Meta-analysis of randomized controlled trials on efficacy and safety of extended thienopyridine therapy after drug-eluting stent implantation
Introduction
Coronary artery disease is very common worldwide and is the leading cause of death for males and females in the USA. Percutaneous coronary intervention is one of its major therapeutic strategies. Drug-eluting stents (DESs) have been widely used because they were considered to dramatically reduce the rates of in-stent restenosis or target lesion revascularization compared with bare metal stents (1). Nevertheless, researchers have also raised concerns about the risks of late and very late stent thrombosis (VLST) after DES implantation, since those events may be catastrophic (2-4). Current guidelines recommend that all patients undergoing DES placements should receive dual antiplatelet therapy, usually referred to as thienopyridine plus aspirin for 12 months if they are not faced high risk of bleeding (5,6).
However, the optimum duration of thienopyridine therapy is still under debate at present. Concerns over late adverse events have driven studies focused on whether extended thienopyridine therapy can be of clinical benefits. But their findings were not consistent. So far, there have been several relevant meta-analysis performed to address this tissue (7,8). However, the reviewers did not follow strict definitions of the durations, e.g., in Elmariah’s meta-analysis (7), some studies included defined 12-month thienopyridine therapy as long-term thienopyridine therapy while 12-month thienopyridine therapy was in the short-term category in some other studies included. When it comes to El-Hayek’s analysis (8), thienopyridine therapy of the short course ranged from 3 to 6 months whereas the prolonged therapy varied from 12 to 24 months among the different studies included. Those mentioned above were certain to have influenced the pooled effects. Therefore, to assess the efficacy and safety of extended thienopyridine therapy after DES implantation, we performed a meta-analysis of related randomized controlled trials (RCTs).
Methods
Potential articles were searched in PubMed, EMBASE, the Cochrane Library, and China National Knowledge Infrastructure from their inception to January 4, 2015 with the following terms: “dual antiplatelet therapy, thienopyridine, prasugrel or clopidogrel” and “stent or drug-eluting stent”. The reference lists of included studies were searched for any additional studies. Language was restricted to English and Chinese. The literature search process was performed by two investigators independently.
Eligible studies in the analysis should meet all the following criteria. Firstly, it should be a RCT and published in a peer reviewed journal. Secondly, participants should be coronary artery disease patients treated with dual antiplatelet therapy after DES implantation. Besides, the thienopyridine in dual antiplatelet therapy regimen should be clopidogrel or prasugrel. Further, the patients should be randomized to receive aspirin alone (12 months group) or extended dual antiplatelet therapy (>12 months group) after 12-month dual antiplatelet therapy after DES implantation. Moreover, comparison of adverse cardiac events and bleeding events should be made between 12 months group and >12 months group. Ongoing trials, case reports, editorials, reviews and duplicated data were excluded.
Study design, country, number of patients, mean age, stent types, end points, durations of dual antiplatelet therapy, incidences of adverse cardiac events and bleeding events were extracted by two investigators independently. In case of suspicion of double reporting of the same patient populations, data from the main publication were extracted. The clinical endpoints were myocardial infarction, all-cause death, cardiac death, stent thrombosis, stroke, repeat revascularization and bleeding events.
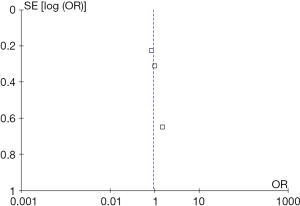
Jadad scale was used to assess the quality of included randomized controlled studies (9,10). A RCT can be awarded a highest score of 5. Funnel plot was used to evaluate the publication bias. All quality assessments of articles were performed by two reviewers. Discrepancies were resolved by contacting a third author.
In this meta-analysis, Cochrane Q-test and I2 were used to assess the heterogeneity. I2 values >25%, >50%, >75% were considered evidence of low, moderate, and severe statistical heterogeneity, respectively. To reduce the potential bias, a random-effect model was chosen in the whole study. Odds ratios (ORs) with 95% confidence interval (95% CI) were used as summary statistics. The P value for significance was set at 0.05 and all the P values were 2-tailed. All statistical tests were performed with Review Manager 5.1 software (Cochrane Collaboration, Copenhagen, Denmark).
Results
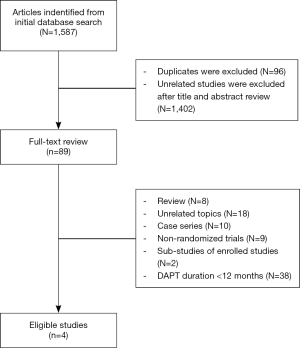
In the initial database search process (Figure 1), a total of 1,587 relevant publications were identified. After title and abstract screening, 1,498 articles were excluded. Finally, four eligible studies were selected after full-text review (11-14). Two studies were from Europe, Australia, New Zealand and North America (12,13). The other two studies were from Asia (11,14). The substudies of included studies were not included (15,16). The types of DESs used in these studies included paclitaxel-eluting, sirolimus-eluting, everolimus-eluting and zotarolimus-eluting stents. The thienopyridines used in the studies were clopidogrel and prasugrel. The patients received both clopidogrel and prasugrel in two studies (12,13) and only received clopidogrel in the other two studies (11,14). However, one of the studies was not enrolled in the analysis process because it was published as an abstract paper and was inferior in quality (14). Finally, the data of three RCTs were used in this meta-analysis (11-13). Totally, 16,265 cases were included in this meta-analysis. Among these patients, about 73.4% of them were males. There were 8,186 patients in >12 months group and 8,079 cases in 12 months group. The main characteristics of included studies are reported in Table 1. According to the funnel plot shown in Figure S1, the publication bias was acceptable.
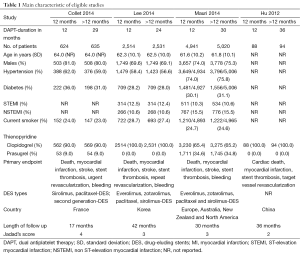
Full table
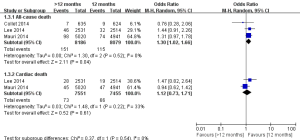
The incidences of myocardial infarction were reported in three studies (11-13). Heterogeneity test results indicated a low heterogeneity among the included studies (I2=35%). As shown in Figure 2, the incidence of myocardial infarction in the >12 months group was significantly lower than the 12 months group (1.55% vs. 2.90%; OR =0.58; 95% CI, 0.40–0.84; P=0.004), indicating that the extended thienopyridine therapy reduced the risk of myocardial infarction after DESs implantation in patients with coronary artery diseases.
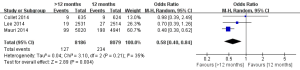
There were three studies incorporating 16,265 participants were included in the comparison of the risk of stent thrombosis between the 12 months group and >12 months group (Figure 3) (11-13). No significant heterogeneity among the studies was found (P=0.30, I2=18%). The risk of stent thrombosis after DESs implantation was significantly reduced in the >12 months group than the 12 months group (0.32% vs. 0.98%; OR =0.35; 95% CI, 0.20–0.62; P<0.001).
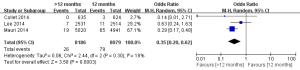
As shown in Figure 4, three studies reported the death rates (11-13) and two of them incorporating 15,006 participants reported the incidences of cardiac death (11,13). There was no significant heterogeneity among the studies (P>0.05, I2<50%). The all-cause death rate was higher in the >12 months group than the 12 months group (1.84% vs. 1.42%; OR =1.30; 95% CI, 1.02–1.66; P=0.04). There was no statistical difference of cardiac death rates between the two groups (0.94% vs. 0.89%, OR =1.12; 95% CI, 0.73–1.71; P=0.61).
Overall, the stroke events were reported in three articles in this analysis. No significant heterogeneity among the studies was found (P=0.69, I2=0%). There was a similar risk of stroke events between the 12 months group and >12 months group (0.78% vs. 0.84%; OR =0.93; 95% CI, 0.66–1.31; P=0.67), indicating that the extended thienopyridine therapy could not further reduce the risk of stroke after DESs implantation in patients with coronary artery diseases (Figure 5).
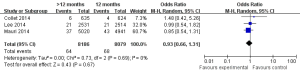
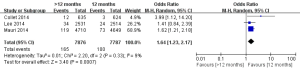
The safety end point mainly analyzed in this study was any bleeding events. As shown in Figure 6, three studies incorporating 15,663 participants reported the incidences of bleeding events. There was no significant heterogeneity among the studies (P=0.33, I2=9%). The extended thienopyridine therapy significantly increased the risk of bleeding in patients with coronary artery diseases after the implantation of DESs (2.09% vs. 1.28%; OR =1.64; 95% CI, 1.23–2.17; P<0.001).
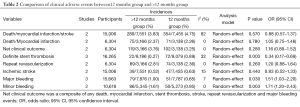
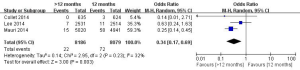
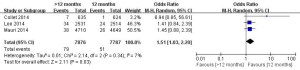
In this meta-analysis, the risk of repeat revascularization, definite stent thrombosis, ischemic stroke, major bleeding, minor bleeding and the composite events of these adverse clinical events in patients after DESs implantation were also pooled and analyzed (Table 2, Figures S2-S9). Random-effect model was used. Global Utilization of Strategies to Open Occluded Arteries (GUSTO) severe bleeding was considered major bleeding in this analysis. The risk of definite stent thrombosis after DESs implantation was significantly reduced in the >12 months group than the 12 months group. However, the extended thienopyridine therapy also markedly increased the risk of major and minor bleeding events (P<0.05). There was no difference in the comparison of repeat revascularization, ischemic stroke and the composite events between the 12 months group than >12 months group (P>0.05).
Full table






Discussion
Our meta-analysis demonstrates that in patients treated with DESs, extended thienopyridine therapy beyond 12 months, as compared to aspirin alone, reduced the risk of stent thrombosis or myocardial infarction but increased the combined incidence of major and minor bleeds. Nevertheless, there was no statistically significant difference between the two groups in the composite of any death, myocardial infarction, stent thrombosis, stroke, repeat revascularization and major bleeding events.
In our study, we defined the currently recommended 12-month thienopyridine therapy by guidelines as “short-term group” and continued thienopyridine therapy beyond 12 months as “extended group”. To our knowledge, this is the first comprehensive meta-analysis to answer whether it is optimal to switch to aspirin alone after 12-month thienopyridine therapy in patients undergoing DESs placement. Moreover, we only included high-quality RCTs and the funnel plot showed no apparent publication bias.
Mainly out of misgivings about VLST and its subsequent cardiovascular complications, some researchers advocate for prolonged use of thienopyridine therapy. Previous studies have shown that VLST, though rare it may be, is associated with high mortality (2). Our study shows extended thienopyridine therapy significantly helps in stent thrombosis or even myocardial infarction but at the cost of increasing bleeding. No statistical difference was detected in the composite of death, myocardial infarction, stent thrombosis, stroke, repeat revascularization and major bleeding events. Thienopyridine therapy does play an important role in the prevention of early stent thrombosis after deployment of a DES. But is it necessary to make it a routine to prolong thienopyridine therapy use for all patients? Our answer is no. To begin with, patients’ adherence to extended thienopyridine therapy beyond 12 month itself is a challenge that we cannot neglect. We think identifying patients who are at high risk for VLST and prolonging thienopyridine therapy use for these people may be more cost-effective. Recent studies have suggested that newer second-generation devices can significantly lower incidence of VLST (17,18), which may be explained by improvements of stent structure can result in better stent apposition, superior endothelialization and consequently, reduced platelet aggregation and thrombus formation (19). In other words, the optimal duration of thienopyridine therapy may need to depend upon stent type. Furthermore, while premature thienopyridine therapy discontinuation after DES deployment is known as an independent predictor for stent thrombosis, Mauri et al. (13) found an increased risk of stent-related myocardial infarction in both 12 months group and >12 months group during the first three months after discontinuation, which may indicate that not only the thienopyridine therapy duration but also the special condition in the early phase after thienopyridine discontinuation should be considered in VLST.
Our study has several limitations. Firstly, only publications in English and Chinese were considered, which may have left out articles in other languages and the unpublished trials. Secondly, although 16,265 patients have been included, there have been only three eligible articles for the synthesis to date, calling for more large-sample multicenter RCTs. Thirdly, different types of DESs or of thienopyridine may have potential effect on clinical outcomes but we have not done the corresponding subgroup analysis as the data was unavailable. Lastly, the RCTs included only enrolled patients free from a major adverse cardiovascular or cerebrovascular event or bleeding in the first year after DESs implantation and it will be inappropriate to extrapolate our findings to populations at high risk for adverse events.
Conclusions
In summary, a meta-analysis of the published RCTs indicates that when compared to aspirin alone, a regimen of extended thienopyridine therapy beyond 12 months may significantly reduce the risks of myocardial infarction and stent thrombosis but increase the risk of bleeding events in the patients who have received DESs implantation.
Acknowledgements
Funding: This work was supported by Natural Science Foundation of Guangdong Province, China (No. S2013040014921).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 2012;379:1393-402. [Crossref] [PubMed]
- Franck C, Eisenberg MJ, Dourian T, et al. Very late stent thrombosis in patients with first-generation drug-eluting stents: a systematic review of reported cases. Int J Cardiol 2014;177:1056-8. [Crossref] [PubMed]
- Huang KN, Grandi SM, Filion KB, et al. Late and very late stent thrombosis in patients with second-generation drug-eluting stents. Can J Cardiol 2013;29:1488-94. [Crossref] [PubMed]
- Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007;369:667-78. [Crossref] [PubMed]
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2012;79:453-95. [Crossref] [PubMed]
- Elmariah S, Mauri L, Doros G, et al. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet 2015;385:792-8. [Crossref] [PubMed]
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014;114:236-42. [Crossref] [PubMed]
- Liu M, Chen J, Huang D, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a meta-analysis of 3 randomized controlled trials. J Cardiovasc Pharmacol 2014;64:41-6. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304-12. [Crossref] [PubMed]
- Collet JP, Silvain J, Barthélémy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577-85. [Crossref] [PubMed]
- Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155-66. [Crossref] [PubMed]
- Hu T, Wang HC. AS-171 Duration of dual antiplatelet therapy and outcomes after left main percutaneous coronary intervention. Am J Cardiol 2012;109:S85-6. [Crossref]
- Garratt KN, Weaver WD, Jenkins RG, et al. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after TAXUS Liberté paclitaxel-eluting coronary stent placement. Circulation 2015;131:62-73. [Crossref] [PubMed]
- Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 2010;362:1374-82. [Crossref] [PubMed]
- Machado C, Raposo L, Dores H, et al. Second-generation versus first-generation drug-eluting stents for the treatment of patients with acute coronary syndromes and obstructive coronary artery disease. Coron Artery Dis 2014;25:208-14. [PubMed]
- Palmerini T, Kirtane AJ, Serruys PW, et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ Cardiovasc Interv 2012;5:357-64. [Crossref] [PubMed]
- Lange RA, Hillis LD. Second-generation drug-eluting coronary stents. N Engl J Med 2010;362:1728-30. [Crossref] [PubMed]