 2001;176:1295-8. [Crossref] [PubMed]
- Alluri K, Joshi PH, Henry TS, et al. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis 2015;239:109-17. [Crossref] [PubMed]
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126-33. [Crossref] [PubMed]
- Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157-62. [Crossref] [PubMed]
- Schmermund A, Denktas AE, Rumberger JA, et al. Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: comparison with cardiac risk factors and radionuclide perfusion imaging. J Am Coll Cardiol 1999;34:777-86. [Crossref] [PubMed]
- O'Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association Expert Consensus Document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol 2000;36:326-40. [Crossref] [PubMed]
- Cassidy-Bushrow AE, Bielak LF, Sheedy PF 2nd, et al. Coronary artery calcification progression is heritable. Circulation 2007;116:25-31. [Crossref] [PubMed]
- Kramer CK, von Muhlen D, Gross JL, et al. Blood pressure and fasting plasma glucose rather than metabolic syndrome predict coronary artery calcium progression: the Rancho Bernardo Study. Diabetes Care 2009;32:141-6. [Crossref] [PubMed]
- Houslay ES, Cowell SJ, Prescott RJ, et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart 2006;92:1207-12. [Crossref] [PubMed]
- Terry JG, Carr JJ, Kouba EO, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am J Cardiol 2007;99:1714-7. [Crossref] [PubMed]
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006;113:427-37. [Crossref] [PubMed]
- Raff GL, Gallagher MJ, O'Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 2005;46:552-7. [Crossref] [PubMed]
- Leber AW, Johnson T, Becker A, et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J 2007;28:2354-60. [Crossref] [PubMed]
- Dewey M, Teige F, Schnapauff D, et al. Noninvasive detection of coronary artery stenoses with multislice computed tomography or magnetic resonance imaging. Ann Intern Med 2006;145:407-15. [Crossref] [PubMed]
- Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol 2006;48:1896-910. [Crossref] [PubMed]
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109:14-7. [Crossref] [PubMed]
- Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26. [Crossref] [PubMed]
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [Crossref] [PubMed]
- Pijls NH, De Bruyne B. Coronary pressure mea- surement and fractional flow reserve. Heart 1998;80:539-42. [Crossref] [PubMed]
- Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive frac- tional flow reserve computed from coronary com- puted tomographic angiograms: results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Non-invasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989-97. [Crossref] [PubMed]
- Kim HJ, Vignon-Clementel IE, Coogan JS, et al. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng 2010;38:3195-209. [Crossref] [PubMed]
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. [Crossref] [PubMed]
- Deng SB, Jing XD, Wang J, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in coronary artery disease: A systematic review and meta-analysis. Int J Cardiol 2015;184:703-9. [Crossref] [PubMed]
- Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145-55. [Crossref] [PubMed]
- Kim KH, Doh JH, Koo BK, et al. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv 2014;7:72-8. [Crossref] [PubMed]
- Renker M, Schoepf UJ, Wang R, et al. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol 2014;114:1303-8. [Crossref] [PubMed]
- Botnar RM, Stuber M, Danias PG, et al. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation 1999;99:3139-48. [Crossref] [PubMed]
- Hofman MB, Paschal CB, Li D, et al. MRI of coronary arteries: 2D breath-hold vs 3D respiratory-gated acquisition. J Comput Assist Tomogr 1995;19:56-62. [Crossref] [PubMed]
- Lobbes MB, Miserus RJ, Heeneman S, et al. Atherosclerosis: contrast-enhanced MR imaging of vessel wall in rabbit model - comparison of gadofosveset and gadopentetate dimeglumine. Radiology 2009;250:682-91. [Crossref] [PubMed]
- Manning WJ, Li W, Boyle NG, et al. Fat-suppressed breath-hold magnetic resonance coronary angiography. Circulation 1993;87:94-104. [Crossref] [PubMed]
- Paschal CB, Haacke EM, Adler LP. Three-dimensional MR imaging of the coronary arteries: preliminary clinical experience. J Magn Reson Imaging 1993;3:491-500. [Crossref] [PubMed]
- Post JC, van Rossum AC, Hofman MB, et al. Three-dimensional respiratory-gated MR angiography of coronary arteries: comparison with conventional coronary angiography. AJR Am J Roentgenol 1996;166:1399-404. [Crossref] [PubMed]
- Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol 1999;34:524-31. [Crossref] [PubMed]
- Scheidegger MB, Muller R, Boesiger P. Magnetic resonance angiography: methods and its applications to the coronary arteries. Technol Health Care 1994;2:255-65. [PubMed]
- Bogaert J, Kuzo R, Dymarkowski S, et al. Coronary artery imaging with real-time navigator three-dimensional turbo-field-echo MR coronary angiography: initial experience. Radiology 2003;226:707-16. [Crossref] [PubMed]
- Schuetz GM, Zacharopoulou NM, Schlattmann P, et al. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med 2010;152:167-77. [Crossref] [PubMed]
- Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol 2010;56:983-91. [Crossref] [PubMed]
- Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. [Crossref] [PubMed]
- Hamdan A, Asbach P, Wellnhofer E, et al. A prospective study for comparison of MR and CT imaging for detection of coronary artery stenosis. JACC Cardiovasc Imaging 2011;4:50-61. [Crossref] [PubMed]
- Fayad ZA. MR imaging for the noninvasive assessment of atherothrombotic plaques. Magn Reson Imaging Clin N Am 2003;11:101-13. [Crossref] [PubMed]
- Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance ima- ging. Circulation 2002;106:1368-73. [Crossref] [PubMed]
- Chu B, Phan BA, Balu N, et al. Reproducibility of carotid atherosclerotic lesion type characterization using high resolution multicontrast weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:793-9. [Crossref] [PubMed]
- Oikawa M, Ota H, Takaya N, et al. Carotid magnetic resonance imaging, A window to study atherosclerosis and identify high-risk plaques. Circ J 2009;73:1765-73. [Crossref] [PubMed]
- Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368-73. [Crossref] [PubMed]
- Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 2000;102:506-10. [Crossref] [PubMed]
- Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for Atherosclerotic carotid artery tissue characterization J Magn Reson Imaging 2002;15:62-7. [Crossref] [PubMed]
- Wasserman BA, Smith WI, Trout HH 3rd, et al. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 2002;223:566-73. [Crossref] [PubMed]
- Yeon SB, Sabir A, Clouse M, et al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J Am Coll Cardiol 2007;50:441-7. [Crossref] [PubMed]
- Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15:62-7. [Crossref] [PubMed]
- Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 2003;107:851-6. [Crossref] [PubMed]
- Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005;112:3437-44. [Crossref] [PubMed]
- Bley TA, Wieben O, Uhl M, et al. High-resolution MRI in giant cell arteritis: imaging of the wall of the superficial temporal artery. AJR Am J Roentgenol 2005;184:283-7. [Crossref] [PubMed]
- Choe YH, Han BK, Koh EM, et al. Takayasu's arteritis: assessment of disease activity with contrast-enhanced MR imaging. AJR Am J Roentgenol 2000;175:505-11. [Crossref] [PubMed]
- Ibrahim T, Makowski MR, Jankauskas A, et al. Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc Imaging 2009;2:580-8. [Crossref] [PubMed]
- Kawasaki T, Koga S, Koga N, et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging 2009;2:720-8. [Crossref] [PubMed]
- Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol 2014;63:989-99. [Crossref] [PubMed]
- Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11:443-57. [Crossref] [PubMed]
- El-Haddad G, Zhuang H, Gupta N, et al. Evolving role of positron emission tomography in the management of patients with inflammatory and other benign disorders. Semin Nucl Med. 2004;34:313-29. [Crossref] [PubMed]
- Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50:1611-20. [Crossref] [PubMed]
- Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med 2002;32:6-12. [Crossref] [PubMed]
- Som P, Atkins HL, Bandoypadhyay D, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 1980;21:670-5. [PubMed]
- Ben-Haim S, Kupzov E, Tamir A, et al. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med 2004;45:1816-21. [PubMed]
- Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1009-16. [Crossref] [PubMed]
- Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818-24. [Crossref] [PubMed]
- Hiari N, Rudd JH. FDG PET imaging and cardiovascular inflammation. Curr Cardiol Rep 2011;13:43-8. [Crossref] [PubMed]
- Font MA, Fernandez A, Carvajal A, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci (Landmark Ed) 2009;14:3352-60. [Crossref] [PubMed]
- Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294-301. [Crossref] [PubMed]
- Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49:871-8. [Crossref] [PubMed]
- Bural GG, Torigian DA, Chamroonrat W, et al. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging 2008;35:562-9. [Crossref] [PubMed]
- Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 2010;3:388-97. [Crossref] [PubMed]
- Derlin T, Wisotzki C, Richter U, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med 2011;52:362-8. [Crossref] [PubMed]
- Derlin T, Toth Z, Papp L, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. J Nucl Med 2011;52:1020-7. [Crossref] [PubMed]
- Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539-48. [Crossref] [PubMed]


