Bone marrow-derived cell therapy in chagasic cardiac disease: a review of pre-clinical and clinical results
Key words: Bone marrow; cardiomyopathy; Chagas disease; cell therapy
Introduction
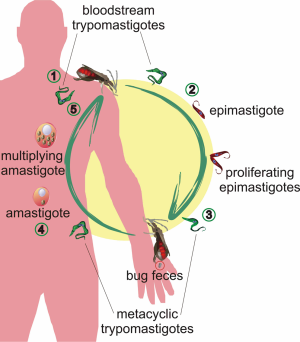
Chagas disease is caused by infection with the protozoan parasite Trypanosoma cruzi. The parasite has a complex life cycle with morphologically and biochemically distinct forms (1). In the blood of an infected mammalian host, trypomastigote forms are found. When the insect vector ingests blood with trypomastigotes, they transform into proliferative epimastigotes in the midgut. After 3-4 weeks, the epimastigotes become non-dividing metacyclic trypomastigotes. These forms are present in the hindgut of the vector and are deposited with the feces during blood meals. Transmission to a new host takes place when the parasite’s infected feces penetrate the skin or other vulnerable surfaces. In the mammalian host, metacyclic trypomastigotes invade cells and transform to amastigote forms that multiply by binary fission. As amastigotes accumulate, the host cell ruptures and disseminates the parasites through the lymphatic system and the bloodstream, where they can invade new cells or be ingested by the insect vectors, thus perpetuating the infective cycle (1) (Figure 1).
Chagas disease is characterized by both acute and chronic phases, separated by a variable-length indeterminate period that can last for many decades and during which patients are relatively asymptomatic (2). In the acute phase, which usually lasts for a few months, symptoms are non-specific, such as fever and myalgia; there is tissue parasitism and high parasitemia. In the chronic phase, parasitemia is hard to detect, but affected organs exhibit an intense inflammatory response. The gastrointestinal tract and heart are the main targets at the chronic stage of the disease and dilatation is present, constituting the so-called mega syndromes (2).
The disease is endemic in all Latin American countries with the exception of the Caribbean nations, but it can be now considered a global disease due to migration of infected individuals to the northern hemisphere. In the USA and Europe, there are almost 400,000 people infected with the parasite, the majority (300,000) living in the USA (3,4). In Latin American countries, it is estimated that 16-18 million individuals are infected with the parasite, with more than 300,000 new cases reported each year. Transmission of T. cruzi to humans has been essentially vector-borne, but while transfusion transmission has been essentially eliminated throughout much of the endemic region by testing of donated blood, this has been the main transmission route in Europe and the USA.
In many aspects chronic chagasic cardiomyopathy (CCC) is similar to other dilated cardiomyopathies, especially those of infectious nature. Not all infected individuals go on to develop the cardiomyopathy, in fact only 10-30% of infected patients develop cardiac symptoms (5). A common aspect of these infectious cardiomyopathies is that current therapy is symptomatic, making use of the arsenal of drugs and devices for the treatment of congestive heart failure (CHF). However, the primary cause of the disease, the infectious agent, is not the usual target of therapy. Additional similarities relate to the outcome of dilated cardiomyopathies once patients develop CHF, when patients present an extremely high mortality rate (5). At this stage, heart transplantation is the only available therapy, regardless of all the problems associated with donor shortage and the need for immunosuppression, but in Chagas disease this option is further limited by persistence of parasite in human tissues and the risk of re-activation of the disease, especially in immune suppressed patients. Therefore, alternative therapies are urgently needed and since cell-based therapies emerged as exciting strategies for ischemic heart diseases, they should also be tested for dilated cardiomyopathies.
Use of cell therapies in animal models of cardiac diseases
The use of cell therapies to improve cardiac function has been attempted experimentally for more than two decades. The use of bone marrow-derived cells to treat cardiac diseases gained impulse based on the observations that stromal bone marrow (BM) cells could be induced to differentiate into cardiomyocytes (CM) in vitro (6) and when transplanted into cryoinjured rat hearts improved myocardial function and promoted angiogenesis (7). Another significant development was achieved by Orlic and colleagues (8), who reported that hematopoietic stem cells from transgenic mice expressing enhanced green fluorescent protein (EGFP), when transplanted into infarcted hearts of syngeneic mice, differentiated into cardiac muscle and vascular cells. Since then, many other laboratories reported that hematopoietic and stromal cells derived from BM improved myocardial function in animal models of both cryoinjured and ischemic heart lesions (9-16).
However, cardiac regeneration by BM-derived cell differentiation into cardiomyocytes has not been reproduced (17-19). Nonetheless, in studies where functional measurements were performed, improvement in heart function was detected after bone marrow-derived cell transplantation, even when differentiation into cardiomyocytes was not observed (18). This led to the hypothesis that the beneficial effects of cell therapies using BM-derived cells in heart disease are promoted by paracrine effects (15,20). Indeed, a recent report by Lee’s group using a double transgenic mouse model showed that c-kit positive cells from the bone marrow induced new cardiomyocyte formation through a paracrine mechanism (16).
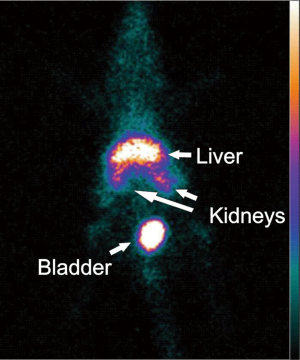
In mouse models of Chagas disease, various areas of the left and right ventricles are affected. For this reason, we opted for intravenous injection of cells in our experiments. To prove cardiac homing of the systemically injected cells, BM cells were labeled with fluorescent dyes or superparamagnetic nanoparticles and tracked in vivo by biofluorescence and magnetic resonance imaging (MRI) respectively. Cells could be detected in the heart until 2 days after injection (Jasmin, 2012, unpublished results). Since BM-derived cells home to the chagasic heart, systemic injection is a viable approach for cell therapy in this model. However, one should be aware that most of the injected cells end up not in the heart, but in organs such as the liver, kidneys and bladder (Figure 2).
After the initial homing experiments, Soares et al. (21) demonstrated that BM mononuclear cells from nonchagasic syngeneic donors significantly reduced cardiac inflammation and fibrosis in mice with chronic T. cruzi infections. This improvement was maintained up to six months after cell therapy. Cell dosing experiments demonstrated that 105 cells were necessary for a significant reduction in the number of inflammatory cells and injection of 106 or 107 cells induced similar effects (21).
Using MRI, we demonstrated that 107 BM mononuclear cells prevent and reverse the right ventricular dilatation induced by T. cruzi infection (22), thus showing that the histopathological improvement reported by Soares et al. (21) had a functional correlate. Furthermore, it was determined that repeated injections of granulocyte-colony stimulating factor (G-CSF), which mobilizes stem cells from the bone marrow, also decreases inflammation and fibrosis in the hearts of chagasic mice (23). We then used BM mononuclear cells followed by G-CSF injections in the infected mice and observed that the combined therapy enhances the reduction of the inflammatory infiltrate (Ribeiro dos Santos R, personal communication).
One of the most striking observations after cell therapy in the chronically infected mice was related to the pattern of gene expression, examined by microarray. While chagasic mice had 1,702 (out of 9,390) cardiac genes with expression altered by infection, after BM mononuclear cell therapy, 96% of these genes were restored to normal levels, although an additional 109 genes had their expression altered by therapy (24).
In another model of chagasic cardiomyopathy, direct left ventricular injection of co-cultured skeletal myoblasts and stromal BM-derived cells improved heart function in chronically infected chagasic rats as measured by echocardiography. Injection of the co-cultured cells increased ejection fraction and decreased end-systolic and diastolic volumes (25).
Clinical trials in chagasic patients with cardiomyopathy
After the encouraging results in the animal models, Vilas- Boas et al. (26) initiated a clinical trial to examine the feasibility and safety of autologous BM cell transplantation in patients with CHF due to CCC. Due to the lack of knowledge regarding the mechanisms of action of the cells, the trial only included patients with end-stage CHF whose sole therapeutic option would be heart transplantation. The trial was an open label, uncontrolled and single center study, enrolling 30 patients with the following inclusion criteria: 18-70 years old, of either gender, with CHF due to Chagas disease, in NYHA class III or IV, with an ejection fraction of less than 40% while on optimized pharmacologic therapy for at least 4 weeks before enrollment (26). All patients received cell therapy and, therefore, there was no control group. BM cell aspiration was performed under local anesthesia on the day of the injection and the mononuclear fraction was obtained through Ficoll density gradient centrifugation. The cell suspension was diluted in 20 mL of saline with 5% autologous serum and injected in the coronary arteries using an angioplasty catheter. Mean number of cells injected was 2.7×108. At the 25th day after cell injection, patients received 5 μg/kg of G-CSF for 5 days. Patients were then followed for six months. Since this was a safety trial, it is important to note that there were no detectable increases in arrhythmias after cell therapy or in troponin I levels during or after the procedure. Results indicated that cell therapy induced a small but significant increase in ejection fraction and in quality of life as determined by the Minnesota Questionnaire and by NYHA class. Six minute walking test also showed significant improvement.
A case report in a patient with chagasic cardiomyopathy showed that BM mononuclear cells delivered by intracoronary route were retained in the diseased, hypoperfused areas of the myocardium (27). Further studies using labeled cells did not confirm these results in six additional chagasic patients (28). In this study, we demonstrated that, although cells were retained in the chagasic heart, they were located preferentially in the well perfused areas.
Based on the promising results of the safety trial, a larger, multicenter, randomized, double-blinded and placebo-controlled trial was designed to test the efficacy of intracoronary delivery of bone marrow-derived mononuclear cells in chronic chagasic cardiomyopathy patients (29). Inclusion criteria were: diagnosis of heart failure by the Framingham criteria, regular visits to a cardiology service with at least two independent serological diagnosis of Chagas disease, ages between 18-75 years, NYHA class II to IV, ejection fraction below 35% by echocardiography according to Simpson’s rule, and optimized pharmacologic therapy. Main exclusion criteria were: valvular diseases (except for functional mitral or tricuspid regurgitation), coronary angiography with significant lesions (more than 50% of obstruction), sustained ventricular tachycardia, abusive use of drugs or alcohol, serum creatinine >2.5 mg/dL, neoplasia and other diseases that might impact life expectancy within two years. The primary endpoint for the trial was the difference in ejection fraction, as determined by echocardiography using Simpson’s rule, between baseline and after cell or placebo injection in the two groups, after 6 and 12 months of follow-up. Trial was powered to detect an absolute 5% difference as significant. The trial enrolled 243 patients who satisfied inclusion criteria. After exclusion of some of the participating centers and of patients who were lost to follow-up, we analyzed 90 patients in the cell group and 93 patients in the placebo group. We did not observe serious adverse events after cell or placebo injections, thus confirming the observations of Vilas-Boas et al. (26). The mean number of cells injected was 2.5×108 mononuclear cells, with 97.5% viability. We observed an improvement in ejection fraction after the procedure, with a mean increase of 3 points in the cell group after 6 months and 3.5 points after 12 months. However, the placebo group also showed an improvement of 2.5 points after 6 months and 3.7 points after 12 months. Therefore, we could not detect differences between the two groups and the trial failed to meet the primary endpoint. Analysis of secondary endpoints also failed to show differences between groups. While left ventricular systolic and diastolic volumes showed no significant differences between baseline and follow-up in the two groups, Minnesota Life Quality Questionnaire, NYHA class and 6-minute walking test showed significant differences between baseline and follow-up, but again both the cell and placebo groups improved and there were no differences between groups. In conclusion, this efficacy trial showed that there is no additional benefit of intracoronary injection of bone marrow mononuclear cells in chagasic patients with low ejection fraction (29).
What went wrong?
This is the question we have been asking ourselves after completion of the multicenter trial. An enormous number of factors may explain why the trial failed: cell type, cell number, injection route and stage of the disease are the most important to consider initially. But another point that deserves attention may be the inadequacy of the animal models used to translate the results to the clinic. In our experience, the mouse model, which is the most used animal model for CCC, may not reproduce faithfully the human disease. We have, throughout these years, used different combinations of mice (syngeneic or not) and T. cruzi strains (cardiotropic or not) and have never been able to consistently show left ventricular dysfunction after infection. In fact, the most consistent dysfunction observed in the infected mice is right ventricular dilatation (22,30,31). This is actually intriguing, since inflammation and fibrosis are present in the left and right chambers (21,32,33). The rat model is not considered a good model of Chagas disease, since it does not recapitulate the degree of inflammation and fibrosis detected in mice and humans. Yet, Guarita- Souza et al. reported LV dysfunction in rats (25). These results should be revisited by other laboratories, preferably using MRI, since the rat model may be more useful than the mouse model for functional studies. An interesting model for translation into the clinic is the dog. The chronically infected dogs develop cardiac inflammation and fibrosis and after 270 days of the initial infection there is a significant decrease in left ventricular ejection fraction (34,35). We are currently starting experiments using bone marrow mesenchymal stem cells (MSC) in chronically infected dogs. This brings us back to the cell type used in the clinical trial. Could another cell type have worked? The current literature shows that bone marrow or adipose tissue-derived MSC have been used with success in animal and human trials. This is certainly an interesting cell type to be tested in CCC, given its immune modulatory properties and the clear involvement of the immune system in the pathogenesis of Chagas disease. We have used both marrow (Jasmin, 2012, unpublished results) and adipose-derived (Mello DB, 2012, unpublished results) MSC in the mouse model of CCC with results similar to those obtained with the mononuclear fraction, but, given the failure to translate the mouse results to humans, we are now planning to test new cell types in the dog model before starting a new clinical trial. Another cell type to be tested is the cardiac stem/progenitor cell (CPC). Preclinical experiments (36-41) and safety clinical trials (42,43) have shown promising results in ischemic heart disease that will hopefully be confirmed in efficacy trials, already planned. This should be the next cell type in the pipeline for CCC, since there is evidence of new cardiomyocyte formation when using these cells (36-41). The use of pluripotent stem cells differentiated into a cardiomyocyte-like phenotype remains an alternative to be tested, if every other cell type fails.
Cell number is another reason that our trial may have failed. However, we think this is highly unlikely, since cell number varied among injected patients (from 108 to almost 109) and we could not detect a correlation between the number of injected cells and the variation in either the primary or secondary endpoints (29). At any rate, this should be considered, since mouse experiments showed that the maximum effect on fibrosis and inflammation was attained at 4×107 cells/kg (21) and, in the human trial, considering a 70 kg patient, we would need 28×108 cells.
Injection route may also explain the failure of the human trial. The animal models show that a low percentage of injected cells (although a significantly higher number than in control animals) home to the heart of infected animals after systemic injection (Jasmin, 2012, unpublished results). Our results in humans confirmed this and further showed that the cells do not reach hypoperfused areas, where they are most needed (28). The use of the intramyocardial route in chagasic patients poses serious safety concerns, since these patients are prone to arrhythmias. Therefore, alternative routes in CCC should be carefully examined and validated in large animal models before any attempt is made to translate this to the clinic.
Disease stage is another reason the trial may have failed. We chose patients with severely compromised left ventricular function. It is possible that, if we had chosen patients at the initial stages of their cardiomyopathy, results would have been better. However, in our view, tests of an experimental therapy, whose mechanism of action is not fully understood, should be done in the patients who are most likely to benefit from it, which are those who, despite optimized pharmacologic therapy, show deterioration of their cardiovascular status. To investigate the influence of disease state in the outcome of cell therapy we will treat dogs both when left ventricular function is starting to show decline and after overt heart failure has been established.
We conclude by stating that we firmly believe that, within the next few years, we will be able to find the best animal model and the appropriate cell type, cell number, injection route and disease state that will result in possible benefits for the chonic chagasic cardiomyopathy patients. These patients, who usually belong to the lower socioeconomic strata of our society, have very limited therapeutic options once they develop congestive heart failure. Heart transplantation, the only current alternative, is plagued with limitations and chagasic patients are not a preferential population due to the need of immune suppression and the possibility of reactivation of the acute disease. Cell therapy may help these patients and we will continue our efforts to make it become a real therapeutic option for this disease.
Acknowledgements
We would like to thank Bruno Diaz Paredes, who provided valuable help in the preparation of the figures for this manuscript. We would also like to thank the following agencies for financial support: CAPES, FAPERJ, CNPq and the Brazilian Ministry of Health.
Disclosure: The authors declare no conflict of interest.
References
- Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol 2005;3:819-23.
- Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 2001;1:92-100.
- Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis 2011;5:e1136.
- Coura JR, Viñas PA. Chagas disease: a new worldwide challenge. Nature 2010;465:S6-7.
- Tarleton RL, Reithinger R, Urbina JA, et al. The challenges of Chagas Disease-- grim outlook or glimmer of hope. PLoS Med 2007;4:e332.
- Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999;103:697-705.
- Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999;100:II247-56.
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5.
- Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430-6.
- Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93-8.
- Olivares EL, Ribeiro VP, Werneck de Castro JP, et al. Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am J Physiol Heart Circ Physiol 2004;287:H464-70.
- Lachtermacher S, Esporcatte BL, Fortes Fda S, et al. Functional and transcriptomic recovery of infarcted mouse myocardium treated with bone marrow mononuclear cells. Stem Cell Rev 2012;8:251-61.
- Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004;104:3581-7.
- Thompson CA, Reddy VK, Srinivasan A, et al. Left ventricular functional recovery with percutaneous, transvascular direct myocardial delivery of bone marrow-derived cells. J Heart Lung Transplant 2005;24:1385-92.
- Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195-201.
- Loffredo FS, Steinhauser ML, Gannon J, et al. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 2011;8:389-98.
- Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004;428:664-8.
- Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004;428:668-73.
- Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004;10:494-501.
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367-8.
- Soares MB, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol 2004;164:441-7.
- Goldenberg RC, Jelicks LA, Fortes FS, et al. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis 2008;197:544-7.
- Macambira SG, Vasconcelos JF, Costa CR, et al. Granulocyte colony-stimulating factor treatment in chronic Chagas disease: preservation and improvement of cardiac structure and function. FASEB J 2009;23:3843-50.
- Soares MB, Lima RS, Souza BS, et al. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle 2011;10:1448-55.
- Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation 2006;114:I120-4.
- Vilas-Boas F, Feitosa GS, Soares MB, et al. Early results of bone marrow cell transplantation to the myocardium of patients with heart failure due to Chagas disease. Arq Bras Cardiol 2006;87:159-66.
- Jacob JL, Salis FV, Ruiz MA, et al. Labeled stem cells transplantation to the myocardium of a patient with Chagas’ disease. Arq Bras Cardiol 2007;89:e10-1.
- Barbosa da Fonseca LM, Xavier SS, Rosado de Castro PH, et al. Biodistribution of bone marrow mononuclear cells in chronic chagasic cardiomyopathy after intracoronary injection. Int J Cardiol 2011;149:310-4.
- Ribeiro Dos Santos R, Rassi S, Feitosa G, et al. Cell therapy in Chagas cardiomyopathy (Chagas arm of the multicenter randomized trial of cell therapy in cardiopathies study): a multicenter randomized trial. Circulation 2012;125:2454-61.
- Mukherjee S, Nagajyothi F, Mukhopadhyay A, et al. Alterations in myocardial gene expression associated with experimental Trypanosoma cruzi infection. Genomics 2008;91:423-32.
- Souza AP, Jelicks LA, Tanowitz HB, et al. The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem Inst Oswaldo Cruz 2010;105:746-51.
- Nagajyothi F, Zhao D, Weiss LM, et al. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res 2012;110:2491-9.
- Melo RC. Acute heart inflammation: ultrastructural and functional aspects of macrophages elicited by Trypanosoma cruzi infection. J Cell Mol Med 2009;13:279-94.
- Maximiano FP, de Paula LM, Figueiredo VP, et al. Benznidazole microcrystal preparation by solvent change precipitation and in vivo evaluation in the treatment of Chagas disease. Eur J Pharm Biopharm 2011;78:377-84.
- Santos FM, Lima WG, Gravel AS, et al. Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas’ disease. J Antimicrob Chemother 2012;67:1987-95.
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763-76.
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 2003;100:12313-8.
- Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004;95:911-21.
- Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA 2007;104:14068-73.
- Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007;115:896-908.
- Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009;120:1075-83.
- Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847-57.
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomized phase 1 trial. Lancet 2012;379:895-904.