
Treatment of advanced heart failure in adults with congenital heart disease: a narrative review and clinical cases
Introduction
Despite all therapeutic advances in the treatment of adults with congenital heart disease (ACHD), they cannot be completely cured and most often sequelae remain (1,2). In particular in complex or hemodynamically relevant ACHD, sequelae often persist or develop over time even after primarily successful cardiac surgery or interventions. In particular, heart failure (HF), cardiac arrhythmias, pulmonary vascular disease, aortopathies, and infectious endocarditis adversely affect the morbidity and mortality of ACHD in the long-term (1-4). In addition, there are often acquired cardiac and noncardiac comorbidities developing in adulthood such as coronary artery disease, acquired valvular heart disease, arterial hypertension, metabolic disorders (diabetes mellitus, hyperlipidemia, metabolic syndrome), or other organ diseases, especially affecting the liver, kidneys, and the brain. Mental illness, post-traumatic stress disorder, depression, and anxiety disorders can further complicate the clinical course (5-7).
HF is a common disease being present and is often the cause of morbidity and mortality (1,2). The review focuses on the treatment of advanced HF with the options available and, especially, on ventricular assist device (VAD) therapy and heart transplantation in the context of increasing numbers of ACHD. In addition, important aspects of HF treatment for non-ACHD patients but also ACHD are discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-230/rc).
Methods—literature search
A literature search in PubMed was executed covering publications up to March 2022. The following combinations of keywords were used: HF and ACHD, heart transplantation in ACHD, guidelines HF and ACHD, VAD in ACHD, comorbidities in ACHD, device therapy in ACHD. These search terms had to be identified anywhere in the text in the articles. Further, the authors selected literature that represents the current guidelines or multicenter trials, however most of the literature derive from observational studies and guidelines for patients with ACHD have the evidence level of expert opinion.
Both qualitative and quantitative studies were considered to elucidate the use of the discussed aspects regarding ACHD, advanced HF, VAD therapy or device therapy in general in ACHD. The search was restricted to original research, humans, and papers published in English at any date. All abstracts were reviewed to assess whether the article met the inclusion criteria. After this selection process, a manual search of the reference lists of all eligible articles was performed. Two authors (i.e., MH and CS with support of HK and KN) assessed independently the importance of the studies prior to their inclusion in the review. A relevant manuscript was defined based on the impact factor of the journal, being a guideline manuscript, a society position paper, or a comprehensive review of the current literature.
The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | The search for literature was conducted during March 2022 and included as well manuscript until 1st of April 2022 |
| Databases and other sources searched | PubMed database |
| Search terms used | Heart failure and ACHD, heart transplantation in ACHD, guidelines heart failure and ACHD, ventricular assist device in ACHD, comorbidities in ACHD, device therapy in ACHD patients |
| Timeframe | All manuscripts until April 2022 |
| Inclusion and exclusion criteria | All study types were included with focus on journals with an impact factor and all manuscripts had to be published in English language |
| Selection process | The literature search was done by Christoph Sinning, Michael Huntgeburth, Harald Kaemmerer and Koichiro Niwa. All authors had to consent to the literature included into the review |
ACHD, adults with congenital heart disease.
Epidemiology and clinical aspects of HF in ACHD
HF has a critical impact on ACHD morbidity and an approximate mortality rate of 25% up to 45% (1,3-6). The reported hospitalization rate for symptomatic HF is 1.2 per 1,000 patient-years (7). Thus, ACHD with specific conditions are at particular risk of developing HF. Among those patients are frequently ACHD with univentricular hearts (in Fontan-Circulation), with a morphologic systemic right ventricle (after atrial redirection for transposition of the great arteries (d-TGA), with congenitally corrected transposition of the great arteries (ccTGA), with severe pulmonary hypertension due to unoperated cardiac shunt lesions (Eisenmenger syndrome), or with severe valvular heart disease, e.g., after Fallot repair, AV (atrioventricular) septal defects post repair, in Ebstein’s anomaly, or in Shone complex (8,9).
Data on the incidence and prevalence of HF in ACHD are unreliable because different definitions exist, such as impaired exercise tolerance, neurohumoral activation, reduced ejection fraction, or the presence of classic clinical signs of right or left ventricular decompensation.
The pathophysiological causes of HF in ACHD are multifactorial. They involve chronic pressure or volume loading, ventricular fibrosis, intracardiac scarring and patch material, valvular abnormalities, arrhythmias, the presence of cyanosis, or pulmonary hypertension in pulmonary vascular disease (1,2,10).
Recognition of HF in ACHD can be challenging because its presentation is often different from acquired heart disease (11). Exercise capacity is often impaired in ACHD and is often unrecognized by affected individuals due to the fact that the process of deterioration often begins in early adolescence and often shows only slow progression (12). Furthermore, the use of established parameters (e.g., end-diastolic and/or end-systolic diameter or volume, ejection fraction) is inappropriate because they depend on the type of cardiac defect and often differ from normal values in adult cardiology.
Current management of HF in ACHD
Current HF treatment is focusing on medical treatment followed by evaluation for additional treatment options, for example, such as cardiac resynchronization therapy (CRT), data regarding treatment medications, especially HF medication is currently lacking (13,14). Sufficient data from randomized controlled trials in ACHD are currently lacking for many therapeutic interventions because of the heterogeneous patient population with underlying congenital heart defects, low number of patients in these study, and that being an ACHD is frequently an exclusion criterion in HF trials (14,15).
The treatment of HF in ACHD requires special expertise (16,17). Before symptom-oriented HF treatment is initiated, surgically or interventional treatable abnormalities of hemodynamics must be excluded. Pharmacological therapy to improve clinical symptoms, limited exercise tolerance, quality of life, and long-term prognosis is done with consideration of heart defect-specific features (15).
If systolic dysfunction of the morphologic left ventricle is present, usually medication with renin angiotensin aldosterone blockers should be established (ACE inhibitors, sartans), together with the class of mineralocorticoid receptor antagonists. Diuretics are most often used in acute decompensation or chronic volume overload (14). The cut-off regarding the decision to begin medical treatment is recommended with 40% ejection fraction (14). Regarding newer medication with sacubitril/valsartan and SGLT-2 inhibitors there are currently no data, however medication should be considered in ACHD with reduced ejection fraction of the left ventricle (13).
If the anatomic right ventricle has an impaired ejection fraction below 40% medical treatment is currently recommended if the patient is symptomatic and follows the same medication recommendations as for the left ventricle. Scarce information is present regarding medication of an univentricular heart and in these patients medication should be according to the anatomic morphology of the ventricle present (14).
Another unique feature relates to the Fontan circulation, in which diastolic and systolic HF, chronotropic incompetence, and an increase in pulmonary vascular resistance may occur during the progression of the disease. While there are no adequately validated data for the use of ACE inhibitors, the use of phosphodiesterase-5 inhibitors or endothelin receptor antagonists (ERA) is now under consideration (18,19).
Advanced HF and therapeutic interventions
Patients at risk for HF can progress to a symptomatic state despite all medical treatment options, interventions and device therapy (2,13). Also the number of ACHD with advanced HF will increase.
The current criteria for advanced HF according to the current European HF guidelines are defined for acquired cardiac disease but also apply for ACHD (Table 2).
Table 2
| No. | Definition |
|---|---|
| 1 | Severe and persistent symptoms of heart failure [NYHA class III (advanced) or IV] |
| 2 | Severe cardiac dysfunction defined by at least one of the following: |
| Left ventricular ejection fraction <30% | |
| Isolated right ventricular failure (for example tetralogy of Fallot, ccTGA, Ebstein’s anomaly, d-TGA with palliation according to Senning or Mustard) | |
| Non-operable severe valvular defects | |
| Non-operable severe congenital abnormalities | |
| High or increasing BNP or NT-proBNP levels and severe diastolic dysfunction or structural abnormalities | |
| 3 | Episodes of pulmonary or systemic congestion requiring high-dose i.v. diuretics or episodes of low output requiring inotropes or arrhythmias causing >1 unplanned visit or hospitalization in the last 12 months |
| 4 | Severe impairment of exercise capacity with inability to exercise or low 6-minute walking distance (<300 m) or pVO2 <12 mL/kg/min or <50% predicted value, due to cardiac disease. |
For diagnosis, the following criteria must be presented despite optimal medical treatment. NYHA, New York Heart Association-Classification; ccTGA, congenitally corrected transposition of the great arteries; BNP, B-type natriuretic peptide; NT-proBNP, N terminal-proBNP.
In addition to the criteria of Table 1, advanced HF in ACHD patients is often complicated by extra-cardiac organ dysfunction like congestive liver disease, renal failure, or pulmonary hypertension. These criteria are currently not obligatory to define advanced HF in patients, but can complicate treatment decisions (2,13).
Since most patients with advanced HF deteriorate in functional status over time, prognostic stratification is important to identify the need of treatment in an expert center, to properly communicate expectations to patients and their families, and to plan treatment and follow-up strategies (2,13). In this context, for most patients with advanced HF, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification is of potential to determine the treatment options (20). Although most commonly in use for decision-making regarding durable VADs, the classification is as well of use for establishing prognosis in patients following urgent heart transplantation and for risk assessment in ambulatory advanced HF patients (2,13) (Table 3). Previous and current reports of INTERMACS show that by collecting data and including centers into the register outcomes of patients with advanced HF are improved and adverse events decreased, although only one publication thus far addresses VAD treatment in ACHD patients (21-23).
Table 3
| Profile | Time for decision |
|---|---|
| 1 Critical cardiogenic shock. Patient with hypotension despite inotrope therapy | Intervention needed in hours—same day |
| 2 Progressive decline. Declining organ function with inotrope support | Intervention needed in days—same week |
| 3 Stable with inotropes. Stable organ function with inotrope support | Elective decision—weeks to months |
| 4 Frequent flyer. Patient can be stabilized with normal volume status but has symptoms at rest or with everyday activities | Elective decision—weeks to months |
| 5 Housebound. Stable at rest | Variable—depends regarding nutrition, activity and organ function |
| 6 Exertion limited. Patient without evidence of fluid overload, comfortable at rest and with activities of daily living | Variable—depends regarding nutrition, activity and organ function |
| 7 Advanced NYHA class III symptoms. Patient without current or recent decompensation | No indication for advanced HF therapy |
NYHA, New York Heart Association-Classification; HF, heart failure.
Alternative therapies for refractory HF include CRT, use of VADs, heart or heart-lung or multiorgan transplantation (4,24).
Arrhythmia management and device therapy in ACHD with HF
In ACHD the whole spectrum of arrhythmias can be encountered and often several types coexist, including disorders of the sinus node, AV-node, His-Purkinje system or intra-atrial propagation (25,26). Arrhythmias might be a consequence of altered anatomy, including the heart conduction tissue, but may also be a result of residual or postoperative sequelae, such as myocardial fibrosis, postoperative intracardiac scars or worsening of the hemodynamic situation (25,27-31). The prevalence of arrhythmias is increasing with the age of the patients. In several types of ACHD, ventricular arrhythmias are the leading cause of sudden cardiac arrest and thus mortality, with an 100-fold increased risk in comparison to an age-matched control population (32,33). However, the incidence for fatal events is low with 0.1% and the highest risk is present in patients with Tetralogy of Fallot, univentricular hearts or patients with transposition of the great arteries after Senning or Mustard procedure (25,29,31,34-36).
The most important ventricular tachyarrhythmias include mono- and polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF). However, supraventricular tachycardia may be also poorly tolerated in ACHD, especially in complex cases (e.g., after atrial redirection, in Fontan circulation, in cyanotic ACHD, in Eisenmenger syndrome) and may result in cardiac decompensation or even death (37).
Cardiac arrhythmias are often managed according to general cardiology guidelines, while taking into consideration ACHD-specific issues like anatomy and placement of catheters or devices (25), including pharmacotherapy, ablation procedures, and the implantation of pacemakers or defibrillators (8).
In the acute setting thus VT or VF are managed according to the respective guidelines for acquired cardiac disease (38). As ACHD have a chronic disease state, and decisions need to be made as to which patient should receive an implantable cardioverter-defibrillator (ICD), as this is the recommended treatment for secondary prevention after sudden cardiac arrest in ACHD (25,26,31,39). However, due to the lack of data, the indications are not yet clearly defined (25,39). In ACHD the medical treatment of choice is amiodarone or procainamide if VT or VF is present (25,26,31). However, treatment in unstable, pulseless VT or VF should be according to the respective resuscitation guidelines (40).
Device therapy with pacemakers or implantable defibrillators is becoming more and more relevant, but the indication for ICD implantation may be difficult to establish. Current recommendation is clear with either level I or IIa for ACHD after cardiac arrest with no other potential cause, spontaneous sustained VT after hemodynamic, electrophysiologic evaluation or ejection fraction <35% of the systemic left ventricle or tetralogy of Fallot with risk factors (25,26,31).
An alternative to defibrillator implantation is the subcutaneous ICD (S-ICD) with a class IIa recommendation in patients with an ICD indication not requiring pacing for bradycardia, CRT or anti-tachycardiac pacing (26,41). Further, class I recommendation for patients with a complex anatomy and venous access problems or at a high risk for infections needing ICD therapy (25,39,41).
Data regarding for the wearable defibrillator, or life-vest, are limited, therefore it can only be recommended in selected cases at this time (42).
Electromechanical dyssynchrony may result in pathologic ventricular remodelling or impaired ventricular-ventricular interaction with resultant HF. In these cases, ventricular activation delay due to bundle branch block or ventricular pacing is characterized by clustering of early and late contracting segments. This may make patients eligible for CRT, where a large, late-contracting area composed of multiple myocardial segments is electrically preexcited via a single pacing lead (25,43).
The effect of CRT in ACHD, however, varies because of the underlying anatomy of the systemic ventricle, scarring of the ventricle, and the presence of hemodynamic relevant valve lesions. However, in some ACHD with systemic right ventricle or single ventricle anatomy and broad QRS complex of 160 msec on ECG, there was an increasing ejection fraction of the systemic ventricle (44,45). Although these older data are promising, the main problem regarding CRT in ACHD is that all of these studies were retrospective, non-randomized, and follow-up was largely limited to a short period. Thus, conclusive analysis of the impact of CRT on long-term morbidity and mortality is still pending (25,44,45). In this regard, current pacing guidelines recommend that the standard indications for CRT may also be used in ACHD, but that the atypical anatomy should be considered in this context (43).
In conclusion, resynchronization therapy is an option in individual cases, although its role is currently unclear and long-term results are lacking (4,46,47).
Ventricular assist devices as treatment option in ACHD
Due to organ shortage and the fact that the need for organ transplantation is increasing in both non-ACHD and ACHD (1,48), the use of VADs is becoming a more established treatment option both as a bridge to transplant and as a destination therapy (48).
These devices have been shown to reduce mortality in end-stage HF in non-transplant candidates and appear to provide a similar benefit to transplant candidates whose condition worsens while waiting for transplantation (23,48). For VAD implantation, ACHD should be considered according to whether they have a morphologic left or right systemic ventricle or a functionally univentricular heart. In the largest study to date, more ACHD were treated with a biventricular ventricular assist or a total artificial heart than non-ACHD patients (23). In this study ACHD had a higher mortality rate and more adverse events than non-ACHD patients, but the increased mortality rate was attributable solely to the higher use of biventricular VADs and total artificial hearts (23). Conversely, the two groups who had VAD implantation to support the systemic ventricle alone, demonstrated similar survival regardless of the underlying cardiac anatomy (23). In particular, patients with d-TGA after atrial-switch operation and a systemic right ventricle may represent a cohort for whom VAD treatment may be considered as bridge-to-transplantation, because patients are often young and have undergone multiple previous surgeries with complex anatomy (2). However, the pyramidal geometry of a morphologic right ventricle often requires resection of trabeculae and/or alternate positioning of the inflow cannula, often on the diaphragmatic surface or free wall, which can be done as destination therapy, as has been shown previously (49).
A major problem with studies of ACHD and VADs, is that patients are described only as having ACHD without accurately classifying the underlying anatomy (50). This is crucial, however, for determining therapeutic decisions. Due to the described heterogeneity of ACHD, more data with a clear description of the underlying defect are needed.
Heart transplantation
Transplantation listing and outcome
Heart or heart-lung transplantation is limited by donor shortage. Here, specific anatomic and pathophysiologic features (e.g., vascular anatomy, vascular anomalies, collateral vessels, type and number of previous operations, intrathoracic scars, immunologic status after previous transfusions) must be given special attention (1,2).
Transplantation criteria may be quite different from those of other cardiac patients and need better definition (2). Further, heart transplantation in ACHD is hard to obtain because of the high likelihood that patients will be rejected due to various comorbidities or multiple organ involvement. Studies could show that up to two-thirds of patients may be rejected for transplantation listing, which is associated with a high mortality rate (51).
As an option to reduce the waiting list time in ACHD patients the United Network of Organ Sharing (UNOS) did revise its organ allocation guidelines in October 2018 with the aim to reduce waiting list times and thus mortality in this patient population (52,53). As a consequence of this revision, candidates supported with a temporary mechanical circulatory assist (MCS) device will receive a higher priority status than candidates with permanent or no MCS devices (52,53). Recent data could show that in the UNOS network the revision led to an increased use of temporary MCS devices. with the result of a relevant decline in waiting list times (54). Although waiting list time was reduced, some aspects could not be addressed in this study, such as information regarding the particular HF medication, the underlying INTERMACS level of the patients. and data on the underlying congenital heart defect. Thus, although the change of the allocation guidelines has resulted in a reduction in waiting times on the transplant list, additional data have to be collected in ACHD to demonstrate benefit in this patient cohort.
After transplantation, early mortality appears increased in ACHD, whereas at 10-year follow-up, prognosis is better in ACHD than in other populations (4). Combined organ transplantation may also be required in the presence of concomitant renal or hepatic disease.
Heart transplantation is often considered and is the method of choice once complex cardiac reconstructive surgery is deemed too risky or ineffective, and then often remains the only viable treatment option (14,55,56). Although ACHD account for a large proportion of patients eligible for heart transplantation, the number of ACHD recipients worldwide has increased from 1,8% in 1992–2000 to 2.7% in 2000–2005 with 3.1% in 2006–2013 (57). In addition, ACHD represent a large proportion of patients receiving heart and lung transplantation, accounting for 40% in 2002–2013 (58). The patients with ACHD listed tend to be younger than non-ACHD considered for heart transplantation. In addition, ACHD have less cardiovascular comorbidities and have unique features characteristic for advanced HF in complex CHD such as protein-losing enteropathy (56,59). Current criteria for ACHD scheduled for heart or heart and combined organ transplantation are listed in Table 4.
Table 4
| Characteristics of ACHD patients listed for heart or heart and combined organ transplantation (1,56,59-61) |
| Younger age of ACHD in comparison to non-ACHD listed for transplantation |
| Lower prevalence of cardiovascular risk factors or life style risk factors causative for coronary artery disease |
| Higher prevalence of increased pulmonary vascular resistance in ACHD in comparison to non-ACHD |
| Impaired nutritional status with patients being underweight especially in patients with protein losing enteropathy |
| Increased risk of postoperative infections or impaired wound healing |
| The type of the underlying heart defect with: |
| • Single ventricle being a common diagnosis in ACHD |
| • Biventricular defects and their sequelae |
| • Different underlying disease as reason for transplantation listing due to the shift from lesions with lower complexity to more complex lesions due to progress of medical therapy |
| Prevalence of previous cardiac surgery is high in ACHD either due to corrective or palliative treatment |
| Increased difficulty of surgical therapy due to extensive scarring in ACHD or formation of collaterals with a high bleeding risk |
ACHD, adults with congenital heart disease.
Previous studies could as well show that more transplantations could be achieved with the use of special organ care systems which allow to deliver organs from donor to recipient in an adequate status and perform transplantation with a low rate of complications. A potential system currently used is the organ care system, which is achieving good results even in patients with advanced HF (62).
Reasons for disparities in transplantation waiting list time and status in adult congenital heart disease
In the context of listing ACHD for transplantation, it is important to emphasize that these patients often do not receive mechanical circulatory support or inotropic treatment due to limited experience and evidence supporting this therapy. However, both of these criteria are often related to a prioritization for heart transplantation (59,63). Thus, the transplantation listing criteria are not as useful in ACHD listed for heart transplantation. Another problem with listing these patients for transplantation is that a high number of patients (over 10%) of patients have antibodies due to previous transfusion of packed red blood cells during previous cardiac surgery (64). Another problem with transplantation in this patients population is that recipient-specific factors contribute to the lower rate of ACHD transplantation. These patient often require reconstructive surgery during transplantation, which is associated with an increased degree of difficulty and should only be done in centers with a high level of experience in treating ACHD (1,65). Furthermore, certain characteristics of the donor organ, such as a longer donor pulmonary artery, often complicate the operation, because a narrowing of the recipient pulmonary arteries might complicate surgery and has to be corrected at the same time (55,56,59,63).
Waiting list morbidity and mortality
ACHD frequently have numerous adverse features including pulmonary arterial hypertension (PAH) and other advanced organ involvement, such as liver or renal disease in patients with Fontan circulation, resulting in a high waiting list mortality (2,61). Patients removed from the waiting list have an especially poor prognosis as they cannot be bridged to transplantation with a VAD or have severe HF with multi-organ failure most commonly involving the liver and/or kidneys (61,66). The low prevalence of ICDs, VADs, and VADs as bridge to transplantation in ACHD may increase waiting list mortality and may be contraindicated or associated with higher risk (56,59,63).
VAD treatment as bridge to transplant or destination therapy
In this context, it should be noted that recently a large study indicated that being an ACHD and being treated with a VAD prolongs the time on the waiting list for an organ transplantation, with a higher probability of successful transplantation (50). Further, a study may provide more evidence that survival rates are similar in ACHD and non-ACHD patients with a VAD (23). However, in this study ACHD more often required biventricular support or a total artificial heart, resulting in a higher mortality rate. Despite the still high mortality on the waiting list, ACHD are not often considered for VAD support because of the complexity of the anatomy, the paucity of VAD programs focused on ACHD, and the need for surgeons to be experienced to establish VAD therapy in ACHD (23,65). The previous published data, however, rise some points of discussion, as the number of patients with single ventricle anatomy, representing those with the most complex underlying congenital heart disease, was only 13% and the reader is not provided with information about the congenital heart disease in the other patients. Adding to this discussion is a study from a paediatric registry with end-stage HF and good outcomes in patients with VAD in end-stage Fontan hemodynamics (67).
In summary, it may be an option to also evaluate ACHD with advanced HF for a VAD if the anatomy is favourable and the patient’s condition worsens.
Clinical cases to illustrate the complexity of advanced HF treatment in ACHD
Clinic case 1: Patient with congenital corrected transposition of the great arteries and successful heart transplantation
A 27-year-old female patient presented to the emergency department with shortness of breath, fatigue and worsening renal function. The diagnosed underlying congenital heart defect was a complex ccTGA with three previous cardiac surgeries. The first procedure was a palliative Blalock-Taussig shunt in 1993, after this surgery a closure of a ventricle septum defect with additional replacement of the pulmonic valve was done using a homograft and an additional patch plasty of the left pulmonary artery in 1995. Following the second operation, the patient needed a pacemaker due to atrioventricular block III. In 2007, severe regurgitation of the systemic ventricle’s morphological tricuspid valve was diagnosed. In 2009, the patient received reconstruction of the tricuspid valve and CRT with an epicardial lead. In the following years, the patient was stable in New York Heart Association-Classification (NYHA) II functional class. In 2019, the patient presented with the question whether a pregnancy could be possible. Subsequent examination revealed relevant stenosis of the homograft in pulmonic valve position and moderately reduced ejection fraction of the systemic right ventricle. With impaired ventricular function and moderate tricuspid regurgitation, the patient was counselled against pregnancy and HF drug treatment was initiated with an ACE inhibitor, a mineralocorticoid receptor antagonist, and loop diuretics. Interventional treatment of the pulmonary homograft was not feasible due to an unfavorable anatomy. She remained stable in NYHA II functional class with cardiac medication until in 2020, when the patient presented several times with cardiac decompensation. After treatment of acute heart failure with diuretics, invasive examination of the patient showed relevant stenosis of the homograft in pulmonary position, severe regurgitation and stenosis of the reconstructed tricuspid valve, and a severe impaired function of the systemic right ventricle with a cardiac index of 1.64 L/min/m². Because of the acute cardiac decompensation and with normal pulmonary vascular resistance, the patient was listed for cardiac transplantation. After a waiting period of one month with high urgent listing status at the HF unit, the patient was successfully transplanted in October 2020.
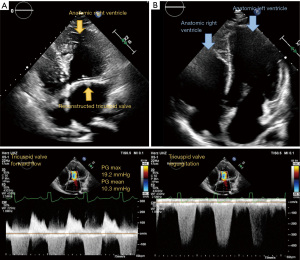
Since her heart transplantation the patient is in regular follow-up and her condition has improved markedly regarding physical activity and she is able to participate in everyday life activities. Figure 1 shows the pre- and posttransplant 4-chamber view. Videos 1,2 show the pretransplant 4-chamber view with and without colour flow. The Video 3 shows the posttransplant donor heart.
Clinical case 2: Coarctation of the aorta, ventricular septal defect with patent ductus arteriosus and development of Eisenmenger syndrome—unsuitable for heart and lung transplantation due to comorbidities and multiorgan failure
This case report summarizes history of a currently 50-year-old woman with complex congenital heart disease. She was born in 1971 with aortic coarctation, bicuspid aortic valve, a large muscular ventricular septal defect (VSD), a patent ductus arteriosus (PDA), and developed PAH during the course of the disease. At the age of 2 years, coarctation of the aorta (CoA) was surgically repaired, and PDA closed.
Over the years, she developed Eisenmenger’s syndrome. Therefore, in 2005, targeted PAH therapy with the ERA bosentan was initiated. By age of 43 (2014), she was diagnosed with severe aortic valve regurgitation and ascending aortic aneurysm (diameter in echocardiography with 53 mm) in association with the bicuspid aortic valve and CoA.
In the same year, her condition deteriorated with right HF. Bosentan was switched to the ERA macitentan, which was not very well tolerated, and therefore changed to the PDE-5-inhibitor (PDE-5-I) tadalafil.
In 2015, targeted PAH monotherapy was extended to a combination therapy with tadalafil and bosentan. Because of side effects, tadalafil was replaced by the PDE-5-inhibitor sildenafil, which was better tolerated. In 2017 a PAH-targeted triple therapy with the oral prostacyclin receptor agonist selexipag was initiated, but shortly after discontinued because of adverse side effects (diarrhea, headache). Instead, PAH treatment was escalated by a dose increase of sildenafil and additional iloprost inhalation. After developing abdominal pain and bloody diarrhea, autoimmune enteropathy and colopathy was suspected. Since 2019, the patient was stable with targeted PAH treatment with bosentan and riociguat until 2021, when she developed right sided cardiac decompensation from Eisenmenger Syndrome, as well as additional acute renal failure and methotrexate-related pneumopathy. Other complicating comorbidities include hemoptysis, hypothyroidism, rheumatoid arthritis, and an IgG type Lambda monoclonal gammopathy. Another severe complication is a massive progress of the ascending aortic aneurysm, which was measured with a diameter of 62 mm at the last measurement.
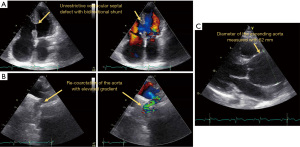
Currently, the patient is only marginal stable under a cardio-pulmonary medical treatment with bosentan, riociguat, digoxin, bisoprolol, spironolactone, and torasemide. Under the diagnosis of recurrent right HF due to Eisenmenger’s syndrome in combination with a large ascending aortic aneurysm at risk of rupture and mild aortic re-coarctation, the patient was presented for heart-lung transplantation and concomitant repair of the ascending aorta. However, due to the complexity of the surgery and the patient’s comorbidities, she was not accepted for transplantation. Figure 2 shows the pathologic features of the patient. Videos 4,5 show the 4-chamber view without and with colour flow. Video 6 shows the aneurysm of the aorta and Video 7 the CoA following the initial repair.
Clinical case 3: Ventricular assist device in a patient with congenital corrected transposition of the great arteries, VSD, atrial septal defect, pulmonary valve stenosis, and aortic valve regurgitation
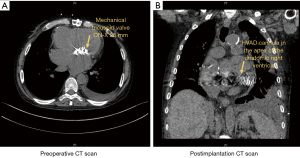
A 59-year-old male patient was evaluated for possible VAD implantation with a diagnosis of congenital corrected transposition of the great arteries, VSD, atrial septal defect, pulmonary valve stenosis, and aortic valve regurgitation. In the past medical history the patient had a left ventricle to pulmonary artery conduit, closure of the ventricular and atrial septal defect and with postoperative complications due to bleeding 22 years ago treated at center elsewhere. Thirteen years ago, he had a second heart surgery (replacement of the left ventricle to pulmonary artery conduit, tricuspid valve replacement, residual VSD closure, aortic valve repair and left pulmonary artery angioplasty) at a national reference center. In 2018 the patient had a third complex cardiac surgery with replacement of the left ventricle to pulmonary artery conduit. As of recent the systemic ventricle of the patient, being an anatomic right ventricle progressively failed and the patient was admitted three times during the last 6 months. The patient developed multiorgan failure with cardiorenal syndrome and cardiac-related liver cirrhosis. During the last admission due to decompensation, the patient presented with cardiogenic shock and was admitted to the intensive care unit multiple times during the admission. Thus, the patient was discussed to be listed for heart transplantation. However, due to rapid deterioration of the patient status in conjunction with the cardiac pathologies and a supposed long waiting time due to blood type “O” treatment with a VAD was decided. The patient was implanted with a Medtronic™ HVAD and concomitant aortic valve closure via double left thoracotomy and partial sternotomy. The patient afterwards had a long hospital stay before further steps regarding rehabilitation could be decided (Figure 3).
Summary
Treatment of advanced HF in ACHD is complex and current results show a poor outcome even in non-ACHD (13). Further challenging in such patients is the presence of an underlying complex congenital heart defect, several previous heart surgeries, a systemic right ventricle and/or significant comorbidities such as pulmonary hypertension, renal or liver failure. The lack of randomized HF studies addressing ACHD is also hindering optimal treatment decisions, as these patients are often excluded from HF research and outcome trials.
Our data review showed, that VAD or heart transplantation are treatment options which have to be considered in ACHD as they have shown to improve outcome (2,23,50,60). However, treatment of these patients has to be planned and scheduled in a specialist center being experienced with the changes of the anatomy and treatment of common comorbidities of ACHD (65). Decades of longitudinal observational data are currently motivating a paradigm shift toward a lifespan perspective and proactive approach to ACHD care, including concepts regarding long-term trajectories and a life course epidemiology framework (68).
An important still unmet gap in knowledge is to gather data of international cohorts how the current standard of treatment is now and to evaluate common approaches to treat advanced HF in ACHD on an international level.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-230/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-230/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-230/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part V” was commissioned by the editorial office without any funding or sponsorship. YVK, HK and KN served as the unpaid Guest Editors of the series. HK has received honoraria for lectures and/or consultancy from Actelion, Bristol Myers Squibb, Janssen. CS receives research funding from the German Foundation for Heart Research and the Dr. Rolf Schwiete Stiftung. Further, he received speaker fees from AstraZeneca and Johnson & Johnson outside the submitted work. EZ reports speaker fees received from AstraZeneca outside the submitted work. CM reports research funding from the German Center for Cardiovascular Research (DZHK) within the Promotion of women scientist program, the Deutsche Stiftung fuer Herzforschung and the Dr. Rolf Schwiete Stiftung, and speaker fees from AstraZeneca, Novartis, Heinen&Loewenstein, Boehringer Ingelheim/Lilly, Bayer, Pfizer, Sanofi, Aventis, Apontis, Abbott outside of this study. SB reports grants and personal fees from Abbott Diagnostics, Bayer, Thermo Fisher, grants from SIEMENS, Singulex, personal fees from Abott, Astra Zeneca, AMGEN, Medtronic, Pfizer, Roche, Novartis, Siemens Diagnostics, outside the submitted work. PK receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last three years. PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). HR is a consultant to Medtronic Inc. and received speaker- and travel honoraria from Abiomed Inc. and Edwards Inc. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Menachem JN, Schlendorf KH, Mazurek JA, et al. Advanced Heart Failure in Adults With Congenital Heart Disease. JACC Heart Fail 2020;8:87-99. [Crossref] [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e698-800. [PubMed]
- Engelings CC, Helm PC, Abdul-Khaliq H, et al. Cause of death in adults with congenital heart disease - An analysis of the German National Register for Congenital Heart Defects. Int J Cardiol 2016;211:31-6. [Crossref] [PubMed]
- Ministeri M, Alonso-Gonzalez R, Swan L, et al. Common long-term complications of adult congenital heart disease: avoid falling in a H.E.A.P. Expert Rev Cardiovasc Ther 2016;14:445-62. [Crossref] [PubMed]
- Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220-9. [Crossref] [PubMed]
- Zomer AC, Vaartjes I, van der Velde ET, et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol 2013;168:2487-93. [Crossref] [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease-reflections on a global problem-part II: infective endocarditis, pulmonary hypertension, pulmonary arterial hypertension and aortopathy. Cardiovasc Diagn Ther 2018;8:716-24. [Crossref] [PubMed]
- Faccini A, Micheletti A, Negura DG, et al. Heart failure in grown-up congenital heart disease. Minerva Cardioangiol 2018;66:329-36. [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e81-e192. [Crossref] [PubMed]
- Van De Bruaene A, Meier L, Droogne W, et al. Management of acute heart failure in adult patients with congenital heart disease. Heart Fail Rev 2018;23:1-14. [Crossref] [PubMed]
- Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828-35. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Budts W, Roos-Hesselink J, Rädle-Hurst T, et al. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2016;37:1419-27. [Crossref] [PubMed]
- Brida M, Diller GP, Gatzoulis MA. Systemic Right Ventricle in Adults With Congenital Heart Disease: Anatomic and Phenotypic Spectrum and Current Approach to Management. Circulation 2018;137:508-18. [Crossref] [PubMed]
- Perloff JK, Warnes CA. Challenges posed by adults with repaired congenital heart disease. Circulation 2001;103:2637-43. [Crossref] [PubMed]
- Derk G, Houser L, Miner P, et al. Efficacy of endothelin blockade in adults with Fontan physiology. Congenit Heart Dis 2015;10:E11-6. [Crossref] [PubMed]
- Hjortshøj CS, Jensen AS, Sørensen K, et al. Epidemiological changes in Eisenmenger syndrome in the Nordic region in 1977-2012. Heart 2017;103:1353-8. [Crossref] [PubMed]
- Kaemmerer H, Apitz C, Brockmeier K, et al. Pulmonary hypertension in grown-ups with congenital heart disease: Recommendations of the Cologne Consensus Conference 2016. Dtsch Med Wochenschr 2016;141:S70-9. [PubMed]
- Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 2009;28:535-41. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant 2008;27:1065-72. [Crossref] [PubMed]
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. [Crossref] [PubMed]
- VanderPluym CJ, Cedars A, Eghtesady P, et al. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant 2018;37:89-99. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm 2014;11:e102-65. [Crossref] [PubMed]
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793-867. [Crossref] [PubMed]
- Kaemmerer H, Bauer U, Pensl U, et al. Management of emergencies in adults with congenital cardiac disease. Am J Cardiol 2008;101:521-5. [Crossref] [PubMed]
- Kaemmerer H, Fratz S, Bauer U, et al. Emergency hospital admissions and three-year survival of adults with and without cardiovascular surgery for congenital cardiac disease. J Thorac Cardiovasc Surg 2003;126:1048-52. [Crossref] [PubMed]
- Khairy P. Ventricular arrhythmias and sudden cardiac death in adults with congenital heart disease. Heart 2016;102:1703-9. [Crossref] [PubMed]
- Mohan S, Moffett BS, Lam W, et al. Analysis of adults with congenital heart disease presenting to pediatric emergency departments with arrhythmias. Congenit Heart Dis 2017;12:507-11. [Crossref] [PubMed]
- Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719-53. [Crossref] [PubMed]
- Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111-6. [Crossref] [PubMed]
- Silka MJ, Hardy BG, Menashe VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998;32:245-51. [Crossref] [PubMed]
- Gleva MJ, Wang Y, Curtis JP, et al. Complications Associated With Implantable Cardioverter Defibrillators in Adults With Congenital Heart Disease or Left Ventricular Noncompaction Cardiomyopathy (From the NCDR® Implantable Cardioverter-Defibrillator Registry). Am J Cardiol 2017;120:1891-8. [Crossref] [PubMed]
- Santharam S, Hudsmith L, Thorne S, et al. Long-term follow-up of implantable cardioverter-defibrillators in adult congenital heart disease patients: indications and outcomes. Europace 2017;19:407-13. [PubMed]
- Tutarel O. Acquired heart conditions in adults with congenital heart disease: a growing problem. Heart 2014;100:1317-21. [Crossref] [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease-reflections on a global problem-part I: development of congenital cardiology, epidemiology, clinical aspects, heart failure, cardiac arrhythmia. Cardiovasc Diagn Ther 2018;8:705-15. [Crossref] [PubMed]
- Soar J, Nolan JP, Böttiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015;95:100-47. [Crossref] [PubMed]
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190-252. [Crossref] [PubMed]
- Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021;161:115-51. [Crossref] [PubMed]
- Kaya E, Wakili R, Rassaf T. Journey of the S-ICD to first-line therapy. Herzschrittmacherther Elektrophysiol 2018;29:228-32. [Crossref] [PubMed]
- Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N Engl J Med 2018;379:1205-15. [Crossref] [PubMed]
- Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427-520. [Crossref] [PubMed]
- Janousek J, Gebauer RA, Abdul-Khaliq H, et al. Cardiac resynchronisation therapy in paediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart 2009;95:1165-71. [Crossref] [PubMed]
- Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol 2005;46:2277-83. [Crossref] [PubMed]
- Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018;104:1568-74. [Crossref] [PubMed]
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Tadokoro N, Fukushima S, Hoashi T, et al. Durable ventricular assist device implantation for systemic right ventricle: a case series. Eur Heart J Case Rep 2020;4:1-9. [Crossref] [PubMed]
- Cedars A, Tecson KM, Zaidi AN, et al. Impact of Durable Ventricular Assist Device Support on Outcomes of Patients with Congenital Heart Disease Waiting for Heart Transplant. ASAIO J 2020;66:513-9. [Crossref] [PubMed]
- Lo Rito M, Poretti G, Varrica A, et al. The Challenging Pathway Toward Heart Transplant Listing for Adult Congenital Heart Disease Patients. Artif Organs 2018;42:911-7. [Crossref] [PubMed]
- Zhou AL, Etchill EW, Giuliano KA, et al. Bridge to transplantation from mechanical circulatory support: a narrative review. J Thorac Dis 2021;13:6911-23. [Crossref] [PubMed]
- Bernhardt AM. The new tiered allocation system for heart transplantation in the United States-a Faustian bargain. J Heart Lung Transplant 2019;38:870-1. [Crossref] [PubMed]
- Zhou AL, Menachem JN, Danford DA, et al. UNOS listing status-related changes in mechanical circulatory support utilization and outcomes in adult congenital heart disease patients. J Heart Lung Transplant 2022;41:889-95. [Crossref] [PubMed]
- Cohen S, Houyel L, Guillemain R, et al. Temporal trends and changing profile of adults with congenital heart disease undergoing heart transplantation. Eur Heart J 2016;37:783-9. [Crossref] [PubMed]
- Goldberg SW, Fisher SA, Wehman B, et al. Adults with congenital heart disease and heart transplantation: optimizing outcomes. J Heart Lung Transplant 2014;33:873-7. [Crossref] [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996-1008. [Crossref] [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:951-64. [Crossref] [PubMed]
- Davies RR, Russo MJ, Yang J, et al. Listing and transplanting adults with congenital heart disease. Circulation 2011;123:759-67. [Crossref] [PubMed]
- Becher PM, Schrage B, Weimann J, et al. Clinical characteristics and outcomes of patients with adult congenital heart disease listed for heart and heart‒lung transplantation in the Eurotransplant region. J Heart Lung Transplant 2020;39:1238-49. [Crossref] [PubMed]
- Alshawabkeh LI, Hu N, Carter KD, et al. Wait-List Outcomes for Adults With Congenital Heart Disease Listed for Heart Transplantation in the U.S. J Am Coll Cardiol 2016;68:908-17. [Crossref] [PubMed]
- Verzelloni Sef A, Sef D, Garcia Saez D, et al. Heart Transplantation in Adult Congenital Heart Disease with the Organ Care System Use: A 4-Year Single-Center Experience. ASAIO J 2021;67:862-8. [Crossref] [PubMed]
- Everitt MD, Donaldson AE, Stehlik J, et al. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the United Network for Organ Sharing (UNOS). J Heart Lung Transplant 2011;30:395-401. [Crossref] [PubMed]
- Nwakanma LU, Williams JA, Weiss ES, et al. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg 2007;84:1556-62; discussion 1562-3. [Crossref] [PubMed]
- Diller GP, Orwat S, Lammers AE, et al. Lack of specialist care is associated with increased morbidity and mortality in adult congenital heart disease: a population-based study. Eur Heart J 2021;42:4241-8. [Crossref] [PubMed]
- Crossland DS, Jansen K, Parry G, et al. Outcome following heart transplant assessment in adults with congenital heart disease. Heart 2019;105:1741-7. [Crossref] [PubMed]
- Auerbach SR, Simpson KEAction Learning Network Investigators. HVAD Usage and Outcomes in the Current Pediatric Ventricular Assist Device Field: An Advanced Cardiac Therapies Improving Outcomes Network (ACTION) Analysis. ASAIO J 2021;67:675-80. [Crossref] [PubMed]
- Diller GP, Arvanitaki A, Opotowsky AR, et al. Lifespan Perspective on Congenital Heart Disease Research: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;77:2219-35. [Crossref] [PubMed]


