Therapeutic modulation of the natural history of coronary atherosclerosis: lessons learned from serial imaging studies
Introduction
As the principle cause of coronary artery disease (CAD) and myocardial infarction (MI), atherosclerosis is a leading cause of mortality and morbidity worldwide. Atherosclerosis describes a chronic, systemic inflammatory process, in which plaques develop in arteries and limit blood flow. Thrombotic occlusion of atherosclerotic vessels most commonly occurs as a consequence of plaque rupture or erosion. Studies with intracoronary imaging techniques have revealed that plaques that are vulnerable to rupture have hallmark features, including high plaque volume, necrotic core, lipid and inflammatory cell content, thin fibrous caps, intraplaque neovascularization and hemorrhage (1). Multiple unstable plaques often co-exist in the coronary vasculature. Although large clinical trials have demonstrated the benefits of primary and secondary prevention therapies targeted against CAD risk factors, such as dyslipidemia (2) and blood pressure (BP) (3), the prevalence of plaque rupture or erosion leading to initial or recurrent MI remains high (4).
In order to optimize management of atherosclerosis and its complications, there is a continued need to better understand the natural history of this disease, and its responsiveness to different treatments. This has been made increasingly possible by advances in various coronary imaging techniques that allow the serial assessment of plaque burden and composition over time. These modalities include invasive intravascular ultrasound (IVUS), optical coherence tomography (OCT) and near infrared spectroscopy (NIRS), and less invasive coronary computed tomography angiography (CTA), magnetic resonance imaging (MRI) and molecular imaging with positron emission tomography (PET). Unlike conventional coronary angiography (CAG), which informs about the degree of luminal stenosis caused by plaque, these other techniques also provide assessment of the vessel wall itself and its atherosclerotic substrate. Their ability to measure and characterize baseline plaque burden and the rate of progression in serial observations over time provides a means of predicting risk for adverse cardiovascular outcomes, namely coronary revascularization, MI and death (5). These coronary imaging modalities therefore play an integral role in preclinical and clinical studies designed to evaluate the natural history of CAD and its responsiveness to traditional and experimental therapeutic interventions. In this review, we discuss the lessons learned from these imaging studies and how they continue to shape our understanding of anti-atherosclerotic therapies.
Invasive modalities for serial CAD assessment
Coronary angiography
CAG has traditionally been used to identify stenotic lesions within the coronary arteries. Randomized trials using serial CAG have shown the effects of medical therapies on coronary disease (6). However, there are limitations to using this imaging technique to quantify atherosclerotic plaque burden. Quantitative coronary angiographic measurements are associated with a lack precision due to variations in equipment and inter-observer differences (7). The imaging produced by CAG is a two-dimensional silhouette of the lumen, and does not include the vessel wall. The reduction in the size of the lumen does not occur until the disease has reached an advanced stage. This limitation has led to a need for vessel wall-based imaging modalities.
Intravascular ultrasound (IVUS)
IVUS allows the placement of a catheter containing a high-frequency ultrasound transducer within the lumen of coronary arteries. Early use of IVUS focused on the role of assisting in the deployment of stents to improve procedural and longer-term clinical outcomes (8). The ability to place an ultrasound transducer in close proximity to the vessel wall enables cross-sectional imaging (Figure 1A). Therefore IVUS provides a more extensive view than CAG of the distribution and nature of plaque burden within the vessel wall (10). Early autopsy studies confirmed that coronary artery segments with a normal angiographic appearance invariably harbor plaque visible on IVUS (11). The ability to visualize and perform serial measurements of the lumen and surrounding vessel wall in multiple contiguous cross-sections enables reproducible quantification of plaque burden, and IVUS represents the current gold standard for this purpose. As described below, numerous studies have used serial IVUS imaging to evaluate the impact of medical therapies, such as statins for cholesterol lowering, upon progression or regression of coronary atherosclerosis.
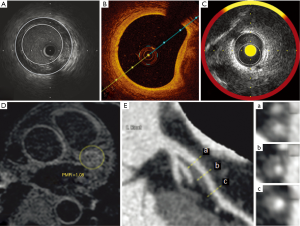
Optical coherence tomography (OCT)
OCT is another intravascular imaging modality that uses near-infrared light, usually of a wavelength of approximately 1,300 nm, to create images of plaque atheroma in the coronary arteries (Figure 1B). Its greatest advantages over other imaging modalities is its high spatial resolution (approximately ten-times higher than that of IVUS) with an axial resolution of up to 10 µm and a lateral resolution of up to 20 µm (12,13). This increase in resolution provides high-quality imaging and quantitative analysis of various components of atheroma below the intimal endothelial surface, including fibrous cap, vascular microchannels, microcalcification, lipid content and macrophage burden (14,15). Imaging of these features gives OCT a potential role in assessing factors that increase the vulnerability of atherosclerotic plaques. However, the benefits of high imaging resolution are somewhat offset by OCT's poor tissue penetration, making it difficult to image the full thickness and deeper layers of atherosclerotic plaques, especially in large vessels, while interference from blood can also adversely affect image quality.
Virtual histology (VH)
There is now increasing interest to look beyond the effect of medical therapies on plaque burden, and also investigate how they modify the composition of plaque. Studies have demonstrated that lesions containing more lipid, inflammation and necrotic material are more vulnerable (16), with greater propensity to plaque rupture and ischemic events (1). Although conventional ultrasonography is able to detect calcium and characterize the degree of echogenicity of plaque, it lacks the resolution to comprehensively monitor therapeutic-induced changes in plaque composition.
Virtual histology IVUS (VH-IVUS) is a technique based on advanced radiofrequency analysis of reflected ultrasound signals. A reconstructed color-coded tissue map of plaque composition is superimposed onto cross-sectional images of the coronary artery obtained by grayscale IVUS, that distinguishes between fibrous, fibrofatty, necrotic core and dense calcific material (17). VH-IVUS has been applied in clinical settings to demonstrate the association between cardiovascular events and plaque containing greater quantities of both necrotic and lipidic material (18). These associations have led to the integration of VH-IVUS in studies evaluating the biological effects of anti-atherosclerotic therapies.
Near-infrared spectroscopy (NIRS)
NIRS is based on the absorbance of light by organic molecules, and determines characteristics of the chemical components of tissue samples. Advances in catheter techniques have enabled NIRS imaging within coronary arteries (Figure 1C) (19). Early development of this imaging technique has been used to assess lipid and protein content within atherosclerotic plaque (20). While the NIRS signal has the capability to detect the spectra of the different molecules within plaque, at this stage it has primarily been applied to detect lipid. Spectroscopic information obtained from raw spectra is transformed into a probability of lipid core that is mapped to a red-to-yellow color scale, with low probability of lipid shown as red and high probability of lipid shown as yellow. Yellow pixels within the analyzed segment are then divided by all viable pixels to generate the lipid-core burden index (LCBI). Further work is required to broaden the application of NIRS to provide more comprehensive assessment of plaque, so that it can be used in serial studies to quantify plaque response to therapies.
Less invasive modalities for serial CAD assessment
Non-contrast computed tomography
The accumulation of calcium generally occurs relatively late in the atherosclerotic process. Non-contrast CT allows detection of calcium as a surrogate means of assessing coronary atheroma burden. Quantification of coronary artery calcium (CAC), most commonly using the Agatston score, has been well demonstrated to correlate with coronary atheroma burden on histology (21), and predict risk of cardiovascular events in asymptomatic patients (22,23). Although the utility of non-contrast CT calcium scoring has been studied extensively for risk stratification, it has limited ability to measure treatment effect and cannot be recommended for the evaluation of plaque progression in treated individuals.
Coronary computed tomography angiography (CTA)
Multidetector row CT (MDCT) has evolved rapidly resulting in improvements in spatial and temporal resolution with current scanners and prospective gating techniques, radiation exposure has been reduced to 2–5 mSv. Administration of iodinated contrast is required with associated risk of contrast-induced nephropathy. While the resolution of coronary CTA itself remains inferior to invasive atherosclerotic imaging techniques, it is still able to detect luminal stenosis of ≥50% anatomic severity with sensitivity approaching 100%, and specificity in the order of 85%, compared to CAG on a per patient basis (24,25). In contrast, it performs less well when compared against intracoronary pressure assessment, with 80% sensitivity and 67% specificity for detecting fractional flow reserve measurements of <0.80 (25). Owing to its ability to characterize plaque composition (calcified versus non-calcified) and arterial remodeling, coronary CTA may also identify patients at high risk of future cardiovascular events (26). In particular the presence of positive remodeling, low attenuation plaque (<30 Hounsfield units), spotty calcification, and the napkin ring sign each confer a high positive predictive value for future acute coronary syndrome (ACS) episodes, while their absence has a high negative predictive value (27).
In addition to its noninvasiveness, coronary CTA carries the distinct advantage of being able to qualitatively and quantitatively assess overall plaque burden in the entire coronary vasculature, with recent data showing that widespread, multivessel, nonobstructive CAD confers similar risk of event rates to localized obstructive disease (28). Various semi-quantitative methods have been used to score the degree of plaque in coronary segments using coronary CTA (24), with the best results shown for semiautomatic expert-guided measurements, as determined by comparison against IVUS (29). Although coronary CTA provides excellent correlation of individual plaque measurements with IVUS (29), it is limited in detecting small noncalcified plaques (<1 mm) and may misclassify plaque subcomponents (30). This is especially the case at the low radiation doses (≤1 mSv) which have become achievable with contemporary coronary CTA technology, and which otherwise would enable patients to more safely undergo repeat CCTA examinations.
Although consensus statements recommend that coronary CTA is “adequate” for excluding significant CAD in patients with chest pain symptoms and intermediate pretest probability of CAD, its use is not approved in asymptomatic individuals. Similarly, although implemented in the experimental setting to monitor plaque change over time, further data are required to validate the accuracy and reproducibility of serial coronary CTA before it can be used routinely for follow-up imaging of plaque.
Magnetic resonance imaging (MRI)
MRI is a noninvasive technique that offers the opportunity to visualize vessel anatomy and differentiate atherosclerotic tissue without exposure to radiation, using features such as chemical composition, water content, molecular motion or diffusion. The recent improvements in MRI techniques such as T1-, T2- and proton-density-weighted imaging have provided the ability to produce information not only about plaque volume, but also composition in multiple arterial territories, including the aorta, carotids and main peripheral arteries, as demonstrated in animal models and human subjects (31-33). However, the resolution of 1.5-Tesla MRI with or without contrast is lower than coronary CTA (1.0–1.5 vs. 0.5–1.0 mm), limiting the accurate visualization of coronary arteries. Other limitations of coronary MR angiography include its relatively long acquisition times and operator dependency, although these are being addressed by ongoing refinements.
In one multicenter study the sensitivity of non-contrast-enhanced whole-heart coronary MR angiography was 88% on a patient-based analysis for detecting ≥50% stenosis shown by invasive CAG, although specificity was only 72% (34). High-field-strength 3.0-T systems improve the signal-to-noise ratio of coronary MR angiography, such that diagnostic accuracy of 3.0-T contrast-enhanced imaging approaches that of some coronary CTA systems (35). However administration of contrast is not without risks, such as the potential for nephrogenic systemic fibrosis in patients with severe renal dysfunction who receive gadolinium agents, resulting in a recent FDA advisory. Repeat gadolinium-enhanced MRI studies have also been linked to the presence of deposits in the dentate nucleus and globus pallidus regions of the brain, which are as yet of uncertain significance.
Noncontrast T1-weighted imaging (T1W1) has been used recently to detect high-intensity coronary plaques (HIPs), which are thought to represent the presence of intraplaque lipid, with or without hemorrhage (36). Studies have now validated measurement of the plaque-to-myocardium signal-intensity ratio (PMR) of HIPs has been recently validated as a method for predicting acute coronary events (37), and for monitoring the response of atheroma to cholesterol-lowering therapy (Figure 1D,E) (9).
The status of coronary MR angiography is that its use is considered “inappropriate” for the routine, non-invasive assessment of CAD, except to assess for anomalous coronary arteries and coronary artery aneurysms in patients with Kawasaki disease (38). By extension, in the absence of more substantial data it can also not be recommended for the surveillance of coronary atherosclerosis in current clinical practice.
Positron emission tomography (PET)
Nuclear imaging techniques, such as PET, have the ability to target distinct mediators and regulators involved in the evolution of atherosclerosis. Several radionuclide-labeled tracers have been developed to image inflammation, angiogenesis, apoptosis and lipid metabolism (39). PET imaging with 18F-fluorodeoxyglucose (FDG) has been considered to be especially promising imaging modality for the identification of vulnerable lesions. However, coronary artery imaging remains difficult due to background FDG uptake by the myocardium, along with the low spatial resolution of PET and small size of coronary arteries, and artefactual noise from cardiac motion, making FDG-PET inadequate for clinical assessment of CAD. Furthermore, there is also the matter of total body radiation exposure which is in the order of 7 mSv with contemporary myocardial 18F-FDG PET imaging
Experimental studies have demonstrated greater FDG uptake in plaques containing a large proportion of macrophages, suggesting that this approach may be used to evaluate the impact of therapies on the inflammatory composition of atherosclerotic plaques (40). More recently PET-based imaging of 18F-sodium fluoride uptake has been used to detect high risk features of unstable plaque in patients with carotid and coronary atherosclerosis (41). Although far from primetime, the integration of less invasive imaging techniques, such as MRI and PET with molecular targeted agents holds potential for evaluating new therapies on specific targets in atherosclerotic plaque that are associated with rupture (42).
Treatment targets to modulate plaque progression
The above imaging techniques have been used to assess modulation of coronary atherosclerosis in response to different established and novel medical therapies. As described below, the greatest body of work has been performed with serial IVUS imaging following cholesterol-lowering treatment with HMG-CoA reductase inhibitors (“statins”).
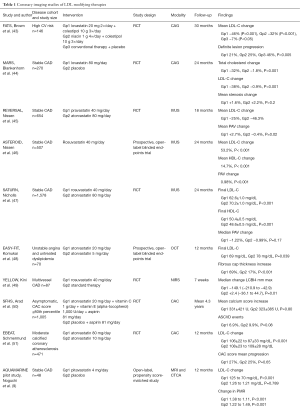
Lowering low-density lipoprotein (LDL) (Table 1)
Full table
The correlation between lowering levels of LDL cholesterol (LDL-C) and a reduction of cardiovascular event rates has been demonstrated in numerous clinical trials (2). These studies have tested a number of lipid-lowering interventions, most notably different types of statins. Early studies used quantitative CAG to demonstrate that LDL-C lowering is associated with less progression of obstructive CAD (6). In the Familial Atherosclerosis Treatment Study (FATS), men at high risk for cardiovascular events were assessed by quantitative angiography to determine the effect of intensive lipid-lowering therapy on coronary atherosclerosis (43). The mean change in the levels of LDL-C was slight in the conventional-therapy group (7%), but more substantial among patients treated with lovastatin and colestipol (46%), or niacin and colestipol (32%). In the conventional-therapy group, 46% of the patients had definite lesion progression. By comparison, progression was less frequent among patients who received lovastatin and colestipol (21%) and those who received niacin and colestipol (25%). Although a benefit on CAD progression was shown, this study evaluated men at a high risk of cardiovascular event rates and very high levels of LDL-C, with a mean LDL-C level of 189 mg/dL at baseline. Such high levels of LDL-C are not typical in most patients with CAD.
Other groups have explored the effect of lipid-modifying therapy on plaque progression in patients with mildly or moderately elevated LDL-C. In the Monitored Atherosclerosis Regression Study (MARS), the mean baseline LDL cholesterol was 151 mg/dL, and lovastatin did not produce a significant benefit on the primary endpoint of per-patient change in percent diameter stenosis measured by quantitative CAG (44). Lovastatin lowered LDL-C by 38%, and the average percent diameter stenosis increased 2.2% in the placebo group and 1.6% in the lovastatin group (P>0.20). This result, consistent with other angiographic trials of similar design, highlighted the limitations of CAG as an imaging technique to evaluate changes in atherosclerotic plaque burden, and underlined the need for studies to be conducted using vessel wall rather than lumen-based imaging modalities.
Serial coronary imaging by IVUS has shown that LDL-C lowering with high-intensity statin therapy provides benefit to atherosclerotic plaque burden (Figure 2). The REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study was a direct comparison of moderate lipid-lowering with pravastatin 40 mg and intensive lipid-lowering with atorvastatin 80 mg for 18 months (45). Baseline LDL-C (mean 3.9 or 150.2 mg/dL) levels were reduced to 2.8 mmol/L (110 mg/dL) in the pravastatin group compared with 2.0 mmol/L (79 mg/dL) in the atorvastatin arm (P<0.0001). The inflammatory biomarker C-reactive protein (CRP) decreased 5.2% with pravastatin and 36.4% with atorvastatin (P<0.0001). For all IVUS endpoints, progression occurred in the moderate-treatment cohort, and plaque volume was unchanged in the intensive arm. Direct relationships were shown between the degree of LDL-C lowering, slowing of disease progression and reduction of CRP (53). These results reflect the notion that aggressively treating CAD with statins has favorable anti-inflammatory effects in addition to lowering LDL-C levels.
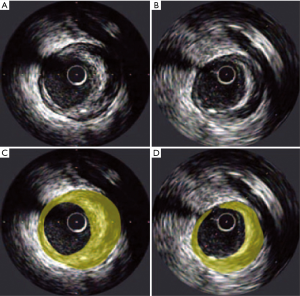
The ability of very high intensity statin therapy to alter the progression of plaque burden was investigated further in the ASTEROID (A Study To evaluate the Effect of Rosuvastatin On Intravascular ultrasound-Derived coronary atheroma burden) trial (46). A total of 349 patients who received 40 mg/d rosuvastatin had evaluable IVUS examinations at baseline and after 24 months. Rosuvastatin therapy was associated with a mean reduction of LDL-C of approximately 53%, along with an increase of HDL-C by almost 15%. Significant plaque regression was observed with a 6.8% median reduction in total atheroma volume. As an extension of these results, rosuvastatin 40 mg/day was then directly compared to atorvastatin 80 mg/day for 24 months in the SATURN (Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin) trial (47). The rosuvastatin group had lower levels of LDL-C than the atorvastatin group [62.6 vs. 70.2 mg/dL (1.62 vs. 1.82 mmol per liter), P<0.001], and higher levels of HDL-C [50.4 vs. 48.6 mg/dL (1.30 vs. 1.26 mmol per liter), P=0.01]. Despite these modest differences in final lipid profile, plaque regression was achieved to a comparable extent in both treatment groups, occurring in two thirds of the trial participants overall. Treatment with high intensity statins favorably impacted plaque progression in all subgroups of patients irrespective of baseline lipoprotein or CRP levels (54).
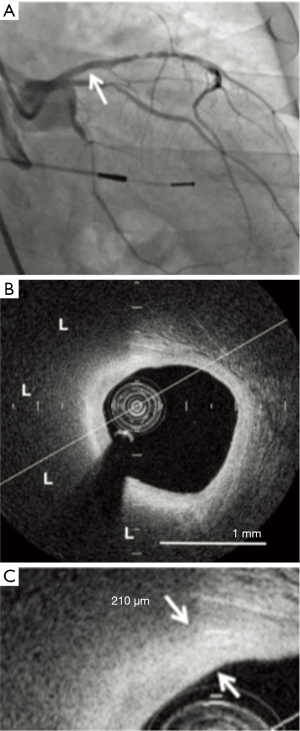
Further analysis of these IVUS-based statin trials has revealed that not only do these atherosclerotic plaques regress, they also become more stable (55). Statin use is pro-calcific irrespective of net plaque regression or progression, with the greatest increase of calcium found in those treated with high intensity statin therapy. Other imaging modalities have also been used to examine the effects of lipid lowering therapy on modification of atherosclerotic plaque composition. The EASY-FIT study investigated plaque stability by using OCT to serially image intermediate nonculprit lesions in 70 patients presenting with unstable angina and dyslipidemia, who were randomized to either 20 or 5 mg/d of atorvastatin. Imaging was performed at baseline and at 12-month follow-up (48). Lower LDL-C levels were obtained in the higher dose group (69 vs. 78 mg/dL, P=0.039), and this was associated with a significantly greater increase in fibrous cap thickness of 69% compared to 17% (P<0.001), suggesting more stable atheroma. The increase in fibrous cap thickness correlated with the decrease in serum LDL-C levels, along with decreases in high-sensitivity CRP, matrix metalloproteinase-9 and grade of OCT-measured macrophages. One single center study investigated the effects of potent statin therapy on plaque microstructures imaged by OCT (Figure 3). Fibrous cap thickness (P=0.01) was greater and the lipid arc was smaller (P=0.02) in nonculprit lipid plaques in stable patients receiving high dose statin therapy. High-dose statin therapy was associated with a greater fibrous cap thickness in patients with smaller (148.2±30.5 vs. 105.3±41.1 µm, P=0.004) but not larger lipid index (91.1±32.6 vs. 78.1±43.3 µm, P=0.21) (56). Similar results were seen in another single center study that examined the effects of the addition of ezetimibe (10 mg) to treatment with fluvastatin (30 mg) on plaque stabilization (57). The reduction in serum LDL-C was significantly larger in the ezetimibe and fluvastatin group compared with fluvastatin alone (−34.0±32.0 vs. −8.3±17.4 mg/dL, P<0.001). While fibrous cap thickness imaged by OCT significantly increased in both treatment groups after nine months of therapy, this change was also greater with the combination therapy (0.08±0.08 vs. 0.04±0.06 mm, P<0.001).
The PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) trial provided the first prospective natural history study of VH-IVUS-derived vulnerable plaque (58). Six hundred and ninety-seven patients with successful percutaneous coronary intervention underwent three-vessel imaging of the coronary arteries with quantitative angiography, grayscale IVUS and VH-IVUS. Thin-cap fibroatheroma (defined as fibrous cap thickness <65 µm, a minimal luminal area of 4.0 mm2, and a large plaque burden of at least 70%, were associated with higher occurrence of cardiovascular events. In the HEAVEN study, 89 patients with stable angina were treated with aggressive lipid lowering therapy of atorvastatin 80 mg and ezetimibe 10 mg or standard therapy (59). Coronary plaque burden regressed in the aggressive treatment group and progressed in the standard treatment group. However, by VH-IVUS there were no significant differences between the two arms with respect to plaque composition, with both groups showing a continuous plaque shift from fibrous and fibro-fatty to necrotic with calcification.
A subset of 71 patients from the SATURN trial underwent collection of VH-IVUS imaging along with grayscale IVUS. There were no changes in fibrous or necrotic core tissue volumes. However, a reduction in estimated fibro-fatty tissue volume accompanied atheroma regression, while dense calcium tissue volume increased, supporting the notion that statins have plaque-stabilizing effects (60).
The YELLOW (Reduction in Yellow Plaque by Intensive Lipid Lowering Therapy) trial was an open label study that compared impact of short-term intensive statin therapy on lipid content of atherosclerotic plaque (49). NIRS and IVUS were performed at baseline and after 7 weeks of treatment with either 40 mg of rosuvastatin or standard-of-care lipid-lowering therapy. Although neither group displayed a difference in plaque burden percentage measured by IVUS (standard group: from 75.6% to 74.9%; intensive group: from 75.9% to 75.3%), intensive therapy was accompanied by a greater median reduction in LCBI4 mm max (lipid-core burden index at the 4 mm maximal segment) measured by NIRS [−149.1 (−210.9 to −42.9) vs. 2.4 (−36.1 to 44.7); P=0.01], meeting the study’s primary endpoint. However, it is important to note that the baseline LCBI was significantly higher in patients randomly allocated to the intensive therapy arm, and these results should be viewed as hypothesis generating rather than definitive.
Studies using non-contrast CT have also investigated plaque stabilization by measuring the amount of coronary calcium. Pooled data from SFHS (50) and EBEAT (51) studies reported that despite the increase in CAC scores after high dose and long-term statin therapy, cardiovascular event rates did not increase, suggesting plaque repair and stabilization, not expansion (61). The well-recognized relationship between increased plaque calcification and reduced event risk, as highlighted in patients on statins, limits the ability of non-contrast CT to meaningfully evaluate CAD progression. Therefore for this type of study coronary CTA is more helpful.
Assessment of the natural history of coronary plaque by coronary CTA has generally been in the context of ACS patients or in retrospective studies. In ACS studies, such as ROMICAT (62) and a CT-substudy of PROSPECT (63), serial CT imaging at 2–3 year intervals has shown increased plaque burden due to progression of noncalcified plaque (62), along with positive remodeling (63). In another study, retrospective analysis of 60 patients who had undergone clinically indicated coronary CTA s showed that statin therapy was associated with reduction in noncalcified plaque after approximately 1 year, without affecting calcified plaque volume (64). A marked reduction of 19.4% in mean size for non-calcified plaque was also achieved following 1 year of atorvastatin therapy in a recent prospectively randomized trial of lipid lowering in HIV patients (65). Notably, the active treatment group also showed attenuation of high-risk coronary CTA features, such as low attenuation of plaque and positive remodeling.
In another statin trial, Noguchi et al. investigated the anti-atherosclerotic effect of pitavastatin using both noncontrast T1-weighted MRI and coronary CTA (9). Twelve months of therapy resulted in lowering of LDL-C from 125 to 70 mg/dL, accompanied by an 18.9% reduction in the PMR signal of coronary HIPs, and a decrease in low-attenuation plaque volume and the percentage of total atheroma volume on coronary CTA.
Early data have also indicated that FDG uptake of plaque decreases in association with statin treatment. Forty-three patients who underwent 18FDG-PET for cancer screening and had radio-tracer uptake in the thoracic aorta and/or carotid arteries, were either treated with simvastatin or dietary management (52). Simvastatin reduced LDL-C by 30% (P<0.01) and increased HDL-C by 15% (P<0.01), whereas LDL-C and HDL-C levels were not changed in the diet group. Plaque inflammation visualized by 18FDG-PET was attenuated by simvastatin, with the decrease in SUV (standard uptake value) found to correlate with HDL-C elevation (P<0.01) but not LDL-C reduction.
A summary of the above studies is provided in Table 1. Despite a wealth of evidence to show that statin therapy and LDL-C lowering beneficially alters CAD burden and plaque composition, one in every five patients who achieve very low LDL-C levels continues to experience CAD progression (66). This phenomenon may be explained by the presence of other uncontrolled risk factors and highlights the multifactorial nature of atherosclerotic disease, and the need to also modify non-LDL-C risk factor targets.
Targeting high-density lipoprotein (HDL) (Table 2)
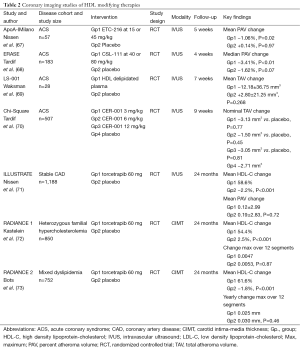
Full table
Several lines of evidence have strongly indicated that HDL plays a protective role in atherosclerosis. An inverse relationship between HDL-C and prospective cardiovascular risk has been demonstrated in population studies (74). This connection is demonstrated in patients with lower levels of HDL-C who continue to experience cardiovascular events despite reaching very low LDL-C levels (75). The independent link that statins possess the ability to slow plaque progression in those with modest increases in HDL-C complements similar findings from clinical event studies (76). These and other observations have led to the development of HDL-targeted anti-atherosclerotic therapies.
The promotion of HDL can be accomplished through transgenic expression of its major proteins or direct infusion, and both of these methods have been used in preclinical models. In many studies, delipidated HDL has been shown to have pleiotropic beneficial effects on atherosclerotic burden, including through promotion of rapid cholesterol efflux (77) and improvement of endothelial cell function (78).
Niacin is the most effective treatment to raise low levels of HDL-C in current clinical practice. In the pre-statin era, experience with niacin suggested benefit with immediate release formulations (79). Demonstration of anti-atherosclerotic effects of niacin was evaluated in a small Korean study that compared the effects of a combination of niacin and simvastatin to simvastatin alone, on plaque regression as measured by IVUS and inflammatory makers (80). There were no intergroup differences for normalized TAV or PAV either before or after nine months of treatment. However, the degree of change for both parameters was greater in the niacin and simvastatin combination group compared to the simvastatin monotherapy group (ΔTAV: −21.6±10.7 vs. 5.3±42.2, P=0.024; ΔPAV: −1.2±2.5 vs. −0.6±5, P=0.047). This occurred in parallel with greater changes in hs-CRP, MMP-9, and sCD40L in the combination group. Surprisingly, data for the effect of niacin on HDL and LDL were not provided. While these results are positive, the use of niacin is limited because of patient intolerance and an ongoing perception that it does not confer additional benefit to statin therapy (81). In particular, a recent study failed to show an additive benefit of extended-release niacin either as monotherapy, or in combination with a prostanoid receptor antagonist to reduce flushing, on cardiovascular events in statin-treated patients (82). Niacin is also associated with other adverse effects, including septic and hemorrhagic complications (83). It must be emphasized that the failure of niacin to demonstrate a clinical benefit should not be interpreted as a failure of the HDL hypothesis, as it has multiple additional mechanisms of action.
The potential benefit of infusing lipid-deplete forms of HDL into humans has been demonstrated in four clinical trials. In the first of these, ACS patients were treated with intravenous infusions of reconstituted HDL containing apoA-I Milano (AIM) or placebo (saline) for 5 weeks (67). IVUS imaging was conducted at baseline and at the end of treatment, and rapid regression of atherosclerosis was observed in those who received the AIM infusions. Notably, these benefits were observed without any change in lumen size, suggesting that they would not have been detected by CAG alone. This finding also supports the notion that plaque regression is accompanied by reverse remodeling of the artery wall (84). In the second study, ERASE (The Effect of rHDL on Atherosclerosis Safety and Efficacy), patients received 4 weekly infusions of HDL particles containing wild-type apoA-I or a placebo of saline (68). After the short period of infusions, there was no significant difference detected by IVUS for coronary atheroma volume between active and placebo groups.
In the Lipid Sciences Selective Delipidation Trial (LS-001), 28 ACS patients underwent plasma apheresis and were randomized to 7 weekly autologous reinfusions of selective HDL delipidated plasma or control plasma (69). IVUS was performed at baseline and up to fourteen days after the last infusion. Reinfusions were well tolerated. There was a numerical, non-significant trend toward regression in the total atheroma volume in the delipidated group compared to a small increase in the control group. In the latest HDL infusion study, Chi-Square (Can HDL Infusions Significantly Quicken Atherosclerosis Regression?), patients were allocated to receive 6 weekly infusions of either saline, or three different doses of CER-001 (Cerenis Therapeutics, Labège, France), a novel engineered HDL-mimetic comprised of recombinant human apoA-I and phospholipids, and designed to mimic the benefits of nascent pre-β HDL. Although there was no statistically significant difference between the active treatment groups and placebo, CER-001 was associated with an inverse, dose-dependent response for the change in coronary atheroma volume (70). This has prompted ongoing evaluation in the recently launched Phase II CARAT study in patients with ACS (www.clinicaltrials.gov NCT02484378). Pending further large-scale evaluation, each of the above-mentioned studies supports the feasibility and safety of treating human patients with HDL infusions, while hinting at their anti-atherosclerotic benefits.
The HDL field has also seen the development of cholesteryl ester transfer protein (CETP) inhibitors, which can substantially raise HDL-C levels. CETP promotes the transfer of cholesteryl esters from HDL to lipoproteins, including LDL and VLDL particles (85). Therefore inhibiting CETP leads to increased HDL-C levels and can prevent cholesterol enrichment of atherogenic lipoproteins. CETP exists in the plasma of a few species, including humans and rabbits, but not rodents. Transgenic mice have been engineered to express CETP, resulting in the development of atherosclerosis (86,87). Different murine models have provided discordant results, with some indicating that CETP is antiatherogenic (88-90), and others that it is proatherogenic (91-93). Rabbits are highly susceptible to the development of diet-induced atherosclerosis, and have a naturally high level of CETP. In one study, CETP was inhibited in rabbits by infusing anti-CETP antibodies, which led to a rise in HDL cholesterol ester, and a fall in triglyceride levels (94). Despite this, levels of HDL protein did not change, suggesting a cholesterol ester for triglyceride exchange. Another study used a chemical inhibitor of CETP in cholesterol-fed rabbits, which reduced CETP activity by >90% and almost doubled serum HDL-C, while decreasing non-HDL-C by approximately 50% (95). These lipid changes were accompanied by a 70% reduction in aortic atherosclerotic burden, supporting the anti-atherogenic potential for CETP inhibitors.
Human data also provided impetus for the clinical evaluation of CETP inhibitors, including the observation that populations with a high prevalence of low CETP activity have fewer cardiovascular events, when accompanied by high HDL-C levels (96). The effect of the CETP inhibitor, torcetrapib, on atherosclerosis was evaluated in number of imaging trials. The ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) study used IVUS to assess the effect of torcetrapib on the progression of atheroma burden in patients treated with atorvastatin for a LDL-C goal of 100 mg/dL (71). B-mode ultrasound was also used to assess the effects of torcetrapib on carotid intima-media thickness (CIMT) in patients with familial hypercholesterolemia in the RADIANCE 1 (The Rating Atherosclerotic Disease Change by Imaging with a New CETP Inhibitor) study (72), and mixed hyperlipidemia in the RADIANCE 2 (The Rating Atherosclerotic Disease Change by Imaging with a New CETP Inhibitor) study (73). The Torcetrapib-treated patients showed an increase in HDL-C concentration by approximately 60% in all three of these imaging trials. LDL-C levels were also reduced by approximately 20% more than that achieved by atorvastatin alone. Despite these results, treatment with torcetrapib had no effect on slowing disease progression or promoting regression of the coronary arteries (71) or on CIMT (72,73).
The first large-scale clinical trial with torcetrapib, conducted in parallel with these imaging studies, was prematurely terminated due to high rates of cardiovascular events and mortality (97). A post-hoc analysis of ILLUSTRATE demonstrated an inverse relationship between the extent of HDL-C increases in those treated with torcetrapib and the rate of atherosclerotic progression, with plaque regression observed in patients who achieved the highest levels of HDL-C (98). These and other results suggest an intact capacity of HDL to reduce the size of atheroma burden and to promote cholesterol efflux after torcetrapib treatment and in CETP-deficient individuals (98,99). The adverse effects seen with torcetrapib may reflect a molecule-specific problem, while the drug has also been shown to have a number of off-target toxic effects at the level of the artery wall and adrenal gland (100). Despite the cautionary tale of torcetrapib, there are ongoing efforts to develop and refine other CETP inhibitors that do not contain these toxicities.
Generating nascent functional HDL particles is another strategy to target HDL. Upregulating endogenous synthesis of apoA-I should create nascent HDL particles that would travel in the systemic circulation, and carry out physiological activities, such as cholesterol efflux and anti-inflammatory functions. This process should theoretically avoid the potential impact of modifying complex remodelling pathways involved in lipid metabolism. Preclinical studies conducted by treating nonhuman primates with RVX-208 showed an increase in hepatic apoA-I synthesis, and enhanced cholesterol efflux capacity (101). Early studies that treated CAD patients with statins and RVX-208 or placebo for 12 weeks showed an increase in apoA-I, HDL-C, and concentration of large HDL particles in those receiving RVX-208. Although these encouraging results indicated facilitation of cholesterol mobilization (102), they were not associated with a positive impact on coronary plaque burden, and the clinical benefit of this therapeutic strategy remains undetermined (103).
Taken together, there have been numerous efforts to modify HDL levels and functionality with variable success at altering the atherosclerotic substrate (Table 2). The completed studies in the field have had important limitations and their results have been far from definitive. Nevertheless, there remains enthusiasm for the development of effective HDL-modifying therapies and the application of atherosclerotic imaging techniques will be integral to their evaluation.
Targeting diabetes (Table 3)
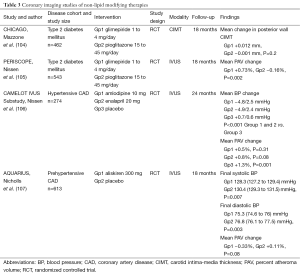
Full table
The increased prevalence of type 2 diabetes is a major factor contributing to the prediction that cardiovascular disease will become the leading cause of mortality worldwide by 2020 (108). In addition to having a substantially increased risk of adverse cardiovascular events (109), diabetic patients also suffer from less favorable outcomes post-MI (110) and following coronary interventions (111). The association between CAC and future cardiovascular events has been observed in patients with diabetes (112). The diabetes heart study (DHS) showed an increase in mortality with increasing levels of CAC in 1,051 diabetic patients (113). There is also an association of greater plaque burden and disease progression in the arterial wall of diabetic patients, including a higher prevalence of impaired compensatory remodeling which is associated with adverse plaque events (114). While aggressively lowering LDL-C levels is associated with a favorable impact on plaque progression, diabetic patients have traditionally demonstrated greater increases in atheroma volume (114). However, in a recent analysis of the SATURN trial, the diabetic patients demonstrated regression of atherosclerotic plaque. Disease regression was greater in the nondiabetic patients when LDL-C levels were >70 mg/dL [(–0.31±0.23)% vs. (–1.01±0.21)%, P=0.03] but similar when LDL-C levels were ≤70 mg/dL [(–1.09±0.16)% vs. (–1.24±0.16)%, P=0.50] (115). This reaffirms the antiatherogenic benefits of tight lipid control in the setting of diabetes.
Peroxisome proliferation-activated receptor gamma (PPARγ) agonists are widely used as insulin sensitisers in diabetes. PPARγ activation encompasses several biological functions in different cell types and tissues, such as regulating metabolism, reducing inflammation, improving endothelial function, and inhibiting apoptosis and oxidative stress. These functions not only have a positive effect on diabetes, but on atherosclerosis as well. A number of trials have investigated this for the PPARγ agonist, pioglitazone, which can also raise HDL-C levels. The CHICAGO (Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone) study, and the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study both directly compared the impact of pioglitazone and the sulphonylurea agent, glimepiride, in patients with diabetes (Table 3). The halting of CIMT (104) and coronary atheroma progression, as assessed by IVUS (105), was associated with lower levels of triglycerides and CRP, and higher levels of HDL-C, along with improvement in glycemic control in the pioglitazone group. Further analyses of both studies revealed that raising HDL-C was the strongest independent predictor of the ability of pioglitazone to slow plaque progression (116,117). The incremental benefit shown by targeting multiple metabolic risk factors supports the need to aggressively treat all cardiovascular risk factors in patients with diabetes (118).
Blood pressure (BP) lowering (Table 3)
Hypertension is a major contributing risk factor to the worldwide escalation of cardiovascular disease, with well-established evidence to support the clinical benefit of different BP-lowering therapies in hypertensive patients (119). Although a few studies have also reported that progression of CIMT is highly sensitive to BP reduction (120), the effect of anti-hypertensive treatments on CAD progression has not been investigated in great detail.
The CAMELOT (Comparison of Amlodipine vs. Enalapril to Limit Occurrences of Thrombosis) study evaluated the effect of amlodipine, enalapril and placebo on clinical event rates in patients with established CAD and BP that was considered to be optimally controlled (diastolic BP <100 mmHg) (106). Its IVUS sub-study revealed that amlodipine treatment slowed atherosclerotic disease progression, as well as lowering cardiovascular event rates (121). There was also a direct relationship between the achieved levels of systolic BP and disease progression, with a trend towards atheroma regression in patients who achieved a systolic BP <120 mmHg.
Preclinical data have demonstrated that activation of the renin-angiotensin-aldosterone system (RAAS) plays a particularly important role in atherogenesis (122) and that RAAS inhibition may therefore have favourable effects on the artery wall (123). However, in the AQUARIUS (Aliskiren Quantitative Atherosclerosis Regression Intravascular Ultrasound Study) study, the direct renin inhibitor, aliskerin, failed to have any effect on IVUS-measured plaque progression when compared to placebo, in patients with prehypertensive BP (107). Although as yet under investigated, there is scope for future imaging studies to compare the anti-atherosclerotic properties of different anti-hypertensive agents, including angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists, in patients with stable and unstable CAD.
Novel therapies and future directions
Serial imaging of the vessel wall has provided the opportunity to explore the effects of novel therapies on atherosclerotic plaque. As effective as statins are for lowering levels of LDL-C, there is still an unmet need for additional or alternative LDL-C-lowering therapies. This is especially so in patients with statin intolerance and those with severe hypercholesterolemia (e.g., familial syndromes) who fail to achieve target cholesterol levels with conventional agents and doses. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a promising new class of drugs for LDL-C reduction that are approved for use and are currently being evaluated in clinical trials including the IVUS-based GLAGOV study (http://clinicaltrials.gov NCT01663402).
Another therapeutic target that has yet to be fully explored is triglyceride- rich lipoprotein particles that have potent atherogenic effects (124). Elevated triglyceride levels predict patients at increased vascular risk (125). To date, there is a paucity of information on the ability of triglyceride-lowering drugs (e.g., fibrates) to modulate the natural history of coronary atheroma in human patients.
The effects of lifestyle interventions such as smoking and weight loss have yet to be investigated fully. However, a study done by Madssen et al. investigated effects of aerobic interval training (AIT) versus moderate continuous training (MCT) on coronary atherosclerosis in patients with significant CAD on optimal medical treatment (126). Patients were randomized to AIT or MCT for 12 weeks after intracoronary stent implantation. Grayscale and VH-IVUS were performed at baseline and follow-up. Necrotic core was reduced in both groups in defined coronary segments (AIT −3.2%, MCT −2.7%, P<0.05) and in separate lesions (median change −2.3% and −0.15 mm3, P<0.05). Plaque burden was also reduced by 10.7% in separate lesions independent of intervention group (P=0.06). Although there was a lack of difference in plaque regression between the two modes of exercise training, the overall favorable effects of exercise should encourage further evaluation.
Other treatments with potential anti-atherogenic benefits, including anti-inflammatory drugs, omega-3 fatty acids, and various experimental agents (e.g., small molecules, gene and cell-based therapies) may also be future candidates for serial coronary imaging studies. The ability to determine whether such agents have a beneficial effect on plaque progression can provide important support to either advance or halt clinical development of new therapies. Examples of the latter include the discontinuation of active research programs investigating the use of compounds such as endocannabinoid receptor antagonists (127) and acyl-coenzyme A:cholesterol acyltransferase inhibitors (128), after negative results.
Summary
The technological advances that have been made with vessel wall imaging modalities have greatly increased the ability to evaluate the full extent of atherosclerotic plaque within the coronary vasculature. Some of the anti-atherosclerotic therapeutic strategies currently available have demonstrated slowing of disease progression and even plaque regression. However, there continues to be a need to develop novel therapies to aggressively target high-risk atherosclerotic lesions, and the evolution and increasing use of coronary imaging techniques will play a central role in determining their effectiveness.
Acknowledgements
Funding: Dr. Psaltis receives funding from the National Health and Medical Research Council (PG1086796) and Heart Foundation (FLF100412) of Australia. Dr. Puri is currently supported by a Neil Hamilton Fairley Early Career Fellowship grant from the National Health and Medical Research Council.
Footnote
Conflicts of Interest: Dr. Nicholls; Research Grant; Modest; Anthera, AstraZeneca, Cerenis, Eli Lilly, InfraReDx, Roche, Resverlogix, Novartis, Amgen, and LipoScience. Speakers Bureau; Modest; AstraZeneca, Pfizer, Merck Schering-Plough, and Takeda. Consultant/Advisory Board; Modest; AstraZeneca, Abbott, AtheroNova, Esperion, Amgen, Novartis, Omthera, CSL Behring, Boehringer Ingelheim, Pfizer, Merck Schering-Plough, Takeda, Roche, Novo Nordisk, LipoScience, and Anthera. None of the other authors has relevant disclosures.
References
- Virmani R, Burke AP, Kolodgie FD, et al. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol 2002;15:439-46. [Crossref] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. [Crossref] [PubMed]
- Turnbull F; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35. [Crossref] [PubMed]
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005;46:1225-8. [Crossref] [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [Crossref] [PubMed]
- Ballantyne CM. Clinical trial endpoints: angiograms, events, and plaque instability. Am J Cardiol 1998;82:5M-11M. [Crossref] [PubMed]
- Keane D, Haase J, Slager CJ, et al. Comparative validation of quantitative coronary angiography systems. Results and implications from a multicenter study using a standardized approach. Circulation 1995;91:2174-83. [Crossref] [PubMed]
- Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J 2008;29:1851-7. [Crossref] [PubMed]
- Noguchi T, Tanaka A, Kawasaki T, et al. Effect of Intensive Statin Therapy on Coronary High-Intensity Plaques Detected by Noncontrast T1-Weighted Imaging: The AQUAMARINE Pilot Study. J Am Coll Cardiol 2015;66:245-56. [Crossref] [PubMed]
- Lavoie AJ, Bayturan O, Uno K, et al. Plaque progression in coronary arteries with minimal luminal obstruction in intravascular ultrasound atherosclerosis trials. Am J Cardiol 2010;105:1679-83. [Crossref] [PubMed]
- Mintz GS, Painter JA, Pichard AD, et al. Atherosclerosis in angiographically "normal" coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol 1995;25:1479-85. [Crossref] [PubMed]
- Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39:604-9. [Crossref] [PubMed]
- Kawasaki M, Bouma BE, Bressner J, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol 2006;48:81-8. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary intima--media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J 2005;69:903-7. [Crossref] [PubMed]
- Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002;106:1640-5. [Crossref] [PubMed]
- Kolodgie FD, Virmani R, Burke AP, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004;90:1385-91. [Crossref] [PubMed]
- Nair A, Kuban BD, Tuzcu EM, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002;106:2200-6. [Crossref] [PubMed]
- Rodriguez-Granillo GA, McFadden EP, Valgimigli M, et al. Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J 2006;151:1020-24. [Crossref] [PubMed]
- Waxman S, Dixon SR, L'Allier P, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovasc Imaging 2009;2:858-68. [Crossref] [PubMed]
- Cassis LA, Lodder RA. Near-IR imaging of atheromas in living arterial tissue. Anal Chem 1993;65:1247-56. [Crossref] [PubMed]
- Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157-62. [Crossref] [PubMed]
- Pletcher MJ, Tice JA, Pignone M, et al. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004;164:1285-92. [Crossref] [PubMed]
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336-45. [Crossref] [PubMed]
- Sandfort V, Lima JA, Bluemke DA. Noninvasive Imaging of Atherosclerotic Plaque Progression: Status of Coronary Computed Tomography Angiography. Circ Cardiovasc Imaging 2015;8:e003316. [Crossref] [PubMed]
- Gorenoi V, Schonermark MP, Hagen A. CT coronary angiography vs. invasive coronary angiography in CHD. GMS Health Technol Assess 2012;8:Doc02. [PubMed]
- Hoffmann U, Moselewski F, Cury RC, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient-versus segment-based analysis. Circulation 2004;110:2638-43. [Crossref] [PubMed]
- Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319-26. [Crossref] [PubMed]
- Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282-91. [Crossref] [PubMed]
- Park HB, Lee BK, Shin S, et al. Clinical Feasibility of 3D Automated Coronary Atherosclerotic Plaque Quantification Algorithm on Coronary Computed Tomography Angiography: Comparison with Intravascular Ultrasound. Eur Radiol 2015;25:3073-83. [Crossref] [PubMed]
- van der Giessen AG, Toepker MH, Donelly PM, et al. Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Invest Radiol 2010;45:693-701. [Crossref] [PubMed]
- Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368-73. [Crossref] [PubMed]
- Chu B, Phan BA, Balu N, et al. Reproducibility of carotid atherosclerotic lesion type characterization using high resolution multicontrast weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:793-9. [Crossref] [PubMed]
- Oikawa M, Ota H, Takaya N, et al. Carotid magnetic resonance imaging. A window to study atherosclerosis and identify high-risk plaques. Circ J 2009;73:1765-73. [Crossref] [PubMed]
- Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol 2010;56:983-91. [Crossref] [PubMed]
- Yang Q, Li K, Liu X, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-T: a comparative study with X-ray angiography in a single center. J Am Coll Cardiol 2009;54:69-76. [Crossref] [PubMed]
- Yahagi K, Joner M, Virmani R. Should CMR Become the New Darling of Noninvasive Imaging for the Monitoring of Progression and Regression of Coronary Heart Disease? J Am Coll Cardiol 2015;66:257-60. [Crossref] [PubMed]
- Noguchi T, Kawasaki T, Tanaka A, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol 2014;63:989-99. [Crossref] [PubMed]
- Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008;118:586-606. [Crossref] [PubMed]
- Narula J, Garg P, Achenbach S, et al. Arithmetic of vulnerable plaques for noninvasive imaging. Nat Clin Pract Cardiovasc Med 2008;5 Suppl 2:S2-10. [Crossref] [PubMed]
- Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708-11. [Crossref] [PubMed]
- Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705-13. [Crossref] [PubMed]
- Hyafil F, Cornily JC, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med 2007;13:636-41. [Crossref] [PubMed]
- Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med 1990;323:1289-98. [Crossref] [PubMed]
- Blankenhorn DH, Azen SP, Kramsch DM, et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann Intern Med 1993;119:969-76. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071-80. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556-65. [Crossref] [PubMed]
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078-87. [Crossref] [PubMed]
- Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol 2014;64:2207-17. [Crossref] [PubMed]
- Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol 2013;62:21-9. [Crossref] [PubMed]
- Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166-72. [Crossref] [PubMed]
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006;113:427-37. [Crossref] [PubMed]
- Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825-31. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29-38. [Crossref] [PubMed]
- Puri R, Nissen SE, Shao M, et al. Impact of baseline lipoprotein and C-reactive protein levels on coronary atheroma regression following high-intensity statin therapy. Am J Cardiol 2014;114:1465-72. [Crossref] [PubMed]
- Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273-82. [Crossref] [PubMed]
- Kataoka Y, Puri R, Hammadah M, et al. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol 2014;114:549-54. [Crossref] [PubMed]
- Habara M, Nasu K, Terashima M, et al. Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe. Am J Cardiol 2014;113:580-7. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Kovarnik T, Mintz GS, Skalicka H, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J 2012;76:176-83. [Crossref] [PubMed]
- Puri R, Libby P, Nissen SE, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging 2014;15:380-8. [Crossref] [PubMed]
- Henein M, Granasen G, Wiklund U, et al. High dose and long-term statin therapy accelerate coronary artery calcification. Int J Cardiol 2015;184:581-6. [Crossref] [PubMed]
- Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging 2009;2:1262-70. [Crossref] [PubMed]
- Papadopoulou SL, Neefjes LA, Garcia-Garcia HM, et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging 2012;5:S28-37. [Crossref] [PubMed]
- Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis 2013;231:198-204. [Crossref] [PubMed]
- Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52-63. [Crossref] [PubMed]
- Bayturan O, Kapadia S, Nicholls SJ, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol 2010;55:2736-42. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Tardif JC, Gregoire J, L'Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007;297:1675-82. [Crossref] [PubMed]
- Waksman R, Torguson R, Kent KM, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 2010;55:2727-35. [Crossref] [PubMed]
- Tardif JC, Ballantyne CM, Barter P, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014;35:3277-86. [Crossref] [PubMed]
- Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304-16. [Crossref] [PubMed]
- Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620-30. [Crossref] [PubMed]
- Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007;370:153-60. [Crossref] [PubMed]
- Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707-14. [Crossref] [PubMed]
- Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301-10. [Crossref] [PubMed]
- Cui Y, Watson DJ, Girman CJ, et al. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study). Am J Cardiol 2009;104:829-34. [Crossref] [PubMed]
- Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol 2004;24:421-8. [Crossref] [PubMed]
- Bisoendial RJ, Hovingh GK, Levels JH, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation 2003;107:2944-8. [Crossref] [PubMed]
- Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360-81. [Crossref] [PubMed]
- Lee K, Ahn TH, Kang WC, et al. The effects of statin and niacin on plaque stability, plaque regression, inflammation and oxidative stress in patients with mild to moderate coronary artery stenosis. Korean Circ J 2011;41:641-8. [Crossref] [PubMed]
- Birjmohun RS, Hutten BA, Kastelein JJ, et al. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol 2005;45:185-97. [Crossref] [PubMed]
- HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203-12. [Crossref] [PubMed]
- AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol 2006;47:992-7. [Crossref] [PubMed]
- Barter PJ, Brewer HB Jr, Chapman MJ, et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160-7. [Crossref] [PubMed]
- Agellon LB, Walsh A, Hayek T, et al. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem 1991;266:10796-801. [PubMed]
- Jiang XC, Masucci-Magoulas L, Mar J, et al. Down-regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein. Mechanism to explain accumulation of lipoprotein B particles. J Biol Chem 1993;268:27406-12. [PubMed]
- Föger B, Chase M, Amar MJ, et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J Biol Chem 1999;274:36912-20. [Crossref] [PubMed]
- MacLean PS, Bower JF, Vadlamudi S, et al. Cholesteryl ester transfer protein expression prevents diet-induced atherosclerotic lesions in male db/db mice. Arterioscler Thromb Vasc Biol 2003;23:1412-5. [Crossref] [PubMed]
- Hayek T, Masucci-Magoulas L, Jiang X, et al. Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J Clin Invest 1995;96:2071-4. [Crossref] [PubMed]
- Westerterp M, van der Hoogt CC, de Haan W, et al. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler Thromb Vasc Biol 2006;26:2552-9. [Crossref] [PubMed]
- Plump AS, Masucci-Magoulas L, Bruce C, et al. Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler Thromb Vasc Biol 1999;19:1105-10. [Crossref] [PubMed]
- Marotti KR, Castle CK, Boyle TP, et al. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature 1993;364:73-5. [Crossref] [PubMed]
- Whitlock ME, Swenson TL, Ramakrishnan R, et al. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J Clin Invest 1989;84:129-37. [Crossref] [PubMed]
- Okamoto H, Yonemori F, Wakitani K, et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 2000;406:203-7. [Crossref] [PubMed]
- Boekholdt SM, Kuivenhoven JA, Wareham NJ, et al. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation 2004;110:1418-23. [Crossref] [PubMed]
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109-22. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Brennan DM, et al. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 2008;118:2506-14. [Crossref] [PubMed]
- Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol 2007;27:257-60. [Crossref] [PubMed]
- Vergeer M, Stroes ES. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am J Cardiol 2009;104:32E-8E. [Crossref] [PubMed]
- Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol 2010;55:2580-9. [Crossref] [PubMed]
- Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol 2011;57:1111-9. [Crossref] [PubMed]
- Nicholls SJ, Pisaniello AD, Kataoka Y, et al. Lipid pharmacotherapy for treatment of atherosclerosis. Expert Opin Pharmacother 2014;15:1119-25. [Crossref] [PubMed]
- Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 2006;296:2572-81. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292:2217-25. [Crossref] [PubMed]
- Nicholls SJ, Bakris GL, Kastelein JJ, et al. Effect of aliskiren on progression of coronary disease in patients with prehypertension: the AQUARIUS randomized clinical trial. JAMA 2013;310:1135-44. [Crossref] [PubMed]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-504. [Crossref] [PubMed]
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73-8. [Crossref] [PubMed]
- Donnan PT, Boyle DI, Broomhall J, et al. Prognosis following first acute myocardial infarction in Type 2 diabetes: a comparative population study. Diabet Med 2002;19:448-55. [Crossref] [PubMed]
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005;293:1501-8. [Crossref] [PubMed]
- Perrone-Filardi P, Achenbach S, Möhlenkamp S, et al. Cardiac computed tomography and myocardial perfusion scintigraphy for risk stratification in asymptomatic individuals without known cardiovascular disease: a position statement of the Working Group on Nuclear Cardiology and Cardiac CT of the European Society of Cardiology. Eur Heart J 2011;32:1986-93, 1993a, 1993b.
- Agarwal S, Morgan T, Herrington DM, et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes Care 2011;34:1219-24. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008;52:255-62. [Crossref] [PubMed]
- Stegman B, Puri R, Cho L, et al. High-intensity statin therapy alters the natural history of diabetic coronary atherosclerosis: insights from SATURN. Diabetes Care 2014;37:3114-20. [Crossref] [PubMed]
- Polonsky T, Mazzone T, Davidson M. The clinical implications of the CHICAGO study for the management of cardiovascular risk in patients with type 2 diabetes mellitus. Trends Cardiovasc Med 2009;19:94-9. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Wolski K, et al. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol 2011;57:153-9. [Crossref] [PubMed]
- Kataoka Y, Shao M, Wolski K, et al. Multiple risk factor intervention and progression of coronary atherosclerosis in patients with type 2 diabetes mellitus. Eur J Prev Cardiol 2013;20:209-17. [Crossref] [PubMed]
- Blood Pressure Lowering Treatment Trialists' Collaboration, Sundström J, Arima H, et al. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384:591-8. [Crossref] [PubMed]
- Zanchetti A. Antiatherosclerotic effects of antihypertensive drugs: recent evidence and ongoing trials. Clin Exp Hypertens 1996;18:489-99. [Crossref] [PubMed]
- Sipahi I, Tuzcu EM, Schoenhagen P, et al. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol 2006;48:833-8. [Crossref] [PubMed]
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 2006;98:121-8. [Crossref] [PubMed]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145-53. [Crossref] [PubMed]
- Quispe R, Manalac RJ, Faridi KF, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis 2015;242:243-50. [Crossref] [PubMed]
- Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2008;51:724-30. [Crossref] [PubMed]
- Madssen E, Moholdt T, Videm V, et al. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol 2014;114:1504-11. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 2008;299:1547-60. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Brewer HB, et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006;354:1253-63. [Crossref] [PubMed]


