
Risk factors for severe acute kidney injury post complication after total arch replacement combined with frozen elephant trunk, in acute type A aortic dissection
Introduction
Acute type A aortic dissection (ATAAD) is an emergency and life-threatening condition with high mortality and major postoperative complications (1). Aortic arch involvement dictates a stringent surgical approach and decision-making framework. Limited aortic arch repair entails hemiarch replacement while other authorities advocate extended repair paired with frozen elephant trunk (FET) use (2). Recent studies have demonstrated that total arch replacement combined with FET [total arch replacement (TAR) + FET] yields good results through improved organ protection techniques (3). In China, this gradually became a routine surgical procedure to treat complex aortic arch disease (2-5).
Acute kidney injury (AKI) patients after aortic surgery have a considerably worse short- and long-term prognosis, resulting in a higher risk of mortality and major adverse outcomes (6). AKI incidence after thoracic aortic surgery varies between 26.0–77.6%, depending on different definitions of AKI and confounding patient selection (7-14). Severe AKI (AKI stage 3) is associated with higher postoperative mortality, more renal replacement therapy (RRT) treatments, and longer intensive care unit (ICU) stays for patients than in non-severe AKI individuals (8,15-17).
Reported series on renal outcomes of patients undergoing TAR + FET are underscored in current literature (12,18,19). However, indifferent attention has been directed to severe postoperative AKI in ATAAD patients receiving TAR + FET procedure (20). Hence, this study aims to evaluate the incidence and risk factors of severe AKI after TAR + FET procedure in ATAAD patients. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-313/rc).
Methods
Study design
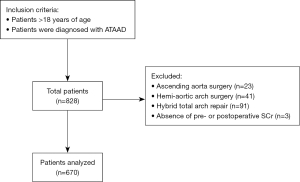
We conducted a retrospective cross-sectional analysis of patients with ATAAD admitted to our institute, a high volume tertiary referral hospital, between January 2013 and December 2018. During this period, 670 consecutive patients with ATAAD involving the aortic arch received the traditional TAR + FET procedure (Figure 1). Inclusion criteria included: (I) adult patients (age >18 years); (II) ATAAD patients who received TAR + FET procedure. All the patients underwent preoperative aortic computed tomography angiography (CTA) and coronary CTA/coronary angiography.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee Institute of Fuwai Hospital approved the present study (No. 2021-1557). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Data definition
We retrospectively reviewed clinical records and data on patient demographics, and preoperative risk factors, as well as intraoperative and postoperative data from the electronic medical records system of our institution. A recent classification for AKI, based on the RIFLE and AKIN criteria, was proposed by Kidney Disease Improving Global Outcome (KDIGO) (21-23). We defined AKI according to KDIGO consensus criteria to classify patients as severe AKI (AKI stage 3) or non-severe AKI (no AKI or AKI stage 1–2). Severe AKI was defined when the postoperative serum creatinine (SCr) increased more than three times baseline in the first week, or there was an increase in SCr to ≥4.0 mg/dL (353.6 µmol/L), or the initiation of RRT within 48 h postoperatively, or urine output <0.3 mL/kg/h for ≥24 h, or anuria ≥12 h. Preoperative SCr values closest to the date of surgery were chosen as baseline SCr levels. Prior cardiovascular surgery was defined as surgery on the heart or great vessels. Hypotension or shock was defined as a permanent deterioration in systolic blood pressure <90 mmHg (1). Lower limb symptoms included pain, decreased muscle strength, and sensory dysfunction. The assessment of branch vessel involvement by aortic dissection was based on preoperative imaging data by two radiologists and intraoperative exploration by surgeons. When there was an aortic root surgery, coronary ostium could be simultaneously reconstructed in the Bentall or David procedure using the button technique. We combined the variables for Bentall and David procedures into one variable for a similar procedure, including ascending aorta replacement and coronary ostium reconstruction (the button technique). According to the International Aortic Arch Surgery Study Group, major adverse outcomes were defined as events ranging from Grade III complications to death (24).
Surgical technique
It has been a complex operation to replace a total aortic arch. However, after successful operations, many patients require further treatment of the distal aorta. Repairing the descending aorta with an open surgical procedure is difficult, especially if the proximal anastomosis needs to be connected to an implanted aortic arch graft. This operation was greatly simplified when the FET was introduced. The FET technique used a stent graft to secure the distal elephant trunk section, contributing to true lumen expansion and coverage of entry tears into the false lumen. It helped diminish the risk of proximal endoleak while facilitating false lumen thrombosis to ensure aortic remodeling. Moreover, it allowed for the treatment of complex pathologies of the aortic arch and the proximal descending aorta in one step and facilitated the possibility of second-stage and more extensive repairs, if required (2).
The chest was opened via median sternotomy in all patients. Arterial cannulation was accessed through one side of the femoral artery and/or the right axillary artery. The right or left common carotid artery was cannulated to perform unilateral selective antegrade cerebral perfusion. Based on the patient’s condition, management was either moderate, or deep hypothermic circulatory arrest occurred. The TAR + FET procedure has previously been described in detail (25). The entire procedure included two steps: the total aortic arch, with or without the ascending aorta, is replaced with a tetrafurcate graft (Vascutek Terumo, Tokyo, Japan), followed by a stented graft (MicroPort Medical Co, Ltd., Shanghai, China) implantation. Selective antegrade cerebral perfusion was discontinued after distal arch reconstruction, cardiopulmonary bypass (CPB) flow was resumed, and systemic warming began until body temperature reached 35 ℃.
Statistical analysis
Categorical characteristics were compared using the χ2 test or Fisher’s exact test, where appropriate. With Student’s t-test, continuous variables were analyzed based on their mean and standard deviation. All statistical tests were 2-sided, and P values were considered statistically significant at 0.05 or below. Univariate analyses were conducted using univariate logistic regression analysis. No correction was made for multiple testing. Multiple logistic regression analysis analyzed the influence of different parameters on severe AKI. All variables including statistically significant and clinically essential variants were included in the multiple logistic regressions, and a forward stepwise selection method was then performed. The logistic regression results are presented as odds ratios (OR) with 95% confidence intervals (CI). All statistics were analyzed using SPSS version 22 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 670 ATAAD patients who received TAR + FET operation were enrolled in this retrospective study and divided into the non-severe AKI group and severe AKI group (Figure 1). The mean age of the study patients (n=670) was 46.9±10.2 years (range, 19–77 years) and 144 (21.5%) were women. Patients’ characteristics are presented in Table 1. After surgery, there were 80 patients (11.9%) with severe AKI. The mean ventilator time in the study cohort was 55.5±103.1 min, and the mean ICU time was 5.5±6.0 days. In total, major adverse outcomes were present in 169 patients (25.2%), and 67 patients (10.0%) required RRT. The whole in-hospital mortality rate was 4.3% (Table 2).
Table 1
| Variables | Total (n=670) | Non-severe AKI (n=590) | Severe AKI (n=80) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years)* | 46.9±10.2 | 46.5±10.0 | 49.4±11.1 | 0.019* |
| Female gender | 144 (21.5) | 121 (20.5) | 23 (28.8) | 0.092 |
| BMI (kg/m²) | 26.5±4.3 | 26.4±4.3 | 26.9±4.5 | 0.338 |
| Medical history | ||||
| Marfan syndrome | 60 (9.0) | 55 (9.3) | 5 (6.3) | 0.367 |
| Hypertension | 561 (83.7) | 495 (83.9) | 66 (82.5) | 0.750 |
| Diabetes | 20 (3.0) | 18 (3.1) | 2 (2.5) | 0.786 |
| Prior cardiovascular surgery | 24 (3.6) | 22 (3.7) | 2 (2.5) | 0.579 |
| Prior CAD | 2 (0.3) | 1 (0.2) | 1 (1.3) | 0.096 |
| Family history of dissections or aneurysms | 10 (1.5) | 9 (1.5) | 1 (1.3) | 0.849 |
| Current smoker | 304 (45.4) | 274 (46.4) | 30 (37.5) | 0.132 |
| ATAAD presentation | ||||
| Chest pain | 562 (83.9) | 494 (83.7) | 68 (85.0) | 0.772 |
| Back pain | 301 (44.9) | 261 (44.2) | 40 (50.0) | 0.331 |
| Abdominal pain | 119 (17.8) | 106 (18.0) | 13 (16.3) | 0.706 |
| Head or neck pain | 16 (2.4) | 13 (2.2) | 3 (3.8) | 0.395 |
| Preoperative malperfusion of organ | ||||
| Brain ischemia | 67 (10.0) | 55 (9.3) | 12 (15.0) | 0.112 |
| Myocardial ischemia | 3 (0.4) | 2 (0.3) | 1 (1.3) | 0.252 |
| Cardiac failure | 15 (2.2) | 14 (2.4) | 1 (1.3) | 0.524 |
| Hypotension or shock* | 13 (1.9) | 9 (1.5) | 4 (5.0) | 0.034* |
| Lower limb symptoms* | 61 (9.1) | 41 (6.9) | 20 (25.0) | <0.001* |
| Echocardiography | ||||
| DAA (mm) | 45.0±7.4 | 45.0±7.5 | 45.5±7.1 | 0.583 |
| LVEDD (mm) | 51.3±6.3 | 51.5±6.2 | 50.2±6.7 | 0.086 |
| LVEF (%) | 60.1±4.5 | 60.2±4.4 | 59.9±5.5 | 0.613 |
| Involvement of vessel branches | ||||
| Coronary artery* | 140 (20.9) | 106 (18.0) | 34 (42.5) | <0.001* |
| Innominate artery* | 387 (57.8) | 331 (56.1) | 56 (70.0) | 0.018* |
| Left common carotid artery | 332 (49.6) | 285 (48.3) | 47 (58.8) | 0.080 |
| Left subclavian artery* | 299 (44.6) | 254 (43.1) | 45 (56.3) | 0.026* |
| Celiac trunk | 283 (42.2) | 249 (42.2) | 34 (42.5) | 0.960 |
| Superior mesenteric artery | 175 (26.1) | 151 (25.6) | 24 (30.0) | 0.400 |
| Right renal artery | 191 (28.5) | 169 (28.6) | 22 (27.5) | 0.832 |
| Left renal artery* | 302 (45.1) | 254 (43.1) | 48 (60.0) | 0.004* |
| Laboratory results | ||||
| White blood cell count (×109/L) | 12.6±5.0 | 12.5±5.0 | 13.6±4.8 | 0.052 |
| Platelets (×109/L)* | 180.5±61.7 | 182.5±62.3 | 165.6±55.8 | 0.021* |
| SCr (μmol/L)* | 100.3±47.0 | 96.9±45.3 | 125.1±51.4 | <0.001* |
| Combined surgery | ||||
| Bentall or David procedure | 173 (25.8) | 149 (25.3) | 24 (30.0) | 0.363 |
| CABG* | 88 (13.1) | 64 (10.8) | 24 (30.0) | <0.001* |
| Duration of procedure (min) | ||||
| CPB time* | 181.1±53.4 | 175.8±46.2 | 219.9±80.6 | <0.001* |
| Cross-clamp time* | 106.2±34.2 | 104.0±30.8 | 122.3±50.2 | <0.001* |
| HCA time* | 17.9±7.0 | 17.6±6.8 | 20.3±7.7 | 0.001* |
Data are presented as the mean ± SD or n (%). *, non-severe AKI vs. severe AKI, P<0.05. AKI, acute kidney injury; BMI, body mass index; CAD, coronary artery disease; ATAAD, acute type A aortic dissection; DAA, diameter of ascending aorta; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; SCr, serum creatinine; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; HCA, hypothermic circulatory arrest; SD, standard deviation.
Table 2
| Variables | Total (n=670) | Non-severe AKI (n=590) | Severe AKI (n=80) | P value |
|---|---|---|---|---|
| In-hospital death* | 29 (4.3) | 18 (3.1) | 11 (13.8) | <0.001* |
| RRT* | 67 (10.0) | 0 (0.0) | 67 (83.8) | <0.001* |
| Major adverse outcomes* | 169 (25.2) | 89 (15.1) | 80 (100.0) | <0.001* |
| Perioperative myocardial infarction* | 22 (3.3) | 11 (1.9) | 11 (13.8) | <0.001* |
| Cerebrovascular accident | 22 (3.3) | 18 (3.1) | 4 (5.0) | 0.359 |
| Paraplegia* | 33 (4.9) | 22 (3.7) | 11 (13.8) | <0.001* |
| Gastrointestinal ischemia or bleeding* | 15 (2.2) | 8 (1.4) | 7 (8.8) | <0.001* |
| Mediastinal infection | 3 (0.4) | 3 (0.5) | 0 (0.0) | 0.523 |
| Respiratory complications* | 47 (7.0) | 26 (4.4) | 21 (26.3) | <0.001* |
| Aortic rupture | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0.712 |
| Osteofascial compartment* | 5 (0.7) | 0 (0.0) | 5 (6.3) | <0.001* |
| Postoperative limb ischemia* | 1 (0.1) | 0 (0.0) | 1 (1.3) | 0.007* |
| Femoro-femoral artery bypass | 12 (1.8) | 10 (1.7) | 2 (2.5) | 0.610 |
| Thoracotomy exploration* | 20 (3.0) | 14 (2.4) | 6 (7.5) | 0.011* |
| ICU time (days)* | 5.5±6.0 | 4.4±4.7 | 13.9±8.2 | <0.001* |
| Ventilator time (min)* | 55.5±103.1 | 42.7±81.9 | 149.8±172.6 | <0.001* |
Data are presented as the mean ± SD or n (%). *, non-severe AKI vs. severe AKI, P<0.05. AKI, acute kidney injury; RRT, renal replacement therapy; ICU, intensive care unit; SD, standard deviation.
In-hospital outcomes
In-hospital mortality in the severe AKI group (13.8%) was 4.5 times higher than in the non-severe AKI group (3.1%). Compared with the non-severe AKI patients, the severe AKI patients had a longer duration of mechanical ventilation (min) (149.8±172.6 vs. 42.7±81.9, P<0.001), a higher incidence of major adverse outcomes (100% vs. 15.1%, P<0.001), and more frequent use of RRT (83.8% vs. 0.0%, P<0.001). Therefore, the severe AKI patients had longer ICU stays (days) (13.9±8.2 vs. 4.4±4.7, P<0.001) (Table 2).
Univariate analysis
Patients who developed severe AKI were more likely to be older (years) (49.4±11.1 vs. 46.5±10.0, P=0.019), were also more likely to present preoperatively with preoperative malperfusion of the organ: hypotension or shock (5.0% vs. 1.5%, P=0.034), and lower limb symptoms (25.0% vs. 6.9%, P<0.001). The proportion of branch vessel involvement by aortic dissection was significantly higher for patients with severe AKI than non-severe AKI patients: coronary artery (42.5% vs. 18.0%, P<0.001), innominate artery (70.0% vs. 56.1%, P=0.018), left subclavian artery (56.3% vs. 43.1%, P=0.026) and left renal artery (60.0% vs. 43.1%, P=0.004). Laboratory results revealed that patients with severe AKI had higher SCr (µmol/L) (125.1±51.4 vs. 96.9±45.3, P<0.001) and lower platelet numbers (×109/L) (165.6±55.8 vs. 182.5±62.3, P=0.021) than non-severe AKI patients. Patients with severe AKI more frequently had concomitant coronary artery bypass graft (CABG) (30.0% vs. 10.8%, P<0.001) than non-severe AKI patients. The severe AKI group had a significantly longer duration CPB time (min) (severe AKI 219.9±80.6 vs. non-severe AKI 175.8±46.2, P<0.001) than the non-severe AKI group (Table 1). The results of the univariate logistic regression analysis showed that age, hypotension or shock, lower limb symptoms, coronary artery involvement, innominate artery involvement, left subclavian artery involvement, left renal artery involvement, platelets, SCr, CABG, CPB time, cross-clamp time, and hypothermic circulatory arrest (HCA) time were significantly correlated with severe AKI (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Demographics | |||||
| Age (years)* | 1.028 (1.004–1.052) | 0.020* | 1.029 (1.002–1.056) | 0.032* | |
| Female gender | 1.564 (0.926–2.641) | 0.094 | – | – | |
| BMI (kg/m²) | 1.025 (0.975–1.078) | 0.338 | – | – | |
| Medical history | |||||
| Marfan syndrome | 0.648 (0.252–1.672) | 0.370 | – | – | |
| Hypertension | 0.905 (0.488–1.677) | 0.751 | – | – | |
| Diabetes | 0.815 (0.186–3.579) | 0.786 | – | – | |
| Prior cardiovascular surgery | 0.662 (0.153–2.870) | 0.582 | – | – | |
| Prior CAD | 7.456 (0.462–120.388) | 0.157 | – | – | |
| Family history of dissections or aneurysms | 0.817 (0.102–6.536) | 0.849 | – | – | |
| Current smoker | 0.692 (0.428–1.119) | 0.133 | – | – | |
| ATAAD presentation | |||||
| Chest pain | 1.101 (0.574–2.113) | 0.772 | – | – | |
| Back pain | 1.261 (0.790–2.012) | 0.322 | – | – | |
| Abdominal pain | 0.886 (0.472–1.664) | 0.706 | – | – | |
| Head or neck pain | 1.729 (0.482–6.205) | 0.401 | – | – | |
| Preoperative malperfusion of organ | |||||
| Brain ischemia | 1.717 (0.875–3.366) | 0.116 | – | – | |
| Myocardial ischemia | 3.722 (0.334–41.515) | 0.286 | – | – | |
| Cardiac failure | 0.521 (0.068–4.015) | 0.531 | – | – | |
| Hypotension or shock* | 3.398 (1.022–11.301) | 0.046* | – | – | |
| Lower limb symptoms* | 4.463 (2.456–8.111) | <0.001* | 4.384 (2.240–8.582) | <0.001* | |
| Echocardiography | |||||
| DAA (mm) | 1.009 (0.978–1.040) | 0.582 | – | – | |
| LVEDD (mm) | 0.967 (0.931–1.005) | 0.086 | – | – | |
| LVEF (%) | 0.987 (0.939–1.038) | 0.612 | – | – | |
| Involvement of vessel branches | |||||
| Coronary artery* | 3.375 (2.066–5.512) | <0.001* | 2.478 (1.432–4.288) | 0.001* | |
| Innominate artery* | 1.826 (1.102–3.026) | 0.019* | – | – | |
| Left common carotid artery | 1.524 (0.949–2.447) | 0.081 | – | – | |
| Left subclavian artery* | 1.701 (1.062–2.724) | 0.027* | – | – | |
| Celiac trunk | 1.012 (0.631–1.623) | 0.960 | – | – | |
| Superior mesenteric artery | 1.246 (0.746–2.081) | 0.400 | – | – | |
| Right renal artery | 0.945 (0.561–1.593) | 0.832 | – | – | |
| Left renal artery* | 1.984 (1.233–3.194) | 0.005* | – | – | |
| Laboratory results | |||||
| White blood cell count (×109/L) | 1.034 (0.995–1.075) | 0.091 | – | – | |
| Platelets (×109/L)* | 0.995 (0.990–0.999) | 0.020* | – | – | |
| SCr (μmol/L)* | 1.009 (1.005–1.013) | <0.001* | 1.008 (1.003–1.013) | 0.001* | |
| Combined surgery | |||||
| Bentall or David procedure | 1.268 (0.759–2.119) | 0.364 | – | – | |
| CABG* | 3.522 (2.044–6.069) | <0.001* | – | – | |
| Duration of procedure (min) | |||||
| CPB time* | 1.013 (1.009–1.017) | <0.001* | 1.011 (1.006–1.015) | <0.001* | |
| Cross-clamp time* | 1.013 (1.007–1.019) | <0.001* | – | – | |
| HCA time* | 1.058 (1.023–1.095) | 0.001* | – | – | |
*, non-severe AKI vs. severe AKI, P<0.05. AKI, acute kidney injury; OR, odds ratio; CI, confidence interval; BMI, body mass index; CAD, coronary artery disease; ATAAD, acute type A aortic dissection; DAA, diameter of ascending aorta; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; SCr, serum creatinine; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; HCA, hypothermic circulatory arrest; SD, standard deviation.
Multivariate analysis
The multivariable analyses of risk factors for severe postoperative AKI were carried out by including all candidate variables derived from the univariable analysis and clinically important variables (age, hypotension or shock, lower limb symptoms, coronary artery involvement, innominate artery involvement, left subclavian artery involvement, left renal artery involvement, white blood cell count, SCr, platelets, Bentall or David procedure, CABG, and CPB time). Multivariate analysis revealed that severe AKI was predicted by advanced age (OR =1.029; 95% CI: 1.002–1.056; P=0.032), lower limb symptoms (OR =4.384; 95% CI: 2.240–8.582; P<0.001), coronary artery involvement (OR =2.478; 95% CI: 1.432–4.288; P=0.001), preoperative SCr (OR =1.008; 95% CI: 1.003–1.013; P=0.001), and prolonged CPB time (OR =1.011; 95% CI: 1.006–1.015; P<0.001) (Table 3).
Discussion
Chinese ATAAD patients were younger than those in Europe and North America (7,9,10,26). Thus, the TAR + FET, as a one-stage repair technique, has become a routine procedure to facilitate future surgery for the distal aorta (25-31). A solid understanding of surgical therapies for ATAAD and optimal organ protection is required to perform aortic arch replacement, albeit limited versus extended. Our study used the KDIGO criteria, previously tested for predicting of AKI in the population undergoing cardiac surgery (27,28). As such, we identified the incidence, risk factors, and in-hospital outcomes for ATAAD patients undergoing TAR + FET, who are complicated with severe postoperative AKI. Some previous studies have depicted that the incidence of severe AKI and in-hospital mortality following ATAAD surgery ranged from 5.5% to 37.8% and from 4.7% to 27%, respectively, according to different diagnostic criteria (7,10,15,32). In addition, a small number of studies revealed that the incidence of severe AKI and in-hospital mortality after TAR + FET was reported to be 19.3% and 5.7%, respectively (17,18). However, few studies focused on severe AKI after TAR + FET in ATAAD patients. In the present study, the incidence of severe postoperative AKI and the overall in-hospital mortality was 11.9% and 4.3%, respectively. Despite the incidence of severe AKI and in-hospital mortality being high in our study, they do not seem to be high after TAR + FET compared to other thoracic aortic surgeries (7,15). To some extent, TAR + FET can be considered a relatively safe procedure.
Previous research has reported the predictive risk factors for postoperative AKI, including age, gender, body mass index, hypertension, left ventricular ejection fraction, preoperative SCr, CPB time, perioperative sepsis, thoracotomy exploration, and coronary involvement (7,9,10,28-30). Nevertheless, there is no agreement on it yet. On the one hand, most of the attention has been paid to AKI, and only a few studies have reported specifically on severe AKI, which was strongly associated with the development of adverse events and in-hospital mortality (8,15,28). Our results also found that severe AKI was associated with significantly higher in-hospital mortality and incidence of in-hospital adverse events, including longer duration of mechanical ventilation, higher incidence of major adverse outcomes, and more frequent use of RRT. On the other hand, TAR + FET with a long duration and complicated operation posed a high risk of periprocedural complications in ATAAD patients. Thus, we focused on severe AKI in ATAAD patients receiving TAR + FET. The main finding of our study was that advanced age, lower limb symptoms, coronary artery involvement, preoperative SCr, and prolonged CPB time are independent risk factors for severe AKI after TAR + FET for ATAAD. Among these, lower limb symptoms is an emerging risk factor. In clinical practice, identifying risk factors for severe postoperative AKI is meaningful to adjust or modify the treatment strategy.
Partly following these previous findings (7,9-12,18,19,28,29,31,33,34), advanced age, preoperative SCr, and prolonged CPB time were also found to contribute to the development of severe AKI in our study. Elevated SCr indicated impaired kidney function (32). Moreover, the population in our study was younger than those conducted in Europe and North America, reflecting differences in demographics (7,10,26,35). The relatively low incidence of severe AKI and in-hospital mortality were considered to be related to the youth of our subjects, extensive prior experience with this procedure, and meticulous perioperative management. In addition to surgical skills, high-volume centers also have greater experience with administering extracorporeal circulation, anesthetic, and intensive care. Our surgical team in TAR + FET procedure has a shorter CPB time, cross-clamp time, and hypothermic circulatory arrest time than other studies (26,36-38). Experience with this procedure may help to shorten CPB time and improve short-term outcomes (2).
Lower extremity ischemia was a risk factor for postoperative AKI in previous studies (10,39-43). Better renal and survival outcomes might be achieved with shorter lower body ischemic times. Moreover, spinal cord injury had also been demonstrated to play an important role in AKI (44,45). In our study, multivariate analysis showed preoperative lower limb symptoms were a risk factor for severe AKI. Limb ischemia or spinal cord injury might associate severe extension of the aortic dissection with renal malperfusion. We surmised that limb ischemia or spinal cord injury was the cause of lower limb symptoms, including pain, a decline of muscle strength, and sensory disturbances. In addition, it was likely that ischemia-reperfusion injury was an important pathophysiological change in severe AKI. The ischemia-reperfusion injury did not only affect the lower extremity but also the kidney, as a nearby organ (39). Rhabdomyolysis, which might be caused by ischemia-reperfusion injury, also might contribute to the development of severe AKI in previous studies (41,43,46). The results of this study confirmed the significant impact of lower limb symptoms on severe AKI for ATAAD patients receiving TAR + FET.
A previous study had correlated the risk of AKI with coronary artery involvement in patients with type A aortic dissection, which was confirmed through the results of multivariate analysis in this study (9). Based on Neri’s classification (47), coronary ostium repair, coronary ostium reconstruction (the button technique), and CABG were performed in patients with coronary artery involvement. Our findings suggest that coronary artery involvement occurs in 20.9% of patients, similar to previous studies (9,48). These additional procedures required longer durations of operation, CPB time, and kidney ischemia time, which likely increases the risks of severe AKI. Moreover, even if there is no myocardial ischemia, it does not mean it will not occur. A few patients without myocardial ischemia might develop suspected myocardial ischemia during surgery preparation due to coronary malperfusion, which may be caused by low blood pressure. Acute worsening of cardiac function due to myocardial ischemia may lead to severe AKI, known as cardiorenal syndrome type 1 (49).
Limitations
This study has several potential limitations. First, it is a retrospective observational study. It is very difficult to conduct a randomized controlled trial for patients with ATAAD due to their urgent needs. Second, we used KDIGO as the diagnostic criteria for AKI, whereas some previous studies (7,20) used RIFLE or AKIN classification. This difference may be difficult to compare our results with previous research. Finally, we have not yet had access to long-term follow-up data. Therefore, we plan to conduct a further follow-up study on this topic.
Conclusions
There was a high incidence of severe AKI and high in-hospital mortality after TAR + FET in ATAAD patients. Based on our results, advanced age, lower limb symptoms, coronary artery involvement, preoperative SCr, and prolonged CPB time are the independent risk factors for severe AKI after TAR + FET in ATAAD patients. The risk factors for severe AKI in ATAAD patients undergoing TAR + FET were determined to help identify the high-risk patients and make rational treatment decisions.
Acknowledgments
We acknowledged all co-authors for their hard work on this study. We thank the Home for Researchers editorial team (https://www.home-for-researchers.com) for polishing the article.
Funding: This work was supported by the National Natural Science Foundation of China (No. NSFC8210022135) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2021-1-I2M-016).
Footnote
Provenance and Peer Review: While submitted as a standard submission to the journal, this article is selected as part of the special series “Frozen Elephant Trunk” published in Cardiovascular Diagnosis and Therapy, with joint decision from the editorial office and Guest Editors (Mohamad Bashir, Edward P. Chen and Mohammed Idhrees). The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-313/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-313/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-313/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-313/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee Institute of Fuwai Hospital approved the present study (No. 2021-1557). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg 2021;162:735-58.e2. [Crossref] [PubMed]
- Zhu Y, Lingala B, Baiocchi M, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol 2020;76:1703-13. [Crossref] [PubMed]
- Shrestha M, Haverich A, Martens A. Total aortic arch replacement with the frozen elephant trunk procedure in acute DeBakey type I aortic dissections. Eur J Cardiothorac Surg 2017;51:i29-34. [Crossref] [PubMed]
- Ma WG, Chen Y, Zhang W, et al. Extended repair for acute type A aortic dissection: long-term outcomes of the frozen elephant trunk technique beyond 10 years. J Cardiovasc Surg (Torino) 2020;61:292-300. [Crossref] [PubMed]
- Vijayan A, Abdel-Rahman EM, Liu KD, et al. Recovery after Critical Illness and Acute Kidney Injury. Clin J Am Soc Nephrol 2021;16:1601-9. [Crossref] [PubMed]
- Helgason D, Helgadottir S, Ahlsson A, et al. Acute Kidney Injury After Acute Repair of Type A Aortic Dissection. Ann Thorac Surg 2021;111:1292-8. [Crossref] [PubMed]
- Sasabuchi Y, Kimura N, Shiotsuka J, et al. Long-Term Survival in Patients With Acute Kidney Injury After Acute Type A Aortic Dissection Repair. Ann Thorac Surg 2016;102:2003-9. [Crossref] [PubMed]
- Wang M, Fan R, Gu T, et al. Short-term outcomes of acute coronary involvement in type A aortic dissection without myocardial ischemia: a multiple center retrospective cohort study. J Cardiothorac Surg 2021;16:107. [Crossref] [PubMed]
- Amano K, Takami Y, Ishikawa H, et al. Lower body ischaemic time is a risk factor for acute kidney injury after surgery for type A acute aortic dissection. Interact Cardiovasc Thorac Surg 2020;30:107-12. [Crossref] [PubMed]
- Li L, Zhou J, Hao X, et al. The Incidence, Risk Factors and In-Hospital Mortality of Acute Kidney Injury in Patients After Surgery for Acute Type A Aortic Dissection: A Single-Center Retrospective Analysis of 335 Patients. Front Med (Lausanne) 2020;7:557044. [Crossref] [PubMed]
- Liu T, Fu Y, Liu J, et al. Body mass index is an independent predictor of acute kidney injury after urgent aortic arch surgery for acute DeBakey Type I aortic dissection. J Cardiothorac Surg 2021;16:145. [Crossref] [PubMed]
- Wang Z, Ge M, Chen T, et al. Risk factors and long-term outcomes of elderly patients complicating with acute kidney injury after type A acute aortic dissection surgery: a retrospective study. J Thorac Dis 2020;12:5833-41. [Crossref] [PubMed]
- Zong Q, Ge M, Chen T, et al. Risk factors and long-term outcomes of acute kidney injury complication after type A acute aortic dissection surgery in young patients. J Cardiothorac Surg 2020;15:315. [Crossref] [PubMed]
- Tian Y, Diao X, Wang Y, et al. Prediction Scores for Any-Stage and Stage-3 Acute Kidney Injury After Adult Cardiac Surgery in a Chinese Population. J Cardiothorac Vasc Anesth 2021;35:3001-9. [Crossref] [PubMed]
- Chien TM, Wen H, Huang JW, et al. Significance of preoperative acute kidney injury in patients with acute type A aortic dissection. J Formos Med Assoc 2019;118:815-20. [Crossref] [PubMed]
- Chen Z, Chen L, Yao G, et al. Novel Blood Cytokine-Based Model for Predicting Severe Acute Kidney Injury and Poor Outcomes After Cardiac Surgery. J Am Heart Assoc 2020;9:e018004. [Crossref] [PubMed]
- Zhou H, Wang G, Yang L, et al. Acute Kidney Injury After Total Arch Replacement Combined With Frozen Elephant Trunk Implantation: Incidence, Risk Factors, and Outcome. J Cardiothorac Vasc Anesth 2018;32:2210-7. [Crossref] [PubMed]
- Fang Z, Wang G, Liu Q, et al. Moderate and deep hypothermic circulatory arrest has a comparable effect on acute kidney injury after total arch replacement with frozen elephant trunk procedure in type A aortic dissection. Interact Cardiovasc Thorac Surg 2019;29:130-6. [Crossref] [PubMed]
- Shang W, Ma M, Ge YP, et al. Analysis of risk factors of type a aortic dissection (TAAD) operation of frozen elephant trunk and total arch replacement. Eur Rev Med Pharmacol Sci 2016;20:4586-92. [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [Crossref] [PubMed]
- Kellum JA, Lameire NKDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Lameire N, Kellum JAKDIGO AKI Guideline Work Group. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2). Crit Care 2013;17:205. [Crossref] [PubMed]
- Yan TD, Tian DH, LeMaire SA, et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation 2014;129:1610-6. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Kremer J, Preisner F, Dib B, et al. Aortic arch replacement with frozen elephant trunk technique - a single-center study. J Cardiothorac Surg 2019;14:147. [Crossref] [PubMed]
- Tian M, Liu X, Chen L, et al. Urine metabolites for preoperative prediction of acute kidney injury after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Chen X, Zhou J, Fang M, et al. Incidence- and In-hospital Mortality-Related Risk Factors of Acute Kidney Injury Requiring Continuous Renal Replacement Therapy in Patients Undergoing Surgery for Acute Type a Aortic Dissection. Front Cardiovasc Med 2021;8:749592. [Crossref] [PubMed]
- Wang J, Yu W, Zhai G, et al. Independent risk factors for postoperative AKI and the impact of the AKI on 30-day postoperative outcomes in patients with type A acute aortic dissection: an updated meta-analysis and meta-regression. J Thorac Dis 2018;10:2590-8. [Crossref] [PubMed]
- Gao Y, Wang C, Li J, et al. Mild and moderate to severe early acute kidney injury following cardiac surgery among patients with heart failure and preserved vs. mid-range vs. reduced ejection fraction: A retrospective cohort study. Eur J Anaesthesiol 2022;39:673-84. [Crossref] [PubMed]
- Ma X, Chen S, Yun Y, et al. The Predictive Role of Lymphocyte-to-Monocyte Ratio in Acute Kidney Injury in Acute Debakey Type I Aortic Dissection. Front Surg 2021;8:704345. [Crossref] [PubMed]
- Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation 2006;114:I409-13. [Crossref] [PubMed]
- Zhang K, Shang J, Chen Y, et al. The prognosis and risk factors for acute kidney injury in high-risk patients after surgery for type A aortic dissection in the ICU. J Thorac Dis 2021;13:4427-37. [Crossref] [PubMed]
- Chen Z, Li J, Sun Y, et al. A novel predictive model for poor in-hospital outcomes in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [Crossref] [PubMed]
- Kozlov BN, Panfilov DS, Ponomarenko IV, et al. The risk of spinal cord injury during the frozen elephant trunk procedure in acute aortic dissection. Interact Cardiovasc Thorac Surg 2018;26:972-6. [Crossref] [PubMed]
- Leone A, Di Marco L, Coppola G, et al. Open distal anastomosis in the frozen elephant trunk technique: initial experiences and preliminary results of arch zone 2 versus arch zone 3†. Eur J Cardiothorac Surg 2019;56:564-71. [Crossref] [PubMed]
- Mariscalco G, Bilal H, Catarino PReflection From UK Aortic Group, et al. Frozen Elephant Trunk Technique as Optimal Solution in Type A Acute Aortic Dissection. Semin Thorac Cardiovasc Surg 2019;31:686-90. [Crossref] [PubMed]
- Eygi B, Gokalp O, Kiray M, et al. Direct kidney injury or lower extremity ischemia induced indirect kidney injury: Which one is more harmful for kidneys? Vascular 2021;29:461-7. [Crossref] [PubMed]
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014;3:351-67. [PubMed]
- Mansour Z, Charles AL, Kindo M, et al. Remote effects of lower limb ischemia-reperfusion: impaired lung, unchanged liver, and stimulated kidney oxidative capacities. Biomed Res Int 2014;2014:392390. [Crossref] [PubMed]
- Nota H, Asai T, Suzuki T, et al. Risk factors for acute kidney injury in aortic arch surgery with selective cerebral perfusion and mild hypothermic lower body circulatory arrest. Interact Cardiovasc Thorac Surg 2014;19:955-61. [Crossref] [PubMed]
- Garbaisz D, Turoczi Z, Aranyi P, et al. Attenuation of skeletal muscle and renal injury to the lower limb following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS One 2014;9:e101067. [Crossref] [PubMed]
- Galeiras R, Mourelo M, Pértega S, et al. Rhabdomyolysis and acute kidney injury in patients with traumatic spinal cord injury. Indian J Crit Care Med 2016;20:504-12. [Crossref] [PubMed]
- Parvin S, Williams CR, Jarrett SA, et al. Spinal Cord Injury Increases Pro-inflammatory Cytokine Expression in Kidney at Acute and Sub-chronic Stages. Inflammation 2021;44:2346-61. [Crossref] [PubMed]
- Miller CC 3rd, Villa MA, Sutton J, et al. Serum myoglobin and renal morbidity and mortality following thoracic and thoraco-abdominal aortic repair: does rhabdomyolysis play a role? Eur J Vasc Endovasc Surg 2009;37:388-94. [Crossref] [PubMed]
- Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg 2001;121:552-60. [Crossref] [PubMed]
- Lu S, Zhao Y, Song K, et al. Long-Term Outcomes of Surgical Treatment for Acute Type-A Aortic Dissection with Coronary Artery Involvement. Int Heart J 2021;62:1069-75. [Crossref] [PubMed]
- Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527-39. [Crossref] [PubMed]


