
The implications of an integrated management model of prenatal diagnosis/postnatal treatment for premature infants with critical congenital heart disease—a case-control study
Introduction
Congenital heart disease (CHD) is the most common malformation arising during foetal development, with a rising prevalence of more than 9 per 1,000 live births worldwide affecting millions of newborns each year (1). The incidence of critical CHD (CCHD) in preterm infants (24–32 weeks gestation) is reported as 0.77%, with overall fatality rates of 18.6% (2). It has been reported that 30% of patients die within the neonatal period, and 50–70% die within one year if left untreated (3). The risk of death is higher in preterm neonates with CCHD than in full-term neonates (4). Furthermore, the extremely high medical costs of CCHD have resulted in substantial economic burdens on countries and individuals (5). As such, the early identification and intervention of CCHD remain formidable challenges for medicine and public health (6).
Early therapeutic intervention is needed for patients with CCHD to survive. With advances in neonatal intensive care unit (NICU) medicine and the establishment of a children’s heart centre, more premature infants with CCHD are being successfully treated, thereby attracting a larger focus on the condition. However, the clinical features, interventions, and outcomes of preterm newborns with CCHD have not been adequately studied (7). Furthermore, the incidence rates and severity of CCHD have gradually increased in China over the past 10 years. Consequently, establishing management models for premature patients with CCHD is an urgent clinical requirement.
Since the 1980s, developments in foetal echocardiography technology have revolutionised the prenatal detection of CCHD and are beginning to change the relevant clinical workflow (8) in the form of an integrated management model of prenatal diagnosis/postnatal treatment (9). The integrated management model comprises a combination of prenatal diagnoses, risk classifications, counselling, immediate treatments and transitions following delivery; along with telephone support and follow-up visits to meet the needs of patients and their parents with different levels of health issues during their care by integrating medical assets and optimizing the allocation of medical and care resources.
Studies have demonstrated that an integrated management model can improve the prognosis and survival rate of CCHD in term infants (9). However, evidence that this workflow can increase the rates of early interventional procedures and improve the survival rate among preterm newborns with CCHD remains lacking. Therefore, the clinical data of 79 premature infants at the authors’ institution with CCHD from 2017 to 2019 were retrospectively analysed. The study aimed to investigate the interventions associated with the integrated management model in premature infants with CCHD and their effect on prognoses to establish a scientific basis for clinical diagnosis and treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-74/rc).
Methods
Subjects
This study represents a retrospective analysis of the clinical data of premature infants with CCHD at Guangdong Provincial People’s Hospital from 2017 to 2019.
Inclusion criteria: (I) the diagnosis of CHD was confirmed by clinical manifestations and imaging modalities [ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), or angiography]. Patients with CCHD were defined as those requiring surgical intervention within 28 days of birth (10). The classification included the following defects (11): the complete transposition of the great arteries (TGA), hypoplastic left heart syndrome (HLHS), pulmonary atresia (PA) or severe pulmonary stenosis (PS), severe tetralogy of Fallot (TOF), total anomalous pulmonary venous connection (TAPVC), tricuspid atresia, persistent truncus arteriosus (PTA), interrupted aortic arch, double outlet right ventricle, Ebstein anomaly, single ventricle defects and other major critical heart defects. (II) Preterm birth was defined as a gestational age of <37 weeks at birth. (III) Only hospitalisations with a date of admission within the neonatal period were included. In addition, the participants were grouped according to whether or not their heart echocardiogram results revealed malformations during the course of pregnancy.
Exclusion criteria: (I) the heart malformation of the child was a simple patent ductus arteriosus or atrial septal defect; (II) key information in the medical records was incomplete (e.g., gestational age and birth weight).
According to the diagnostic and exclusion criteria, the subjects were divided into prenatal and postpartum diagnostic groups. The clinical characteristics and survival outcomes of patients were collected and compared. The delivery classification scale was used for risk stratification and patient management.
Antenatal risk assessment
Classification of premature infants with CCHD using a delivery classification scale designed by the Children’s Hospital of Philadelphia to help determine the immediate neonatal needs of infants with a prenatal diagnosis of cardiovascular disease (12). The scale includes four classes as follows: (I) class I—a foetus for which no special care is anticipated at delivery. Examples include a simple large ventricular septal defect, a balanced complete atrioventricular canal defect or truncus arteriosus with normal truncal valve function. (II) Class II—a foetus that is anticipated to be stable at birth but is dependent upon the patency of the ductus arteriosus for either systemic or pulmonic blood flow and requires the initiation of a prostaglandin infusion. Examples include stable neonates with PA or HLHS with an open atrial septum. (III) Class III—a foetus with a type of heart disease for which instability is possible or likely. Examples include TGA or TAPVC with obstruction. (IV) Class IV—immediate postpartum access to cardiac therapy, which is realised for a foetus in whom marked instability is anticipated as soon as it is separated from placental circulation. Examples include HLHS with an intact atrial septum or a complete heart block with marked bradycardia. In this study, the personnel included the NICU, cardiac surgeon and the Paediatric Cardiology Department.
Inpatient treatment
On admission, all patients with clinical manifestations underwent echocardiography and/or cardiac CT/MRI. Treatment was conducted according to the patient’s delivery classification, clinical condition, the severity of CCHD, laboratory examinations and the willingness of their legal guardians. Decisions on CCHD interventions were made by neonatologists, cardiologists and the patients’ families.
All of the hospitalised infants were treated according to international guidelines. If routine preoperative examinations and functional evaluations were in line with the operation indications, the infants were treated with surgery or interventional therapy. In instances where surgery may not have been tolerated (e.g., because of low weight and/or other congenital malformations), a conservative approach was assumed. Following discharge, regular follow-up was conducted to observe the infant’s clinical condition. If hemodynamic instability occurred during the follow-up, emergency surgery was performed immediately.
Observed indicators
Basic information and clinical indicators were obtained from the medical records management system of Guangdong Provincial People’s Hospital with informed consent. The basic demographic information included sex, gestational age, birth weight, Apgar score, cesarean delivery, maternal age, parity, multiple pregnancies and pregnancy disorders (including gestational hypertension, gestational diabetes and a history of adverse pregnancy). Medical variables included age on admission, admission weight, clinical symptoms (intolerance to feeding, dyspnoea, cyanosis, extrauterine growth retardation and cardiac murmur), the date of diagnosis, CCHD type, heart-function grade, length of hospital stay, hospitalisation cost, treatment modalities and case fatality rates. The families were mainly followed up via phone after discharge, and some received outpatient follow-ups. The follow-up deadline was 31 May 2021, and the survival time was calculated from delivery to death or follow-up deadline. Comparisons between the two groups were performed.
Statistical analysis
SPSS 26.0 (IBM Corporation) and R version 3.6.0 statistical software were used to process the data. The counting data were expressed in frequencies (%), and differences between the groups were compared via a chi-square test. The measurement data were expressed as mean ± standard deviation or a median (interquartile range), and group comparisons were performed using a t-test or the Mann-Whitney U test. Survival curves were estimated using Kaplan-Meier analysis, and the differences in survival rates were compared between the prenatal and postnatal diagnosis groups; P<0.05 was considered statistically significant.
Ethical statement
This study was performed in accordance with the Declaration of Helsinki (revised in 2013). The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2018317H), and informed consent was obtained from the guardians of all patients.
Results
Inclusion and grouping of participants
The flow diagram of screening and grouping participants in this study is shown in Figure 1. By May 2021, 71 cases had completed follow-up, while 8 were lost to follow-up (because the families refused contact during follow-up).
Basic information and clinical features
Basic information about the patients is presented in Table 1. Compared with the postnatal diagnosis group, the prenatal diagnosis group included infants that had been born slightly earlier during gestation [35.00 (33.29–35.86) vs. 35.57 (34.14–36.71) weeks, P<0.05], and their mothers were older (33.23±5.22 vs. 30.43±6.37 years, P<0.05). In addition, they also showed significantly lower Apgar scores [Apgar 1 min: 9 [8–10] vs. 10 [9–10], P<0.05; Apgar 5 min: 10 [9–10] vs. 10 [10–10], P=0.009; Apgar 10 min: 10 [9–10] vs. 10 [10–10], P<0.05]. There were no significant differences in sex, gestational age, birth weight, cesarean delivery, parity, multiple pregnancies, history of resuscitation or assisted reproduction and pregnancy disorders between the two groups.
Table 1
| Variables | Prenatal diagnostic group | Postnatal diagnostic group | P value* |
|---|---|---|---|
| Gender | 0.693 | ||
| Male | 30 (62.5%) | 18 (58.1%) | |
| Female | 18 (37.5%) | 13 (41.9%) | |
| Maternal age (years) | 33.23±5.22 | 30.43±6.37 | 0.036* |
| Gestational age (years) | 35.00 (33.29–35.86) | 35.57 (34.14–36.71) | 0.046* |
| Parity | 2 (1–5) | 2 (1–3) | 0.412 |
| Multiple pregnancy | 26 (54.2%) | 10 (32.3%) | 0.056 |
| Cesarean delivery | 34 (70.8%) | 16 (51.6%) | 0.084 |
| Birth weight (g) | 2,105.10±549.76 | 2,164.35±449.36 | 0.618 |
| Apgar 1 min | 9 (8–10) | 10 (9–10) | 0.023* |
| Apgar 5 min | 10 (9–10) | 10 (10–10) | 0.009* |
| Apgar 10 min | 10 (9–10) | 10 (10–10) | 0.011* |
| History of resuscitation | 7 (14.6%) | 2 (6.5%) | 0.267 |
| Gestational diabetes | 10 (20.8%) | 5 (16.1%) | 0.603 |
| Gestational hypertension | 4 (8.3%) | 2 (6.5%) | >0.05 |
| History of adverse pregnancy | 7 (14.6%) | 4 (12.9%) | >0.05 |
| Assisted reproduction | 13 (27.1%) | 6 (19.4%) | 0.433 |
Data are presented as n (%, mean ± SD) or median (range). *, P<0.05, indicating that there was significant difference between the two groups.
The types of CCHD
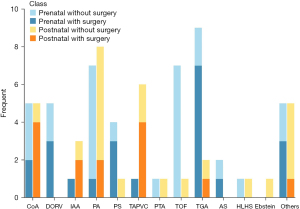
Figure 2 shows the types of CCHD that were included in the study. The top three CCHD types in the prenatal diagnosis group were TGA [9 (18.8%)], TOF [7 (14.6%)] and PA [7 (14.6%)]. In the postnatal diagnosis group, the top three types were PA [8 (25.8%)], TAPVC [6 (19.4%)] and coarctation of the aorta [CoA; 5 (16.1%)]. The foetal echocardiography diagnoses were not in agreement with the final diagnoses in 10 cases. For two of 48 prenatal diagnoses cases (CoA and PA, respectively), the diagnostic results of the newborns disagreed with the foetal echocardiography results. For eight of the 31 postnatal diagnosis cases, no foetal congenital anomalies were observed, and a diagnosis of CCHD was confirmed after birth. There were 3 cases with TAPVC, 2 with CoA, 1 with TGA, 1 with PA and 1 with TOF in the postnatal diagnosis group and their prenatal echocardiography tests were carried out at lower-level hospitals.
Clinical manifestations and hospitalization
Table 2 shows the clinical manifestations and in-hospital treatment among the two groups in this study. In the prenatal diagnosis group, the foetal cardiac ultrasonography fully detected the cardiac malformations at 24 (22.25–26.00) weeks of gestation. Thereafter, the pregnant women received antenatal registration and prenatal counselling at the authors’ hospital, where most of them gave birth, and the complete treatment of newborns was immediately initiated after delivery; alternatively, the parents/guardians opted to seek childbirth services at local community hospitals in combination with appropriate referrals. The patients in the postnatal diagnosis group became symptomatic after birth, and a diagnosis was established within 6 [2–13] days.
Table 2
| Variables | Prenatal diagnostic group | Postnatal diagnostic group | P value* |
|---|---|---|---|
| Admission weight (g) | 2,200 (1,800–2,436) | 2,400 (1,900–2,600) | 0.144 |
| Admission age (days) | 0 (0–5.5) | 7 (5–16) | <0.001* |
| Intolerance to feeding | 9 (18.8%) | 6 (19.4%) | 0.947 |
| Cyanosis | 29 (60.4%) | 15 (48.4%) | 0.293 |
| EUGR | 4 (8.3%) | 2 (6.5%) | 1.000 |
| Cardiac murmur | 29 (60.4%) | 22 (71.0%) | 0.338 |
| Heart function grades | 2 (2–4) | 2 (2–4) | 0.455 |
| Treatment modalities | 0.659 | ||
| Surgery inventions | 21 (43.8%) | 12 (38.7%) | |
| Conservative treatment | 20 (41.7%) | 12 (38.7%) | |
| Abandon treatment | 7 (14.6%) | 7 (22.6%) | |
| Hospitalization cost (yuan) | 82,241.48 (27,997.58–170,333.44) | 37,546.81 (15,949.65–104,228.06) | 0.008* |
| Extra-cardiac malformations | 11 (22.9%) | 3 (9.7%) | 0.132 |
| Length of hospital stay (days) | 21 (9.5–32.75) | 12 (6–25) | 0.023* |
| Death during hospital stay | 9 (18.8%) | 5 (16.1%) | 0.766 |
Data are presented as n (%) or median (range). *, P<0.05, indicating that there was significant difference between the two groups. EUGR, extrauterine growth retardation.
The difference in the admission age between the groups was statistically significant [0 (0–5.5) vs. 7 (5–16) days, P<0.001]. No significant differences were found in the patients’ clinical manifestations, heart function grades and treatment modalities between the two groups. In addition, the length of stay (LOS) of the patients with a prenatal diagnosis was significantly longer than the LOS for the postnatal diagnosis group [21 (9.5–32.75) vs. 12 (6–25) days, P<0.05]. Meanwhile, higher hospitalisation costs were required for the prenatal diagnosis patients [82,241.48 (27,997.58–170,333.44) vs. 37,546.81 (15,949.65–104,228.06), P<0.05], and the differences between the two groups were statistically significant.
Notably, for 7 infants in each group, treatment was abandoned and the mother left the hospital. Among these 14 infants, 2 were still alive at the time of this study; the remaining 12 had died, including six cases with PA or PS (one still alive without surgery), 2 with HLHS, 1 with TGA, 1 with TAPVC, 1 with PTA, 2 with TOF (alive following surgery at a different institute) and 2 with other defects. The reasons for abandoning treatment varied but included parents’ desire to forego surgery, the patient’s poor prognosis and high medical costs. The proportion of abandoned treatment or mortality in hospitals between the two groups were no statistically significant differences.
Classification and survival analysis of CCHD patients
In the prenatal diagnosis group, the authors classified 48 cases with CCHD using the delivery classification scale, i.e., 13 in class I (27.1%), 22 in class II (45.8%), and 13 in class III (27.1%); no class IV cases were included. Using the medical record management system data, the authors analysed whether the treatment of patients with CCHD after birth and the recommended principles varied. The exact agreement was 95.8%. Of the two disagreement cases, one was CAVC and one was TOF.
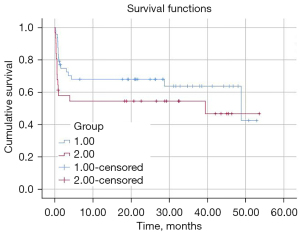
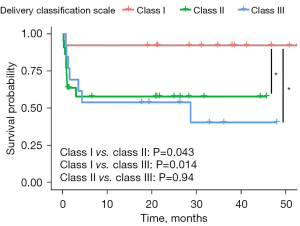
By May 2021, 71 cases had completed follow-up, while 8 were lost to follow-up (because the families refused contact during follow-up) (Table 3). The survival of the patients is presented in Figure 3. The median survival time of the prenatal diagnosis group was 48 months (95% CI: 40.78–57.29), and that of the postpartum diagnosis group was 39 months (95% CI: 34.41–44.32). The study found that survival was higher in the prenatal than in the postnatal diagnosis group, and the difference was statistically significant (P<0.05). The prenatal diagnosis group was also divided into three risk groups according to the delivery classification scale, and survival curves of the three groups were drawn to compare the survival times (Figure 4). The 3-year survival rates of the classes I, II and III were 92.31% (12/13), 59.09% (13/22) and 38.46% (5/13), respectively. The differences between classes I and II and classes I and III according to the delivery classification scale were statistically significant (class I vs. II, P<0.05; class I vs. III, P<0.05). However, no differences were found between classes II and III (P>0.05).
Table 3
| No. | Delivery classification scale | Prenatal diagnosis | Postnatal diagnosis | Operation age (days) | Operation | Follow-up |
|---|---|---|---|---|---|---|
| 1 | I | DORV, VSD | DORV, VSD, ASD, PDA | 13 | Radical surgery | Survive |
| 2 | II | VSD | CoA, VSD, ASD | 14 | Radical surgery | Survive |
| 3 | II | COA | CoA, PDA | 28 | Radical surgery | Died from LCOS after surgery |
| 4 | II | DORV, IAA | DORV, VSD, IAA, PDA, ASD | 13 | Radical surgery | Lost to follow-up |
| 5 | II | PS | PS, PFO, PDA | 16 | Palliative surgery after radical surgery failure | Lost to follow-up |
| 6 | II | PS | PS, PDA, ASD | 13 | Radical surgery | Died from LCOS after surgery |
| 7 | II | PS | PA, PDA, PFO | 9 | Radical surgery | Survive |
| 8 | II | AS | PDA, AS, ASD | 16 | Palliative surgery | Survive |
| 9 | II | COA | CoA, PDA, ASD | 26 | Radical surgery | Lost to follow-up |
| 10 | II | PS, ASD | PS, PDA, PFO | 24 | Ballooning dilatation | Survive |
| 11 | III | TGA, ASD | TGA, PDA, PFO | 8 | Radical surgery | Survive |
| 12 | III | TGA, VSD | TGA, PDA, PFO | 9 | Radical surgery | Survive |
| 13 | III | TGA | TGA, PDA, PFO | 8 | Radical surgery | Survive |
| 14 | III | TGA, VSD | TGA, VSD, ASD, PDA | 9 | Radical surgery | Died from LCOS after surgery |
| 15 | III | TGA | TGA, PDA, ASD | 5 | Radical surgery | Survive |
| 16 | III | TAPVC | TAPVC, PDA, ASD | 20 | Radical surgery | Survive |
| 17 | III | TGA, VSD | TGA, VSD, PDA, PFO | 7 | Radical surgery | Survive |
| 18 | III | CAVC | CAVC, valve defects | 17 | Palliative surgery | Died from LCOS after surgery |
| 19 | III | TGA | TGA, PDA, ASD | 20 | Radical surgery | Died from LCOS after surgery |
DORV, double outlet right ventricle; VSD, ventricular septal defect; ASD, atrial septal defect; PDA, patent ductus arteriosus; COA, coarctation of the aorta; LCOS, low cardiac output syndrome; IAA, interrupted aortic arch; PS, pulmonary stenosis (mostly severe); PFO, patent foramen ovale; PA, pulmonary atresia; AS, aortic stenosis (severe); TGA, transposition of the great arteries; TAPVC, total anomalous pulmonary venous connection; CAVC, complete atrioventricular canal.
Discussion
Based on advancements in prenatal diagnoses technology, the management of preterm newborns with CCHD has in recent years received more attention. The current multicentre retrospective study showed that the adjusted odds of death for very or extremely premature infants with CCHD were 7.5-fold greater than those without CHD (13). The burden of CCHD has always been expressed as an excess number of deaths, the added number of days in the hospital and additional costs (14,15). However, in the absence of evidence related to the effect of an integrated management model for prenatal diagnosis/postnatal treatment for Chinese preterm infants, the results of our study are essential.
The study classified 48 cases with CCHD using the delivery classification scale in the prenatal diagnosis group. A close relationship between foetal echocardiography and postnatal risk stratification was previously reported in infants with CHD (16). However, two cases in our study accepted higher levels of intensive care and surgical intervention, which were at odds with the established recommendations. On the one hand, this resulted from a transformation from foetal to postnatal circulation, which led to some lesions being more severe after delivery. This was, in turn, the result of the inaccessibility of the foetal circulation during an ongoing pregnancy. Hence, interdisciplinary discussions between the neonatal department, the cardiac surgeon, the paediatric cardiology department and obstetrics providers must be made a priority. Standard consultations, teamwork and multidisciplinary management are vital for optimising outcomes (17). The delivery classification scale offers a fundamental principle and opportunity for cooperation and collaboration between disciplines, thereby assisting them to apply medical resources for the treatment of critically ill newborns.
The data in the current study also showed that clinical evaluations after delivery were essential. Existing studies of prenatal diagnoses have focused on reporting the risk factors and benefits (18,19) without reporting postnatal management, which has greater utility for counselling and service planning. With this in mind, the current study highlights that individualised treatment strategies should be adopted according to each newborn’s hemodynamic status and clinical manifestations. The current results also showed that the survival of class I cases, based on the delivery classification scale, was better compared with classes II or III. This distinction is essential for establishing prenatal counselling, as it allows for evaluating the risk of cardiac malformation and proposing appropriate interventions and prevention.
This study focused on survival and found that the prenatal diagnosis group had a higher survival rate than the postnatal diagnosis group, which was statistically significant. This may be because the study sample size was small or because the rapid transfer and advanced intensive care could ameliorate a more dramatic advantage of prenatal diagnosis. Alternatively, as noted in Quartermain, a prenatal diagnosis was significantly associated with lower rates of preoperative risk factors (20). Advances in surgical techniques and treatment modalities may mask the benefits of prenatal diagnosis, as previously noted by Forbess (21). Controversies have long existed regarding the influence of a prenatal diagnosis on CCHD. Holland et al. indicated that newborns diagnosed prenatally with CCHD were less likely to die significantly before planned cardiac surgery (22). Morris et al. also pointed out that infants with HLHS who had been born far away from a cardiac surgical centre had increased neonatal mortality (23).
A prenatal diagnosis of CCHD may be associated with decreased morbidity, similar to the results noted in foreign academic research. In a large retrospective cohort study conducted by Bakker et al., the authors found that prenatal detection ranged from 13% (Slovak Republic) to 87% (some regions in France). Prenatal detection was consistently high for HLHS (64% overall) and was lowest for TAPVC (28% overall) (6). Moreover, the findings of an existing study in Chicago suggested that the positive predictive value increased from 75% in 1992–1996 to 96% in 1996–2001 (24). The current authors detected 10 cases in which a foetal echocardiography diagnosis did not agree with the final diagnosis. However, because the diagnostic accuracy of prenatal ultrasound can be affected by complex cardiovascular anatomy, foetal position, the frequency of foetal movement, the technical level of the instruments used and a poor acoustic window, missed diagnoses and misdiagnoses can easily occur under these circumstances.
Conversely, studies have also indicated that a prenatal diagnosis of CHD was associated with a significant reduction in acidosis but lacked any survival benefits (25,26). If there is no immediate opportunity for surgical intervention, prenatal diagnosis may not always be associated with improved survival in patients with CCHD (27). It is possible that later diagnoses are associated with improved one-year survival, as was also reported in other studies (28). Thus, the authors believe that the survival results presented in this study represent realistic clinical outcomes, and the use of the integrated management model of prenatal diagnosis/postpartum treatment may have an effect on improving survival rates.
We envision that our management model could help contribute to a shift from a reactive monodisciplinary system to a proactive, multidisciplinary and dynamic management paradigm in premature infants with CCHD in the near future. Our scenario also included a significant positive effect of this management model, i.e., shortening the treatment time, reducing medical expenses and improving patient prognoses. According to a meta-analysis published in 2016, prenatal diagnoses in perinatal management is an essential and effective approach for achieving timely intervention (29). Nagata et al. described the significant reduction in the length of time from birth to receiving tertiary care, and a prenatal diagnosis was associated with improved one-year survival (30). However, the results of the present study showed that the time for effective medical service was shorter in the prenatal diagnosis group and, unexpectedly, the hospitalisation cost was lower, and total in-hospital days were shorter in the postnatal diagnosis group. This was consistent with a report compiled by Copel et al., which stated that a prenatal diagnosis of CHD could not reduce the cost and length of hospitalisation (31). This was likely because the required laboratory tests and/or CT/MRI scans had been completed at other institutes. Typically, these examinations will not be conducted for all newborns for economic purposes. Additionally, the analysis was limited to the LOS in one hospital and the related inpatient costs, thereby omitting indirect costs, such as treatment costs at other hospitals, referral fees (the costs of which were vast) and intangible costs. These aspects may have caused underestimation of the LOS and inpatient costs in the postnatal diagnosis group and may partially explain why the length of hospitalisation in the postnatal group was shorter (i.e., due to a shorter examination time).
The present study also found statistically significant differences in maternal age. It had previously been reported that the maternal age in the two groups had not been significant (5,25). This may have been due to imperfections in the maternal health management system in China. Foetal echocardiography is not a routine type of screening and was only conducted in selected regional tertiary (the highest level in China) obstetric centres following positive ultrasound screening examinations for foetal anomalies with other indications (32). Due to the seriousness of CCHD, all high-risk pregnant women must undergo prenatal screening, and advanced age is one of the most critical risk factors (33). That is, in the current study, pregnant younger mothers who lived in remote areas may not have had access to additional foetal echocardiography. This could explain why the maternal age was older in the prenatal diagnosis group.
The limitations of this study are related to its retrospective nature. A separate analysis of CCHD cases was not performed in this study because the number was too small, which may have a certain impact on the results. Prospective, single-centre studies and the findings of this study may not be directly applicable to other centres. Most prenatal echocardiography is carried out in a primary hospital, and its need for accuracy and its advanced nature can make the results difficult to determine. Moreover, long-term neurodevelopmental outcomes may be a better measurement of prenatal diagnosis when transferals are readily available (34). Additionally, long-term studies of the neurological development of CCHD in premature infants are required to assess the impact of prenatal diagnosis. Finally, they were different from other regions (35). This may have been related to the characteristics of the inpatient cases and the sample size of the current study. Meanwhile, the possibility of differences between CCHD types within China could not be excluded altogether. Therefore, further research is required to develop more efficient strategies for prenatal diagnoses in China.
Conclusions
The findings of the current study suggest that combined prenatal diagnosis/postnatal therapy may be effective for preterm infants and that the childbirth classification scale has some prognostic value for CCHD. Therefore, prenatal diagnosis/postpartum combined treatment should be promoted in China; furthermore, the treatment level of CCHD in the country must be improved and have its integrated construction strengthened.
Acknowledgments
We would like to thank Martin C. for his help in polishing our paper.
Funding: This work was supported by National Key R&D Program of China (No. 2018YFC1002603).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-74/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-74/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-74/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Guangdong Provincial People’s Hospital (No. GDREC 2018317H). Written informed consent was obtained from the guardians of all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455-63. [Crossref] [PubMed]
- Norman M, Håkansson S, Kusuda S, et al. Neonatal Outcomes in Very Preterm Infants With Severe Congenital Heart Defects: An International Cohort Study. J Am Heart Assoc 2020;9:e015369. [Crossref] [PubMed]
- Glidewell J, Grosse SD, Riehle-Colarusso T, et al. Actions in Support of Newborn Screening for Critical Congenital Heart Disease–- United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2019;68:107-11. [Crossref] [PubMed]
- Mustafa HJ, Cross SN, Jacobs KM, et al. Preterm Birth of Infants Prenatally Diagnosed with Congenital Heart Disease, Characteristics, Associations, and Outcomes. Pediatr Cardiol 2020;41:972-8. [Crossref] [PubMed]
- Pinto NM, Nelson R, Botto L, et al. Costs, mortality, and hospital usage in relation to prenatal diagnosis in d-transposition of the great arteries. Birth Defects Res 2017;109:262-70. [Crossref] [PubMed]
- Bakker MK, Bergman JEH, Krikov S, et al. Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. BMJ Open 2019;9:e028139. [Crossref] [PubMed]
- Steurer MA, Peyvandi S, Baer RJ, et al. Impaired Fetal Environment and Gestational Age: What Is Driving Mortality in Neonates With Critical Congenital Heart Disease? J Am Heart Assoc 2019;8:e013194. [Crossref] [PubMed]
- Young AA, Sinclair BG. The Critical Importance of Prenatal Diagnosis of Critical Congenital Heart Disease: Toward 100% Detection in All Regions. Can J Cardiol 2020;36:1564-5. [Crossref] [PubMed]
- Zhang X, He S, Liu Y, et al. The significance of an integrated management mode of prenatal diagnosis-postnatal treatment for critical congenital heart disease in newborns. Cardiovasc Diagn Ther 2021;11:447-56. [Crossref] [PubMed]
- Plana MN, Zamora J, Suresh G, et al. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev 2018;3:CD011912. [Crossref] [PubMed]
- Stallings EB, Isenburg JL, Aggarwal D, et al. Prevalence of critical congenital heart defects and selected co-occurring congenital anomalies, 2014-2018: A U.S. population-based study. Birth Defects Res 2022;114:45-56. [Crossref] [PubMed]
- Bhat AH, Sahn DJ. Fetal Cardiovascular Imaging: A Disease-Based Approach. Circulation 2012;125:e1025.
- Chu PY, Li JS, Kosinski AS, et al. Congenital Heart Disease in Premature Infants 25-32 Week’' Gestational Age. J Pediatr 2017;181:37-41.e1. [Crossref] [PubMed]
- Lytzen R, Vejlstrup N, Bjerre J, et al. Mortality and morbidity of major congenital heart disease related to general prenatal screening for malformations. Int J Cardiol 2019;290:93-9. [Crossref] [PubMed]
- Mukerji A, Shafey A, Jain A, et al. Pulse oximetry screening for critical congenital heart defects in Ontario, Canada: a cost-effectiveness analysis. Can J Public Health 2020;111:804-11. [Crossref] [PubMed]
- Narayen IC, Te Pas AB, Blom NA, et al. Cost-effectiveness analysis of pulse oximetry screening for critical congenital heart defects following homebirth and early discharge. Eur J Pediatr 2019;178:97-103. [Crossref] [PubMed]
- Qu Y, Wen S, Liu X, et al. Perinatal and early postnatal outcomes for fetuses with prenatally diagnosed d-transposition of the great arteries: a prospective cohort study assessing the effect of standardised prenatal consultation. Cardiol Young 2018;28:66-75. [Crossref] [PubMed]
- Hancock HS, Romano JC, Armstrong A, et al. Single Ventricle and Total Anomalous Pulmonary Venous Connection: Implications of Prenatal Diagnosis. World J Pediatr Congenit Heart Surg 2018;9:434-9. [Crossref] [PubMed]
- Wright LK, Ehrlich A, Stauffer N, et al. Relation of prenatal diagnosis with one-year survival rate for infants with congenital heart disease. Am J Cardiol 2014;113:1041-4. [Crossref] [PubMed]
- Quartermain MD, Hill KD, Goldberg DJ, et al. Prenatal Diagnosis Influences Preoperative Status in Neonates with Congenital Heart Disease: An Analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Pediatr Cardiol 2019;40:489-96. [Crossref] [PubMed]
- Forbess JM, Cook N, Roth SJ, et al. Ten-year institutional experience with palliative surgery for hypoplastic left heart syndrome. Risk factors related to stage I mortality. Circulation 1995;92:II262-6. [Crossref] [PubMed]
- Holland BJ, Myers JA, Woods CR Jr. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol 2015;45:631-8. [Crossref] [PubMed]
- Morris SA, Ethen MK, Penny DJ, et al. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation 2014;129:285-92. [Crossref] [PubMed]
- Cuneo BF, Curran LF, Davis N, et al. Trends in prenatal diagnosis of critical cardiac defects in an integrated obstetric and pediatric cardiac imaging center. J Perinatol 2004;24:674-8. [Crossref] [PubMed]
- Chakraborty A, Gorla SR, Swaminathan S. Impact of prenatal diagnosis of complex congenital heart disease on neonatal and infant morbidity and mortality. Prenat Diagn 2018;38:958-63. [Crossref] [PubMed]
- Escobar-Diaz MC, Freud LR, Bueno A, et al. Prenatal diagnosis of transposition of the great arteries over a 20-year period: improved but imperfect. Ultrasound Obstet Gynecol 2015;45:678-82. [Crossref] [PubMed]
- Özer Bekmez B, Alyamaç Dizdar E, Okur N, et al. Does prenatal diagnosis of critical congenital heart diseases influence the prereferral mortality in a center without surgical intervention? J Matern Fetal Neonatal Med 2019;32:3431-4. [Crossref] [PubMed]
- Zhang W, Xu HY, Zhang YC, et al. Delayed diagnosis of critical congenital heart defects predicting risk factors and survival rate in newborns in Beijing: a retrospective study. J Int Med Res 2021;49:3000605211028028. [Crossref] [PubMed]
- Li YF, Zhou KY, Fang J, et al. Efficacy of prenatal diagnosis of major congenital heart disease on perinatal management and perioperative mortality: a meta-analysis. World J Pediatr 2016;12:298-307. [Crossref] [PubMed]
- Nagata H, Glick L, Lougheed J, et al. Prenatal Diagnosis of Transposition of the Great Arteries Reduces Postnatal Mortality: A Population-Based Study. Can J Cardiol 2020;36:1592-7. [Crossref] [PubMed]
- Copel JA, Tan AS, Kleinman CS. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? Ultrasound Obstet Gynecol 1997;10:237-41. [Crossref] [PubMed]
- Chu C, Yan Y, Ren Y, et al. Prenatal diagnosis of congenital heart diseases by fetal echocardiography in second trimester: a Chinese multicenter study. Acta Obstet Gynecol Scand 2017;96:454-63. [Crossref] [PubMed]
- Zhang X, Sun Y, Zhu J, et al. Epidemiology, prenatal diagnosis, and neonatal outcomes of congenital heart defects in eastern China: a hospital-based multicenter study. BMC Pediatr 2020;20:416. [Crossref] [PubMed]
- Khoshnood B, Lelong N, Houyel L, et al. Impact of prenatal diagnosis on survival of newborns with four congenital heart defects: a prospective, population-based cohort study in France (the EPICARD Study). BMJ Open 2017;7:e018285. [Crossref] [PubMed]
- Steurer MA, Baer RJ, Keller RL, et al. Gestational Age and Outcomes in Critical Congenital Heart Disease. Pediatrics 2017;140:e20170999. [Crossref] [PubMed]



