Imaging in the context of replacement heart valve development: use of the Visible Heart® methodologies
Key words: interventional cardiology; cardiac surgery; Visible Heart®; heart valve
Introduction
The Visible Heart® laboratory has developed a number of research approaches to image and analyze the valves within normal and diseased human. To date, our laboratory has collected and preserved over 250 human hearts, 51 which were reanimated using Visible Heart® methodologies (1). Via our unique library of: (I) physical specimens (fixed and plastinized); (II) classic anatomical plates; (III) fluoroscopic, echocardiographic, magnetic resonance (MR) and computed tomography (CT) images; (IV) functional video footage; and (V) digital models, our laboratory serves as an unique resource from which one can compare the various anatomies of cardiac valves from a variety of different disease states. Specifically, the use of the Visible Heart® methodologies to reanimate large mammalian hearts provides: (I) device designers a rapid method to critically assess prototypes and (II) clinicians the opportunity to directly visualize the deployment of new or problematic clinical systems/techniques. It is clear that the future clinical applications of cardiac valve therapies will include beating heart procedures, many of which are on the market today, and implantation of these devices will likely utilize simultaneous multiple imaging modalities (e.g., within a hybrid operating room). The combination of multiple imaging modalities will facilitate the various aspects of device deployment, ensuring the correct positioning and orientation of the delivered device and the acute evaluation of the cardiac performance post implantation within both experimental and clinical settings.
Specifically, our laboratory has investigated the deployment of numerous types of transcatheter delivered valves, assessed current surgical repair techniques, and mimicked various cardiac valve pathologies to advance the development of the field. Such valves have been deployed into the aortic, mitral, or pulmonary positions of both the native and modified anatomies to simulate: (I) the potential range of beating heart (“off pump” or minimally invasive) procedures; and (II) the presence of anatomical abnormalities (e.g., ruptured chordae or calcification of the valve).
Background
Valve anatomy and static imaging
With the advent of high resolution noninvasive imaging allowing modern cardiac centers of excellence to collect detailed images of myocardial contractions, blood movement, and valve function; the understanding of functional human heart anatomy has progressed rapidly. An intricate understanding of cardiac valve anatomies and their associated pathologies remains an important fundamental in both cardiovascular medicine and the cardiac device industry (2,3). In other words, a well-developed appreciation of the relevant cardiovascular anatomies (in relation to both vascular approaches and within the heart itself) is critical at all levels of device design, the development process, and eventual clinical use (4). Our laboratory has had the privilege of collecting a large library of fresh human heart specimens for educational and research purposes from: (I) organ donors whose hearts are not deemed viable for transplantation and are donated for research (via LifeSource, the Upper Midwest Organ Procurement Organization); and (II) bodies donated to the University of Minnesota’s Anatomy Bequest Program (5). This collection is an open resource, which continues to provide researchers with important insights as to how potential valve technologies may interact with the human endocardial anatomy. In addition to specific anatomical investigations, such research capabilities allow for the placement of prototype devices and the rapid comparison of how these devices will interact with the surrounding cardiac anatomies in a variety of human specimens.
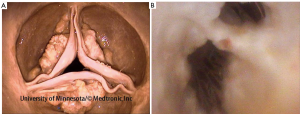
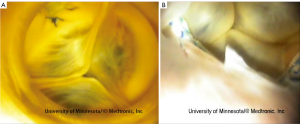
Occasionally, hearts received by our laboratory present with cardiac valve pathologies or congenital defects, for example, fibrous leaflets of the atrioventricular valves, calcified leaflets of the aortic valve (Figure 1A), or double orifice mitral valves (Figure 1B) (5,7).
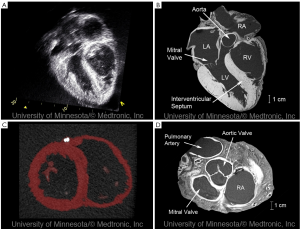
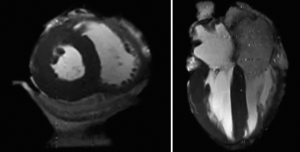
Static imaging of hearts from the Visible Heart® library via multi-slice CT and MR imaging (e.g., 1.5T, 3T, or greater) has allowed us to create a digital library via the nondestructive imaging of each specimen (8). By embedding the hearts in agar gel, our laboratory has been able to acquire high quality images using a variety of clinical modalities. Several advantages of this approach include: (I) the reduction of motion artifacts during imaging; (II) the consistent orientation of the specimens for each imaging modality; and (III) the continued preservation of the specimens over time. Shown in (Figure 2) are some of the results from these imaging studies showcasing the high resolution imaging capabilities.
Recently, a series of 30 perfusion fixed hearts was assessed by Tsang et al. to determine the size of the aortic annulus dimensions using three-dimensional echocardiography, CT, and MR imaging (9). Such studies have provided unique insights into the abilities of each imaging modality to size the native annulus of the valve, a critical step during the implantation of transcatheter delivered aortic valves.
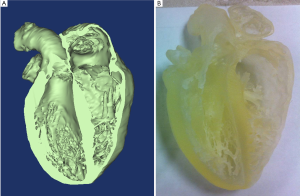
The acquisition of high resolution images has allowed for the creation of 3D reconstructions using software packages such as Mimics (Materialise, Leuven, Belgium). These reconstructions have been used to populate a digital anatomical library of human hearts and selected specimens have in turn been used to print 3D models. The creation of accurate models has provided the laboratory with educational tools for students and engineers alike, see (Figure 3).
The Visible Heart®
For the past 15 years, our laboratory at the University of Minnesota has partnered with Medtronic, Inc. to further develop and utilize the Visible Heart® methodologies for cardiac device development. This approach involves the remaval of the heart using standard transplant procedures as described by Chinchoy and Hill (1,10). Once isolated, the hearts are cannulated, perfused, and defibrillated and can eventually function with all four chambers working (typically eliciting a native sinus rhythm). Following reanimation, cardiac and systemic pressures and outputs are monitored and preloads and afterloads can be adjusted to simulate systemic vascular pathologies such as pulmonary or systemic hypertension. Our laboratory routinely utilizes various imaging techniques (both endoscopes and fiberscopes) and has compared them to clinically available methods (e.g., fluoroscopy and echocardiography). This has provided unique comparative footage of normal and abnormal functional anatomies of both human and animal hearts, not only within a given chamber, but within vessels as well (7).
More specifically, our patented isolated heart method/ apparatus serves multiple roles by allowing operation in either Langendorff, right-side working, or four-chamber working modes (1,11,12). During the Langendorff mode, the left-side afterload is held constant with a coronary perfusion pressure of approximately 60 to 80 mmHg (1). Thus, the flows through the coronaries are determined by dilations or constrictions of the arteries themselves. Right-side working mode combines Langendorff retrograde aortic perfusion with antegrade, or physiologic, flows through the right atrium and right ventricle (adjustable between 3-5 L/min). During four-chamber working mode, the flows through the heart are normally determined by the intrinsic heart rates and contractility of the heart. Note that the intrinsic heart rates can be modified by altering the temperature of the buffer or by adding various chronotropic pharmacological agents (e.g., dobutamine). Although no model can perfectly mimic in vivo conditions, the Visible Heart® methodologies allow for the useful re-creations of various cardiac states through control of preload and afterload pressures. We can also change the physical orientation of a given heart to obtain clinically relevant medical images and/or to simulate the relative heart orientations during planned clinical procedures.
When studying cardiac valves, it is important to understand that, while in four-chamber working mode, the aortic (left side) and pulmonary (right side) valves open during ventricular systole and close during ventricular diastole. Initially, there is a net movement of buffer into the ventricles through the open mitral (left side) and tricuspid (right side) valves during diastole, i.e., relative to the applied preloads. At this same time, the externally applied afterloads in the aorta and pulmonary artery are made to be greater than the pressure in the ventricles, effectively closing the aortic and pulmonic valves, respectively. As systole begins, the mitral and tricuspid valves close due to the pressure increases in the ventricles. Once the pressures in the left ventricle exceed those of the aorta (the applied left heart afterload), the aortic valve opens and the buffer is ejected through the aortic valve. The same is true with the pulmonary valve, as it opens when the pressure in the right ventricle exceeds that of the pulmonary artery (the applied right heart afterload). Typically, there can be some micro-bubbles in the fluid flow observed during ultrasonic imaging within the heart, due to imperfect sealing of the preparation and/or inadvertent introduction of air, yet it is important to note that this does not prohibit imaging and may actually help one to visualize turbulent flow patterns. Furthermore, while in any of these modes, one may add a catecholamine and/or a bolus of calcium ions to upregulate/enhance hemodynamic functioning.
To date, the Visible Heart® model has provided our scientists, engineers, and clinical collaborators with an innovative heart model to better understand how the dynamic forces and complex anatomic structures of the heart interact with a broad range of cardiac devices (e.g., pacing and defibrillation leads, pressure sensors, valves, and occluder devices). We are continually in the process of enhancing the overall utility of our system and methodologies, e.g., from improved mechanics to novel chemical additives to the buffer.
Visualization of functional anatomies and valve therapies®
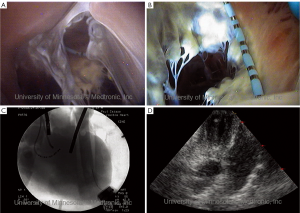
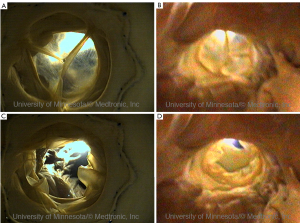
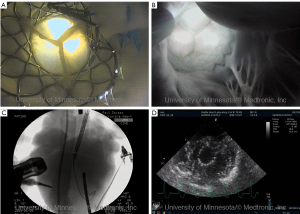
As noted above, by utilizing such endoscopic video systems in conjunction with clinically relevant imaging modalities, such as fluoroscopy (continuous x-ray) and cardiac ultrasound (echocardiography), we have been able to create novel comparative anatomy footage. This has provided a direct visualization of what the physician would see in the clinical setting, and thus has also provided valuable insights into device and delivery system performance (1,10). Again, all footage of human anatomy has been placed on the free-access website “The Atlas of Human Cardiac Anatomy,” for public educational use (7). This site uniquely includes movie clips of functional cardiac anatomies, as well as comparative imaging using echo, fluoroscopy, and MRI. Examples of imaging capabilities within the human heart can be seen in Figures 4-7.
Use of the Visible Heart® in transcatheter valve device development
Additionally the Visible Heart® approach can be used to capture internal and external images of valve implantations during near normal hemodynamic conditions (left ventricular systolic pressures of 70-120 mmHg). These images allow for evaluation of both surgical (Figure 5) and percutaneous (Figures 6,7) device implantations and provide insight into the performance of the delivery catheters, deployment balloons, frames, and tissue valves (see: http://www.vhlab.umn.edu/ atlas/devicetutorial/). Specifically, engineers and scientists are able to evaluate many aspects of transcatheter device design such as deployed frame lengths, frame shapes, relative valve attachments, and/or interaction of the device with native or conduit anatomies. For example, visualization of the delivery of a transcatheter pulmonic valve provided new insights into the design of valve leaflets in the pulmonary position to accommodate the low pressure gradients encountered in this location, see (Figure 6) (13). The implantation of transcatheter aortic valve replacements into the native aortic roots of human hearts has highlighted the interaction of the stent with both native leaflets and the mitral apparatus, thus illustrating the importance of precise stent sizing and positioning, i.e., in order to avoid interaction with the anterior leaflet of the mitral valve and minimize pressure on the cardiac conduction system, see Figure 7 (14).
Aortic valve dysfunctions
With the intense interest in percutaneous aortic valve repair/ replacements, there have been considerable advances in the design and development of transcatheter delivered prosthetic aortic valves: transcatheter aortic valve replacement (TAVR). In recent years, our laboratory has been fortunate to utilize Visible Heart® methodologies to provide a beating heart model for the testing of such devices and their delivery systems in both animal and human hearts (15). However, it should be noted that the routine testing of such devices in healthy swine anatomy does not always satisfactorily approximate the environment and function of the devices’ intended patient populations. As such, our laboratory has been working to develop models of severe aortic stenosis, with the specific aims of determining how large calcific deposits on the leaflets affect the deployment and function of such delivered devices. To approximate severe stenoses of the aortic valve, we have: (I) directly adhered models of calcifications to the leaflets to reduce leaflet motions; and (II) partially adhered the leaflet commissures to reduce the effective orifice areas of these valves (5,16). To date these models have allowed for better procedural testing of transcatheter delivered aortic valves (e.g., from balloon valvuloplasty to device deployments), and has provided useful insights into the potential interactions of deployed devices with the calcific deposits, i.e., relative to the healthy native anatomies.
Combining the Visible Heart® methodologies with clinical imaging modalities
Due to the inherent advantages of MRI and CT assessment of native valve function and anatomy in vivo, it was considered desirable to develop a portable Visible Heart® system which would allow MR or CT imaging of an isolated beating heart. The full details of the development of a portable apparatus and associated methodologies for isolated heart imaging in CT and MRI environments is described by Eggen et al. (17). The system allows for the isolated heart to be placed safely on the patient bed of the MR or CT scanner for the acquisition of functional and static images (Figure 8).
Discussion
Utilization of Visible Heart® methodologies has provided (and will continue to provide) unique visualization for a variety of cardiac valve replacement technologies and their delivery systems. Hence, we consider that the described anatomical preparations and in vitro functional models and images will aid both design engineers and physicians who hope to rapidly and effectively develop and test implant methodologies. Nevertheless, our experimental setup is not without known limitations. For example, ischemic times prior to reanimation can compromise cardiac functions, specifically contractilities and pressure generation. The positioning of the heart on the apparatus may also affect the performance of these isolated hearts: yet, we are constantly modifying our system to allow for changes in spatial orientations. Additionally, the lack of a pericardium may contribute to a degree of over-expansion of the atrial chambers (and the right ventricle), and cause slightly different respective anatomical orientations of the great vessels and chambers and/or differences in contractility compared to in vivo performance (18). Finally, the use of a clear perfusate without a specific oxygen carrier, such as hemoglobin in blood, will result in a continuous low-level global ischemia and the development of tissue edema, which will have an effect on the long-term viability of these reanimated hearts. On the other hand, this experimental approach can also be considered as a potential model for acute heart failure.
The Visible Heart® methodologies for valve visualization are intended to complement the work by others that employ well-established in vivo or in vitro methods to test the reliability, durability, biocompatibility, or other parameters of newly developed transcatheter delivered valves (19-24). Importantly, as clinicians push the current boundaries of transcatheter delivered therapies, it is predicted that multiple imaging modalities will be required to plan and guide these interventions. Nevertheless to date, all standardized imaging modalities utilized during intracardiac interventions exhibit some potential limitations, e.g., low temporal or spatial resolution, excessive exposure to ionizing radiation, and/or interference with the clinical operator’s freedom of movements. In the near term, hybrid operating rooms will employ a combination of imaging modalities providing the information required to guide these complex interventions. Hence, our laboratory in a way may also provide “a glimpse into the future,” by employing simultaneous or comparative imaging in an experimental setting. Here we have provided a small sampling of the direct visualization of transcatheter valve implantations and surgical valve therapies that have been completed in our laboratory and the device testing that can be achieved using Visible Heart® methodologies. It should be noted that we continually add images to our free-access website “The Atlas of Human Cardiac Anatomy” (7).
The complexities of intra-cardiac valve repair/replacements will continue to increase with the continual advancement of transcatheter technologies. It is expected that such procedures will continue to intensify as clinicians become more comfortable with these novel techniques of administering therapies and/or devices within the beating heart. Additionally, clinicians and engineers continue to invent, design, and refine new product solutions at a rapid pace. This is exemplified by the fact that in the field of TAVR alone two devices (CoreValve ReValving® system, Medtronic Inc., Minneapolis, MN and the Edwards SAPIENTM valve, Edwards Lifesciences, Irvine, CA) have achieved market release and are involved in ongoing clinical trials. Additionally, up to 25 other new designs of TAVR devices are currently under development (25). These products approach and attempt to solve the challenges of TAVR by incorporating desirable features to improve the performance and efficacy of implantation. As such, thorough in vitro and pre-clinical testing is essential to the success of these products.
Conclusions
In summary, our laboratory utilizes a variety of research approaches to gain a better understanding of the variability in human cardiac anatomies that can be elicited. Such information is important for both the clinician and medical device designer when developing novel valve replacement therapies. More specifically, we have developed a library of perfusion fixed human hearts to perform a variety of static imaging assessments, allowing for the development of 3D anatomical reconstructions. Additionally, with the use of Visible Heart® methodologies, one can better visualize anatomical and morphological alterations that occur with specified pathologies and/or those that may occur following the deployment of devices within a given heart. By reanimating large mammalian hearts using a clear perfusate, we are able to visualize functional anatomies with endoscopes placed directly within various heart chambers and/or within the large diameter vessels of the heart. When direct visualization is simultaneously coupled with clinically employed imaging modalities, it provides further critical insights that can be used to more quickly and precisely advance such technologies. We consider that the utilization of both fixed specimens and Visible Heart® methodologies for device evaluations should be used in a complementary fashion with other techniques that utilize in vivo or in vitro methods, to test the reliability, durability, biocompatibility, or other parameters of newly developed cardiac valves. Specifically, the continued testing of novel cardiac valve prostheses (e.g., TAVR) via in vitro and in vivo studies will provide clinicians, scientists, and engineers working in this field, with the appropriate tools to drive the required research and developments of the next generation of valve replacement therapies.
Acknowledgements
This research is supported by a research contract with Medtronic, Inc., Minneapolis, MN 55112 USA and the Institute for Engineering in Medicine at the University of Minnesota, Minneapolis, MN 55455 USA.
Disclosure: The authors declare no conflict of interest.
References
- Chinchoy E, Soule CL, Houlton AJ, et al. Isolated fourchamber working swine heart model. Ann Thorac Surg 2000;70:1607-14.
- Anderson RH, Becker AE. The heart: structure in health and disease. London: Gower Medical Publishing 1992.
- Weinhaus AJ, Roberts KP. Anatomy of the human heart. In: Iaizzo PA (ed). The handbook of cardiac anatomy, physiology, and devices, 2nd edn. Humana Press, USA, 2009:59-85.
- Maselli D, Guarracino F, Chiaramonti F, et al. Percutaneous mitral annuloplasty: an anatomic study of human coronary sinus and its relation with mitral valve annulus and coronary arteries. Circulation 2006;114:377-80.
- Bateman MG, Iaizzo PA. Comparative imaging of cardiac structures and function for the optimization of transcatheter approaches for valvular and structural heart disease. Int J Cardiovasc Imaging 2011;27:1223-34.
- Available online: http://www.vhlab.umn.edu/atlas/index. shtml
- Howard S, Bateman MG, Hill A, et al. In vitro images of a double orifice mitral valve in a reanimated human heart. Ann Thorac Surg 2012. [Epub ahead of print].
- Eggen MD, Bateman MG, Iaizzo PA. Methods to prepare perfusion fixed cardiac specimens for multimodal imaging: the use of formalin and agar gels. J Med. Devices 5,2011: 027539.
- Tsang W, Bateman MG, Weiner L, et al. Accuracy of aortic annular measurements obtained from three-dimensional echocardiography, CT and MRI: human in vitro and in vivo studies. Heart 2012;98:1146-52.
- Hill AJ, Laske TG, Coles JA Jr et al. In vitro studies of human hearts. Ann Thorac Surg 2005;79:168-77.
- Langendorff O. Untersuchungen am uberlebenden Saugenthierherzen [Investigations on the surviving mammalian heart]. Pflugers Arch 1895;61:291-332.
- Laske TG, Iaizzo PA, Hjelle MA, et al. Isolated perfused heart preparation and method for use. US 7,045,279.
- Quill JL, Laske TG, Hill AJ, et al. Images in cardiovascular medicine. Direct visualization of a transcatheter pulmonary valve implantation within the visible heart: a glimpse into the future. Circulation 2007;116:e548.
- Iaizzo PA, Hill AJ, Laske TG. Cardiac device testing enhanced by simultaneous imaging modalities: the Visible Heart, fluoroscopy, and echocardiography. Expert Rev Med Devices 2008;5:51-8.
- Quill JL, Hill AJ, Menk AR, et al. Multimodal imaging of a transcatheter aortic valve implantation within an isolated heart. JACC Cardiovasc Imaging 2011;4:1138-9.
- Bateman MG, Torrianni M, Menk A, et al. Development of an aortic stenosis swine model using the Visible Heart® 2011. [Epub ahead of print].
- Eggen MD, Bateman MG, Rolfes CD, et al. MRI assessment of pacing induced ventricular dyssynchrony in an isolated human heart. J Magn Reson Imaging 2010;31:466-9.
- Richardson E, Hill AJ, Skadsnerg ND, et al. The pericardium. In: Iaizzo PA (ed). The handbook of cardiac anatomy, physiology, and devices, 2nd edn. Humana Press, NJ, 2009:125-36.
- Yoganathan AP, Chandran KB, Sotiropoulos F. Flow in prosthetic heart valves: state-of-art and future directions. Ann Biomed Eng 2005;33:1689-94.
- Gallegos RP, Nockel PJ, Rivard AL, et al. The current state of in vivo pre-clinical animal models for heart valve evaluation. J Heart Valve Dis 2005;14:423-32.
- Stachelek SJ, Alferiev I, Connolly JM, et al. Cholesterol- modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo. Ann Thorac Surg 2006;81:47-55.
- Paniagua D, Induni E, Ortiz C, et al. Percutaneous heart valve in the chronic in vitro testing model. Circulation 2002;106:e51-2.
- Martin AJ, Christy JR. An in vitro technique for assessment of thrombogenicity in mechanical prosthetic cardiac valves: evaluation with a range of valve types. J Heart Valve Dis 2004;13:509-20.
- Bakhtiary F, Dzemail O, Steinseiffer U, et al. Opening and closing kinematics of fresh and calcified aortic valve prostheses: an in vitro study. J Thorac Cardiovasc Surg 2007;134:657-62.
- Bande M, Michev I, Sharp AS, et al. Percutaneous transcatheter aortic valve implantation: past accomplishments, present achievements and applications, future perspectives. Cardiol Rev 2010;18:111-24.