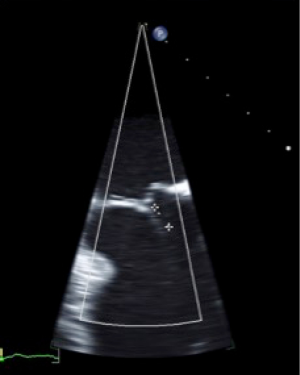
Peri-procedural imaging for transcatheter mitral valve replacement
Introduction
Mitral regurgitation (MR) has a high prevalence in older patient populations of industrialized nations (1). Its etiology can be broadly categorized into primary and secondary MR. Primary MR is characterized by pathologic changes of the valve, causing valvular dysfunction. In contrast secondary MR is defined by a structurally intact mitral valve with functional impairment secondary to ventricular remodeling. With decreasing prevalence of rheumatic heart disease and an increasing percentage of older individuals, degenerative MR is now the most common primary etiology (1). Various cardiomyopathies are the underlying cause of secondary MR, with ischemic heart as the most common etiology. Because of high prevalence of co-morbidities and associated risk, surgical mitral valve repair or replacement is deferred in a significant percentage of patients (2). For these patients transcatheter repair/replacement are emerging as treatment options (3).
During open-heart repair/replacement, direct or video-endoscopic visualization of the mitral valve structures guide surgical management. Because transcatheter approaches lack direct visualization, peri-procedural imaging is critical. Pre-procedural imaging of the mitral valve defines the presence of disease, confirms inclusion and exclusion criteria based on anatomic and functional characteristics, guides selection of appropriate device size, and determines eligibility of peripheral or central vascular access. Intra-procedural imaging guides the procedure, identifies immediate procedural success and complications. Similar to open surgical approaches, post-procedural imaging confirms long-term outcome.
Echocardiography remains the ‘Reference Standard’ for identifying, monitoring and grading mitral valvular disease, in the pre- and post-operative period. Echocardiography and angiography are essential for intra-procedural imaging. Magnetic resonance imaging (MRI) and computed tomography provide complementary information in selected clinical scenarios and patient populations. A strength of multidetector computed tomography (MDCT) is detailed three-dimensional (3D) reconstructions of mitral valve anatomy, including precise measurement of annular dimensions. It has therefore gained increasing acceptance as a component of pre-procedural assessment in the context of transcatheter valve interventions (4). MRI provides additional functional and anatomic analysis.
In this review we summarize anatomy of the mitral complex and current status of various transcatheter mitral valve interventions, and discuss the imaging modalities for characterization of the mitral valve, in the peri-procedural period.
Anatomy of the mitral valve structures
The mitral valve complex includes the annulus, the commissures, the two valve leaflets, the chordae tendineae, and the papillary muscles (Figure 1).
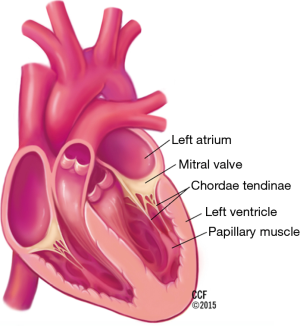
The mitral annulus has a parabolic rather than planar geometry, with peaks toward the anterior and posterior edges (5). This unique shape is thought to lessen the stress exerted on the valve leaflets during systole. The anterolateral and posteromedial commissures are the points where the two mitral leaflets attach to each other (6). Fibrous thickenings external to the mitral valve commissures are known as the right and left trigones. The mitral annulus is divided into the anterior and posterior portions. The anterior portion of the mitral annulus lies between the right and left trigone and is in very close association with the aortic annulus. The fibrous continuity of the anterior mitral valve leaflet with the non-coronary and left-coronary leaflet of the aortic valve is also known as the aortic mitral curtain (Figure 2) (7). The fibrous nature of the anterior mitral annulus makes it less prone to pathological remodeling (8). In contrast, the posterior portion of the annulus is predominantly muscular and thus prone to remodeling (9). It is important to note the close association of the coronary sinus with the posterior portion of the annulus, which is the basis for indirect annuloplasty procedures. The mitral annulus is a dynamic structure which undergoes a variety of deformations during the cardiac cycle including folding and contraction during systole, as well as translation in response to ventricular filling (5). The deformation of the annulus is a significant challenge for device stability in transcatheter mitral valve implantation (2).
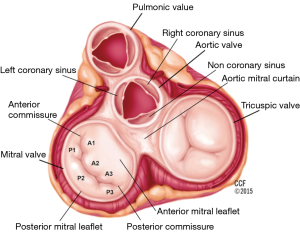
The anterior and posterior mitral valve leaflets border the aortic valve, and the free cardiac wall, respectively. The leaflets attach to each other at the anterolateral and posteromedial commissures. The posterior leaflet, narrower than the anterior leaflet, covers approximately two-thirds of the annular opening. Indentations, better known as scallops, accommodate the curved coaptation line, and define three portions of the posterior leaflet (named from lateral to medial P1, P2 and P3) (7). The anterior leaflet similarly is divided into three portions (named A1, A2 and A3, from lateral to medial) (Figure 2). Each leaflet contains three zones: the basal zone is the area where the leaflet connects to the annular ring, the clear zone is the central portion of the leaflet, and the rough zone is the coapting portion of the leaflet, along its free edge.
The papillary muscles are located in the anterolateral and posteromedial positions and originate from the middle to apical region of the left ventricle. The anterolateral papillary muscle usually has dual blood supply from the circumflex and left anterior descending coronary artery. The posteromedial papillary muscle is most frequently supplied by the right coronary artery and less commonly by a dominant circumflex. Extending from the tip of each papillary muscle are chords known as chordae tendineae, which attach to the leaflets. Each papillary muscle distributes chords to the ipsilateral side of both leaflets. Primary chords attach to the free edge of the leaflet. Secondary chords attach to the ventricular surface of the rough zone. Both primary and secondary chords arise from papillary muscles. In contrast, tertiary chords may arise directly from the left ventricular wall and insert exclusively into the middle and basilar portions of the posterior leaflet. Primary chords are thinner and function to maintain leaflet apposition and valve closure. Dysfunction of these primary chords results in acute MR (7). Secondary chords are thought to play a role in maintaining left ventricular size rather than leaflet apposition (7). Longer and thicker secondary chords are referred to as strut chords and attach only to the anterior leaflet. These strut chords are responsible for the fibrous continuity of mitral annulus with the left ventricular myocardium (10). Strut chords are under constant tension and help maintain size and shape of the left ventricle. Transection of strut chords results in alterations in left ventricular geometry and can lead to left ventricular remodeling (11).
In summary, the mitral valve apparatus is a complex anatomical structure. Detailed pre-procedural and intra-procedural imaging is needed to ensure successful transcatheter procedures, including mitral valve repair and replacement.
Transcatheter mitral valve implantation/replacement (TMVI/TMVR)
Percutaneous treatments for valvular heart disease have undergone rapid development and gained popularity in recent years (12). Current strategies including leaflet repair (13-17), annuloplasty (18-22), percutaneous LV remodeling devices (23) have been reviewed in recent papers.
However, these techniques are often unable to eliminate MR completely and depend on native valve components. TMVI/TMVR is a developing technique for patients with severe mitral valve disease who are poor surgical candidates. If successful, TMVI has the potential to treat a wider range of mitral pathologies compared to percutaneous annuloplasty and leaflet repair technologies, including mitral stenosis (24-26).
However, because of the complex anatomy of the mitral structures, significant technical challenges will need to be solved (2,27). The complex geometry with significant variability between patient and the larger size makes the implantation into the mitral annulus more difficult than valve deployment into the more symmetric, planar, and smaller aortic annulus. The relative lack of calcifications, which serve as a fluoroscopic landmark and is known to provide stability in TAVR valve deployment, will likely affect positioning and anchoring of the mitral valve devices. It is further unknown if subsequent annulus remodeling may impact on medium-term results after implantation. Peripheral access is associated with a highly curved catheter course (both transseptal and transaortic access). Other anatomical considerations include protection of patency of the coronary sinus, circumflex coronary artery and LV outflow tract with device implantation. High transvalvular gradients and dynamic motion of the mitral annulus during the cardiac cycle adds to technical difficulties and raises engineering concerns about device durability. A number of devices are currently in development.
TMVI devices for native mitral valve
Fortis valve
The FORTIS valve (Edwards Life Sciences Corp, CA, USA) is a self-expanding nitinol stent with three bovine pericardial leaflets implanted via a transapical route. Paddles are present on the central valve body, which can be deflected away from the body to attach to the native valve leaflets and then tightened to secure the leaflets between the paddles and the body. An atrial flange portion of the valve rests in the left atrium and functions to prevent the structure from interfering with aortic valve function.
The procedure is performed under general anesthesia using TEE and fluoroscopic guidance. Rapid pacing is not necessary as blood flow is maintained throughout the procedure. Following transapical puncture, a guidewire is placed and TEE and fluoroscopy are used to confirm absence of chordal entanglement. Final echocardiographic evaluation, preferably using two-dimensional (2D) and 3D TEE, is done before the paddles are closed and the device is released allowing full deployment. Post-deployment, TEE is used to confirm positioning of the valve and function.
Initial experiences with the device include a case series of five treated patients (27). These patients were poor candidates for surgery and MitraClip based on anatomical findings. Thorough pre-procedural imaging of patients included coronary angiogram, TEE and ECG-gated CT. Anatomic inclusion criteria for the 29 mm FORTIS valve determined by echocardiography included an intercommissural annular diameter between 30 and 44 mm, posterior leaflet length of >0.5 cm and an anterior leaflet length of <2.3 cm. Anatomical contraindications included P2 leaflet prolapse, predominant commissural MR, and a left ventricular end diastolic diameter less than 4 cm (27). CT was utilized mainly for imaging of the subvalvular apparatus including papillary muscles and chordae. Unfavorable subvalvular anatomy for leaflet capture during device implantation included irregular papillary muscle head branching, fused papillary muscle heads, chordae extending to subannular groove of mitral valve and large strut chordae (27). Assessment of fluoroscopic angles perpendicular to the mitral annulus was difficult, requiring greater reliance on TEE. Following the procedure, patients were placed on dual antiplatelet therapy and anticoagulation with warfarin on day 1 with target INR of >2.5. Single antiplatelet therapy and warfarin was continued after 3 months. Three of the 5 patients survived beyond 30 days. One patient died from intractable heart failure despite procedural success and another patient experienced very high transvalvular pressures resulting in device displacement and heart failure worsening. Current device development is not active.
NaviGate valve
The transcatheter mitral device developed by NaviGate Cardiac Structures Inc. (Irvine, California, USA) is composed of trileaflet equine pericardial leaflets mounted on a self-expandable 21 mm height nitinol stent with a truncated cone configuration and annular winglets for anchoring the native mitral leaflets. Early animal study results have shown successful implantation in the acute swine model. There was no coronary artery or left ventricular outflow obstruction and echocardiographic evaluation showed excellent function and accurate position of the successfully deployed valves. An interesting aspect was that successful implantation could be performed using all three approaches, trans-septal, trans-apical and trans-atrial. Chronic animal studies are in process for this device.
Medtronic valve
The Medtronic valve is a trileaflet nitinol self-expanding valve that has a large atrial inflow portion and a short circular outflow portion. The device is delivered via a transatrial approach. 2D and 3D TEE in combination with fluoroscopy are used to guide delivery of the implant, verify final positioning and evaluate valve function. Successful animal implantation has been performed using echocardiographic and fluoroscopic guidance. The support arms of the device capture the anterior and posterior native mitral leaflets at the A2 and P2 positions. Successful implantation with absence of valvular leakage has been completed in animals and further valve development is underway (6).
CardiAQ valve
The CardiAQ valve (CardiAQ Valve Technologies, CA, USA) has a self-expanding nitonol frame supporting three leaflets of bovine pericardial tissue. Initial experience with this device was collected in swine with successful implantation in 14 of 19 animals (2). The first human experience with the device was on June 12, 2012 in Denmark where an 86 year old gentleman with 4+ MR was declined for surgery and MitraClip placement. A transapical approach was used to implant the CardiAQ valve. Despite a properly positioned and well-functioning valve, the patient died three days following the procedure secondary to multiorgan failure (2). This device currently has an FDA investigational disease exemption to undergo feasibility studies.
Tiara valve
The Tiara valve (Neovasc Inc, British Columbia, Canada) is a self-expanding bioprosthesis with bovine pericardial tissue. It has a D shaped structure to fit the mitral orifice and prevent LV outflow tract obstruction. Initial experience showed successful implantation in 29 of 36 swine with failure mainly due to improper positioning or failure of the valve anchors (28). Two human cases of Tiara valve implantation were performed via transapical approach in January and February 2014 in British Columbia, Canada (29). The procedures were completed without any complication and patients improved in MR and left ventricular volumes (2). The tiara is the first transcatheter mitral valve to receive an FDA conditional investigational device exemption approval for use in a feasibility trial which is currently underway.
Tendyne valve
The Tendyne valve (Tendyne Inc., MN, USA) is a trileaflet pericardial valve that is delivered transapically and secured via a tether near the LV apex using a pad that sits on the epicardium. Following successful porcine implantations, the valve was implanted in two human individuals in 2013 (30). The procedure eliminated severe MR in one patient and reduced the severe MR to grade 1 in the other patient (2). The Tendyne valve system has also been approved for feasibility studies which are currently underway.
Others
Cardiovalve (Valtech Cardio Ltd, Israel) and Endovalve are additional implantable valves that are subject to current evaluation (2). MitrAssist (MitrAssist Medical, Israel) is another valve implant placed on top of the native valve that works in conjunction with the valve to improve valve functionality. Caisson TMVR (Caisson), Cardiovalve (Valtech), MitraCath (Emory University, Atlanta, USA), HighLife Mitral Valve (HighLife) and the MitrAssist valve (MitrAssist) are additional valves in preclinical development (30).
Valve in valve replacement
TMVI using a valve in valve replacement approach, in which prosthetic valves are implanted into a valve annulus which already has a poorly functioning degenerating prosthesis in place, may also be a viable alternative to valve replacement surgery (31). The SAPIEN XT and the Sapien 3 valves (Edwards, CA, USA) as well as the Melody valve (Medtronic, MN, USA) have been used in dysfunctional mitral bioprostheses for valve-in-valve implantation and in annuloplasty rings for valve in ring implantation. These valves have shown excellent hemodynamic results with low transvalvular gradients and low incidences of perivalvular regurgitation (32-37).
Peri-procedural imaging
Imaging can be divided into pre-, intra-, and post- procedural imaging. Pre-procedural imaging is used to establish diagnosis and describe details/severity of pathology. A critical aspect of pre-procedural planning is 3D image reconstruction along defined planes. Intra-procedural imaging guides the procedure and is critical for precise placement of the device. Post-procedural imaging is used for ongoing surveillance.
Pre-procedural imaging
Echocardiography
2D echocardiography remains the standard method for identification of mitral valve disease and grading of its severity. Transthoracic and transesophageal echocardiography (TTE and TEE) allow accurate anatomic and functional assessment.
The geometry and dimensions of the mitral annulus, presence of annular calcification, valve leaflet morphology leaflet motion, anatomy of the subvalvular apparatus including chordae tendineae and papillary muscles, and left ventricular morphology can be assessed. Parasternal long axis or apical long axis views are typically used to measure the anterior-posterior diameter of the annulus while the apical two chamber view (intercommissural) is used to measure inter-commissural diameter (Figures 3,4).
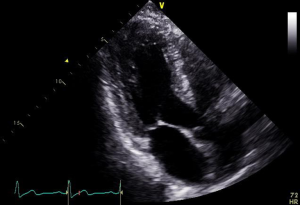
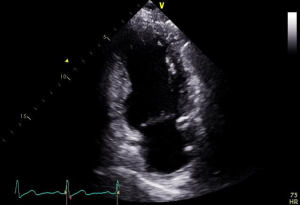
Severity of mitral stenosis is evaluated by continuous wave Doppler-based measurement of pressure half-time, planimetry of valve orifice area and characterization of the mean pressure gradient across the valve by measuring blood flow velocity. Regurgitation severity measurement using 2D and 3D methods involves measuring regurgitant orifice area by PISA methods, proximal regurgitant jet width, diameter of the vena contracta and pulmonary vein flow patterns (38).
Left ventricular size and function are important parameters, describing the impact of left ventricular remodeling and dysfunction on mitral valve function. Notably, severely reduced ejection fraction (<25%) has been an exclusion criteria for many of the landmark clinical trials of transcatheter mitral repair techniques (23).
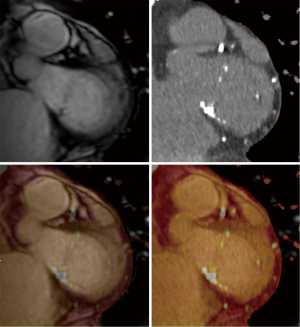
In the context of pre-procedural planning, 3D echocardiography allows dedicated analysis after the image acquisition (Figure 5). In contrast to 2D echocardiography, where the acquisition plane is determined at the time of acquisition, the acquired 3D volumetric datasets centered on the mitral valve can be reconstructed along any plane during data analysis. This allows detailed measurements of the mitral annulus similar to 3D reconstruction of CT data. Data comparing 3D echocardiography with MDCT for mitral valve geometry evaluation showed highly comparable measurements between the two modalities with minimal interobserver variability (39). In addition volume rendered display of the data provides a more intuitive understanding of the 3D relationship and anatomy of the mitral structures in what has been called ‘surgical views’. There is also recent data describing superior accuracy of 3D TEE compared to 2D echocardiographic imaging in quantifying severity of MR by allowing better morphologic evaluation of the regurgitant orifice and flow convergence (40). In degenerative MR, automated quantitative analysis of 3D TEE images is capable of providing data concerning particular mitral valve pathology such as flail gap and flail width.
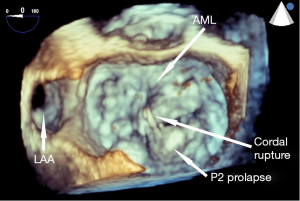
Detailed, structured assessment of leaflet anatomy and motion by echocardiography allows understanding the etiology of MR and classification of valve pathology. In the classification described by Carpentier et al., type I MR is characterized by normal leaflet motion with leaflet perforation responsible for regurgitation, type II involves excessive movements of the leaflets including prolapse and flail, type IIIa involves restricted movements of the leaflets due to fibrosis and IIIb from systolic apical tethering due to ventricular remodeling, annular dilatation and papillary muscle ischemia (41). Functional MR (Carpentier 3b), in which the structure of the papillary muscles, chordae and valve leaflets are normal results from left ventricular dilatation.
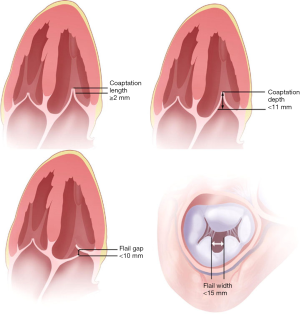
The experience with percutaneous valve repair demonstrates the impact of a detailed assessment on eligibility for the procedure as well as procedural approach. For example, it has been demonstrated that annuloplasty or valve repair devices are unlikely to be able to treat very lateral or very medial leaflet tethering (23). Greater length of the flail segments may necessitate placement of multiple devices in percutaneous leaflet repair procedures (23). Flail leaflet regions in close proximity to the commissures may also complicate percutaneous leaflet repair. Coaptation length and depth relative to the annular plane are also important to evaluate and a coaptation length <2 mm has been found to be insufficient to allow grasping by a valve repair device (42).
Multi-detector computed tomography (MDCT)
In the context of pre-procedural imaging, a particular strength of MDCT is the routine acquisition of 3D data sets with high spatial resolution (43). As described above for 3D echocardiography, these volumetric datasets allow unlimited reconstruction after image acquisition along defined planes and therefore provide detailed visualization of cardiac morphology. Because the orientation of the data volume is standardized by the position of the patient on the scanner table, reconstructed planes can be described relative to the body axes and specifically in angiographic coordinates (LAO/RAO-cranial/caudal).
Cardiovascular CT requires synchronization to the cardiac cycle (‘ECG-synchronization’/’gating’). This can be achieved by prospectively triggering data acquisition only in parts of the cardiac cycle, e.g., systole or diastole. This acquisition mode (‘prospective triggering’) has the advantage of lower radiation exposure, but limits analysis to a single phase of the cardiac cycle. In contrast, acquisition with retrospective gating acquires data from the entire cardiac cycle, with subsequent reconstruction of datasets in different cardiac phases. This acquisition mode (‘retrospective gating’) is associated with higher radiation exposure, but allows more flexibility. Specifically, retrospective gated acquisitions allow time-resolved (4-D) data with a temporal resolution of 75 ms and a spatial resolution of 0.5 mm for dual source scanners (44). These high-end acquisition protocols provide almost isotropic data for detailed 3D and 4D reconstructions along any plane throughout the entire cardiac cycle, and are typically used for imaging in the context of trans-catheter interventions (45,46).
Details such as mitral annular shape, dimensions, and angiographic coordinates, presence and extent of annular calcification, coronary sinus anatomy and spatial relationship, leaflet anatomy, thickening, calcification, tenting height, tethering angles compared to annulus plane, and papillary muscle structure, can be appreciated on MDCT prior to the intervention (Figures 6-11). The CT data can also be co-registered to angiographic data at the time of the procedure, as is described with rotational angiography systems (46,47).
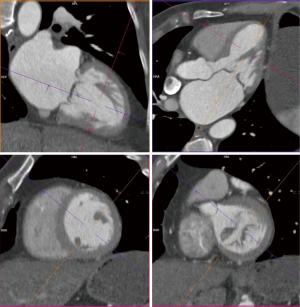
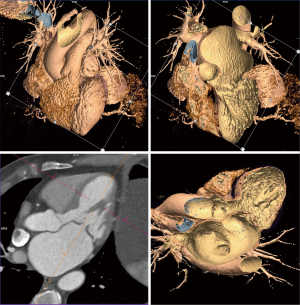
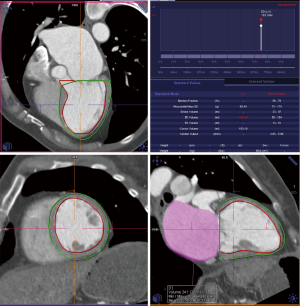
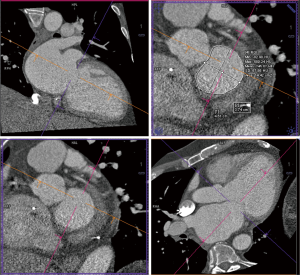
The anatomy of the mitral annulus and valve structures varies between patients and impacts procedural success (27). 3D data sets are particularly useful for exact measurements and assessment of orientation of the mitral annular plane (23). Blanke et al. proposes a D-shaped approach in which the anterior horn of the mitral annulus must be excluded when sizing the annulus for device implantation to minimize the risk for LV outflow tract obstruction with device (48).
MDCT further allows detailed description and quantification of mitral annular calcification, which defines contraindication for certain devices. Papillary muscle displacement can be evaluated by measuring the left ventricular circumference at the base and the tip of the papillary muscle (23).
MDCT allows assessment of the relationship between the mitral annulus, coronary sinus and left circumflex coronary artery, which is particular important for some approaches of transcatheter indirect mitral annuloplasty procedures. A wide angle between the sinus and the mitral annulus indicates poor pressure transmission from the sinus to the annulus and possible procedure failure (23). Studies have noted substantial variability in circumflex anatomy and MDCT features such as increased posterolateral distance between the coronary sinus and left circumflex artery in certain patients with annular dilation (49).
The temporal resolution of 4D MDCT is inferior to that of MRI and echocardiogram and, in contrast to echocardiography, MDCT does not allow real-time imaging (44). Therefore, visualization of thin, mobile structures, such as the valve leaflets and chordae tendineae is limited, in particular in MDCT in comparison to TEE. Furthermore MDCT cannot assess flow and therefore cannot directly detect or grade MR. It is limited to identification of indirect morphological characteristics of regurgitation including, distance between papillary muscles, angle of posterior leaflet and tenting height, which correlate with functional MR (43).
Another consideration with MDCT is the radiation exposure and the use of iodine based contrast material. Modern scanners and imaging protocols, and limiting coverage to the valvular region allow minimization radiation exposure (46). Furthermore, the relatively small risk of radiation exposure has to be considered in the context of the clinical scenario (50).
The amount of contrast varies with specific protocol and should be adjusted to renal function (GFR). Non-contrast imaging is an option in patients with renal insufficiency, but is of limited utility.
Magnetic resonance imaging (MRI)
Pre-procedural MRI provides incremental information for anatomical and functional characterization of the mitral annulus and valve. It is used when echocardiographic findings are in question, and is considered the gold standard for left and right ventricular volumetric assessment. Characterization of functional and hemodynamic parameters is more complex with MRI compared to echocardiogram, but MRI has the ability to detect regurgitant volume and orifice area (40). Flow measurements in Cine MRI can be used to calculate valvular pressure gradients, however MR tends to underestimate the value compared to echocardiography (51). It should be noted that calcification artifacts (signal void), preclude reliable assessment of annular and valvular calcification with MRI. Overall, MRI has the potential to play a role in pre-procedural understanding of mitral anatomy and MR severity, but its incremental value to echocardiography in this context is incompletely understood.
Angiography
Pre-procedural angiography is a routine part of percutaneous valve procedures. The main role is to determine presence and severity of coronary artery disease. At the same time angiographic features of valvular pathology are evaluated, and the spatial relationship of the mitral annulus and valve is described in angiographic coordinates. For certain types of indirect annuloplasty procedures it is also important to define the relationship between the coronary sinus and the left circumflex artery.
Intra-procedural imaging
The goal of intra-procedural imaging in the context of percutaneous mitral valve implantation is to ensure accurate deployment of the device in the mitral annulus. This is critical, because complications such as device embolization and paravalvular regurgitation are mainly associated with imprecise deployment.
Fluoroscopy
Fluoroscopy is a central component of percutaneous valvular interventions. It guides vascular access and guides catheter manipulations in the cardiac chambers (23). If required, it can be used to identify the coronary sinus and left circumflex artery. Angiography can identify mitral annular calcifications and fluoroscopic landmarks surrounding the mitral structures, but because of its limited value in distinguishing soft tissue structures, precise positioning of a device particular at a non-calcified annulus is not feasible with fluoroscopy (27).
TEE
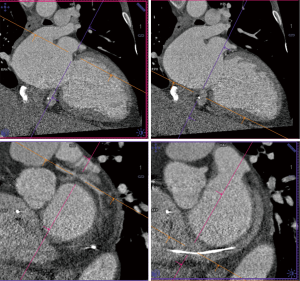
These limitations of fluoroscopy are the rational for the expanded role of intra-procedural TEE, which is the primary modality used for positioning of the device. Common views used on TEE include: the mid-esophageal short axis and bicaval views to visualize atrial septum and guide the catheter, mid-esophageal commissural view (60°–90°) to assess medial-lateral device positioning, mid-esophageal long axis view (120°–150°), also known as left ventricular outflow tract view, to assess the perpendicularity of the devices to the mitral annulus and anterior-posterior device positioning and finally, a transgastric short axis view (0°–30°) to assess coaptation of leaflets (Figures 12,13). An incremental value of 3D TEE for accurately visualizing the interatrial septum, the distance between the septal puncture site and the mitral valve, and guiding deployment of devices at the correct anatomical position has been described (52). X-plane view imaging allows simultaneous visualization of two perpendicular planes, which can facilitate visualization of the interatrial septum for transeptal punctures (Figure 14).
Immediately post-deployment, TEE is used to confirm device position and function. Particular attention is paid to identification of MR and quantification of its severity, complications such as pericardial tamponade, septal rupture, coronary sinus trauma and wall motion abnormalities from left circumflex artery impingement. Limitations of intra-procedural TEE include the need for intubation and general anesthesia.
Intracardiac echocardiogram (ICE)
ICE is a developing tool that has the potential to address some of these pitfalls and shows great promise in aiding interventional procedures for structural heart disease (53). The ICE-catheter, tipped with a miniaturized echocardiographic probe, is placed in the right atrium and notably remains stationary throughout the procedure as workstation software is manipulated to obtain different views (54). However, the experience with 2D-ICE in MitraClip implantation demonstrates that it does not allow adequate left atrial visualization to allow clip steering, leaflet visualization and grasping (55). Transventricular short and long axis ICE-views from the right ventricle have a role in evaluating left ventricular function and the presence of complications like tamponade and residual MR during the procedure. 3D ICE, in the future, may allow a more detailed anatomical characterization to guide interventional procedures for valvular and structural heart disease and in certain scenarios may allow to replace TEE in the guidance of percutaneous structural interventions and reduce the need for angiography (56,57).
The specific role of the different imaging modalities in guiding interventional procedures has not been compared, because most procedures on the mitral valve have been monitored primarily by TEE and fluoroscopy combined. Therefore data regarding the clinical benefit of specific intra-procedural imaging protocols is limited (58).
Conclusions
MR has a high prevalence in older patient populations of industrialized nations. For patient with significant co-morbidities and associated high surgical risk, transcatheter mitral repair/replacement is emerging as a treatment option. Several valve systems are in different stages of development, with some already implanted in the context of clinical trials. Because of the lack of direct visualization and the complex anatomy of the mitral annulus/mitral valve, pre- and intra-procedural imaging is critical for these procedures.
Pre-procedural echocardiography remains the ‘Reference Standard’ for identification and grading of MR, measuring annulus size and studying leaflet anatomy and mobility. Because of its ability for advanced 3D and 4D analysis, MDCT provides incremental anatomical characterization. During the procedure fluoroscopy guides catheter manipulation, but TEE use is more critical for device deployment, secondary to its superior soft tissue visualization. ICE is emerging as an intra-procedural modality that has the potential to guide valvular interventions while minimizing the need for intubation and general anesthesia (53).
As more experience develops with TMVI/TMVR devices, it will be critical to understand the relative role of various imaging modalities used in pre-procedural planning and intra-procedural guidance. Studies addressing this need will help provide uniformity in the evaluation of the mitral apparatus in the context of transcatheter interventions.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jose Navia—Chairman of the Scientific Medical Board of NaviGate Cardiac Structures, Inc.; Thomas Bartel—Consulting Fees/Honoraria/Sponsoring (Edwards Lifescience Biosense, Webster/Johnson & Johnson, Actelion, Siemens, Abbott); Paul Schoenhagen—Editor-In-Chief Cardiovascular Diagnosis and Therapy. The other authors have no conflicts of interest to declare.
References
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [Crossref] [PubMed]
- De Backer O, Piazza N, Banai S, et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv 2014;7:400-9. [Crossref] [PubMed]
- Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275-86. [Crossref] [PubMed]
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J 2012;164:163-76. [Crossref] [PubMed]
- Foster GP, Dunn AK, Abraham S, et al. Accurate measurement of mitral annular dimensions by echocardiography: importance of correctly aligned imaging planes and anatomic landmarks. J Am Soc Echocardiogr 2009;22:458-63. [Crossref] [PubMed]
- Silbiger JJ, Bazaz R. Contemporary insights into the functional anatomy of the mitral valve. Am Heart J 2009;158:887-95. [Crossref] [PubMed]
- McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr 2010;11:i3-9. [Crossref] [PubMed]
- Ho SY. Anatomy of the mitral valve. Heart 2002;88 Suppl 4:iv5-10. [Crossref] [PubMed]
- David TE. Papillary muscle-annular continuity: is it important? J Card Surg 1994;9:252-4. [Crossref] [PubMed]
- Rodriguez F, Langer F, Harrington KB, et al. Importance of mitral valve second-order chordae for left ventricular geometry, wall thickening mechanics, and global systolic function. Circulation 2004;110:II115-22. [Crossref] [PubMed]
- Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc Interv 2011;4:1-13. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [Crossref] [PubMed]
- Silvestry FE, Rodriguez LL, Herrmann HC, et al. Echocardiographic guidance and assessment of percutaneous repair for mitral regurgitation with the Evalve MitraClip: lessons learned from EVEREST I. J Am Soc Echocardiogr 2007;20:1131-40. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Rudolph V, Lubos E, Lubs D, et al. Mitral Regurgitation of degenerative as opposed to functional origin negatively impacts outcomes of MtraClip Therapy: Single-Center Experience with 255 consecutive patients. J Am Coll Cardiol 2012;59:E2004. [Crossref] [PubMed]
- Boronyak SM, Merryman WD. The once and future state of percutaneous mitral valve repair. Future Cardiol 2012;8:779-93. [Crossref] [PubMed]
- Byrne MJ, Kaye DM, Mathis M, et al. Percutaneous mitral annular reduction provides continued benefit in an ovine model of dilated cardiomyopathy. Circulation 2004;110:3088-92. [Crossref] [PubMed]
- Maniu CV, Patel JB, Reuter DG, et al. Acute and chronic reduction of functional mitral regurgitation in experimental heart failure by percutaneous mitral annuloplasty. J Am Coll Cardiol 2004;44:1652-61. [Crossref] [PubMed]
- Gillinov AM, Liddicoat JR. Percutaneous mitral valve repair. Semin Thorac Cardiovasc Surg 2006;18:115-21. [Crossref] [PubMed]
- Siminiak T, Dankowski R, Baszko A, et al. Percutaneous direct mitral annuloplasty using the Mitralign Bident system: description of the method and a case report. Kardiol Pol 2013;71:1287-92. [Crossref] [PubMed]
- Delgado V, Kapadia S, Marsan NA, et al. Multimodality imaging before, during, and after percutaneous mitral valve repair. Heart 2011;97:1704-14. [Crossref] [PubMed]
- Hasan R, Mahadevan VS, Schneider H, et al. First in human transapical implantation of an inverted transcatheter aortic valve prosthesis to treat native mitral valve stenosis. Circulation 2013;128:e74-6. [Crossref] [PubMed]
- Sinning JM, Mellert F, Schiller W, et al. Transcatheter mitral valve replacement using a balloon-expandable prosthesis in a patient with calcified native mitral valve stenosis. Eur Heart J 2013;34:2609. [Crossref] [PubMed]
- Guerrero M, Greenbaum A, O'Neill W. First in human percutaneous implantation of a balloon expandable transcatheter heart valve in a severely stenosed native mitral valve. Catheter Cardiovasc Interv 2014;83:E287-91. [Crossref] [PubMed]
- Bapat V, Buellesfeld L, Peterson MD, et al. Transcatheter mitral valve implantation (TMVI) using the Edwards FORTIS device. EuroIntervention 2014;10 Suppl U:U120-8.
- Banai S, Verheye S, Cheung A, et al. Transapical mitral implantation of the Tiara bioprosthesis: pre-clinical results. JACC Cardiovasc Interv 2014;7:154-62. [Crossref] [PubMed]
- Cheung A, Webb J, Verheye S, et al. Short-term results of transapical transcatheter mitral valve implantation for mitral regurgitation. J Am Coll Cardiol 2014;64:1814-9. [Crossref] [PubMed]
- Maisano F, Alfieri O, Banai S, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur Heart J 2015;36:1651-9. [Crossref] [PubMed]
- Rossi ML, Barbaro C, Pagnotta P, et al. Transapical transcatheter valve-in-valve replacement for deteriorated mitral valve bioprosthesis without radio-opaque indicators: the "invisible" mitral valve bioprosthesis. Heart Lung Circ 2015;24:e19-22. [Crossref] [PubMed]
- Cheung A, Al-Lawati A. Transcatheter mitral valve-in-valve implantation: current experience and review of literature. Curr Opin Cardiol 2013;28:181-6. [Crossref] [PubMed]
- Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol 2013;61:1759-66. [Crossref] [PubMed]
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012;5:341-9. [Crossref] [PubMed]
- Cullen MW, Cabalka AK, Alli OO, et al. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv 2013;6:598-605. [Crossref] [PubMed]
- Hasan BS, McElhinney DB, Brown DW, et al. Short-term performance of the transcatheter Melody valve in high-pressure hemodynamic environments in the pulmonary and systemic circulations. Circ Cardiovasc Interv 2011;4:615-20. [Crossref] [PubMed]
- Descoutures F, Himbert D, Maisano F, et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg 2013;44:e8-15. [Crossref] [PubMed]
- Thavendiranathan P, Liu S, Datta S, et al. Quantification of chronic functional mitral regurgitation by automated 3-dimensional peak and integrated proximal isovelocity surface area and stroke volume techniques using real-time 3-dimensional volume color Doppler echocardiography: in vitro and clinical validation. Circ Cardiovasc Imaging 2013;6:125-33. [Crossref] [PubMed]
- Shanks M, Delgado V, Ng AC, et al. Mitral valve morphology assessment: three-dimensional transesophageal echocardiography versus computed tomography. Ann Thorac Surg 2010;90:1922-9. [Crossref] [PubMed]
- Delgado V, Tops LF, Schuijf JD, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc Imaging 2009;2:556-65. [Crossref] [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [Crossref] [PubMed]
- Feldman T, Cilingiroglu M. Percutaneous leaflet repair and annuloplasty for mitral regurgitation. J Am Coll Cardiol 2011;57:529-37. [Crossref] [PubMed]
- Flachskampf FA, Ropers D. Computed tomography to analyze mitral valve: an answer in search of a question. JACC Cardiovasc Imaging 2009;2:566-8. [Crossref] [PubMed]
- Schoenhagen P, Tuzcu EM, Kapadia SR, et al. Three-dimensional imaging of the aortic valve and aortic root with computed tomography: new standards in an era of transcatheter valve repair/implantation. Eur Heart J 2009;30:2079-86. [Crossref] [PubMed]
- Jakobs TF, Becker CR, Ohnesorge B, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol 2002;12:1081-6. [Crossref] [PubMed]
- Schoenhagen P, Numburi U, Halliburton SS, et al. Three-dimensional imaging in the context of minimally invasive and transcatheter cardiovascular interventions using multi-detector computed tomography: from pre-operative planning to intra-operative guidance. Eur Heart J 2010;31:2727-40. [Crossref] [PubMed]
- Numburi UD, Kapadia SR, Schoenhagen P, et al. Optimization of acquisition and contrast injection protocol for C-arm CT imaging in transcatheter aortic valve implantation: initial experience in a swine model. Int J Cardiovasc Imaging 2013;29:405-15. [Crossref] [PubMed]
- Blanke P, Dvir D, Cheung A, et al. Mitral Annular Evaluation With CT in the Context of Transcatheter Mitral Valve Replacement. JACC Cardiovasc Imaging 2015;8:612-5. [Crossref] [PubMed]
- Choure AJ, Garcia MJ, Hesse B, et al. In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography: implications for percutaneous coronary sinus mitral annuloplasty. J Am Coll Cardiol 2006;48:1938-45. [Crossref] [PubMed]
- Schoenhagen P, Baker ME. Our preoccupation with ultra-low dose radiation exposure. Low contrast resolution and cardiovascular CT imaging. J Cardiovasc Comput Tomogr 2014;8:426-8. [Crossref] [PubMed]
- Webb WR, Higgins CB, editors. Thoracic Imaging: Pulmonary and Cardiovascular Radiology. Philadelphia: Lippincott Williams & Wilkins, 2004.
- Kim JH, Kocaturk O, Ozturk C, et al. Mitral cerclage annuloplasty, a novel transcatheter treatment for secondary mitral valve regurgitation: initial results in swine. J Am Coll Cardiol 2009;54:638-51. [Crossref] [PubMed]
- Bartel T, Müller S, Biviano A, et al. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J 2014;35:69-76. [Crossref] [PubMed]
- Maini B. Real-time three-dimensional intracardiac echocardiography: an early single-center experience. J Invasive Cardiol 2015;27:E5-E12. [Crossref] [PubMed]
- Henning A, Mueller II, Mueller K, et al. Percutaneous edge-to-edge mitral valve repair escorted by left atrial intracardiac echocardiography (ICE). Circulation 2014;130:e173-4. [Crossref] [PubMed]
- Silvestry FE, Kadakia MB, Willhide J, et al. Initial experience with a novel real-time three-dimensional intracardiac ultrasound system to guide percutaneous cardiac structural interventions: a phase 1 feasibility study of volume intracardiac echocardiography in the assessment of patients with structural heart disease undergoing percutaneous transcatheter therapy. J Am Soc Echocardiogr 2014;27:978-83. [Crossref] [PubMed]
- Bartel T, Bonaros N, Edlinger M, et al. Intracardiac echo and reduced radiocontrast requirements during TAVR. JACC Cardiovasc Imaging 2014;7:319-20. [Crossref] [PubMed]
- Bartel T, Konorza T, Arjumand J, et al. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 2003;107:795-7. [Crossref] [PubMed]