
Choosing the right treatment for the right lesion, part I: a narrative review of the role of plain balloon angioplasty in dialysis access maintenance
Introduction
The majority of the nearly 4 million people with end-stage renal disease (ESRD) receiving renal replacement therapy worldwide do so via hemodialysis (HD) (1). The 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines emphasized an individualized approach to HD access options as part of a patient’s ESRD life plan, encouraging “the right access, in the right patient, at the right time, for the right reason” (2). Under this recommendation, the majority of patients remain best served by an arteriovenous fistula (AVF), followed by an arteriovenous graft (AVG), both of which are preferred over a dialysis catheter. Greater than 80% of Americans undergo HD via these two options; 65% with a fistula and 17% with a graft (3).
AVFs and AVGs are prone to dysfunction, most commonly due neointimal hyperplasia (NIH) and subsequent stenosis. Approximately 30% of AVFs and 50% of AVGs will require an intervention within 6 months of HD initiation to maintain patency (4-7). Percutaneous transluminal angioplasty (PTA) using “plain” balloons is the first-line treatment for clinically-significant stenosis (2). While initial success rates are excellent, results after PTA are often not durable. Restenosis requiring repeat intervention is the norm rather than the exception, evidenced by one-year post-angioplasty primary patency rates ranging from 40–60% for AVFs and 20–40% for AVGs (8-16). Efforts to improve outcomes have included PTA technique modifications, device innovations such as ultra-high pressure and cutting balloons, and use of covered stents (17-26). While some have proven effective for specific lesions and in particular scenarios, none have proven to be the “magic bullet” in the treatment of AV access stenosis. Recent research has focused on drug-coated balloons (DCBs), which exploit the antiproliferative effect of paclitaxel on vascular smooth muscle cells in hopes of preventing, or at least delaying, restenosis. While evidence supporting DCBs has grown, heterogeneity in terms of trial design, inclusion criteria, and outcome measures have tempered their implementation in daily practice.
If there is anything the multitude of research regarding arteriovenous (AV) access stenosis has demonstrated, it is that each stenosis is unique in terms of its underlying pathophysiology leading to stenosis development and its response to angioplasty (7,27). Treatment strategies should reflect this and, as the 2019 KDOQI recommends, an individualized approach to treatment of clinically-significant AV access stenosis, including device choice and technique, should be considered in accordance with a patient’s ESRD life plan with the goal of maximizing immediate clinical outcomes while preventing restenosis and reintervention for as long as possible (2). This is especially true if one strives to meet the goals of the 2019 KDOQI, which included a target of ≤3 interventions/year in order to maintain an AV access before considering alternative options (2). This two-part review seeks to provide the reader with (I) a succinct yet comprehensive overview of the mechanisms of AV access stenosis, the role of high-quality plain balloon angioplasty in their treatment, and considerations for specific lesions and (II) the emerging evidence regarding DCBs, with a focus on the multitude of randomized controlled trials (RCTs) published in recent years. With this knowledge, we hope to provide the reader with the knowledge and evidence-basis to perform the right treatment for the right lesion. We present the following article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-375/rc).
Methods
Part one of this review focuses on the underlying pathophysiology of HD access stenosis and its treatment within the mature AVF and AVG using plain balloon angioplasty. Given the extent of this topic, a narrative rather than systematic review of the literature was performed. A literature search was performed using the PubMed and EMBASE databases, from 1980 to 2022, to identify all relevant studies. A combination of search terms included: dialysis, hemodialysis, fistula, graft, angioplasty, PTA, and stenosis (Table 1). Studies focusing on the treatment of central venous stenosis or non-maturing fistulas were not specifically included. Additional studies were identified manually. Articles reflecting the highest available level of evidence regarding stenosis pathophysiology, angioplasty techniques, and approaches to treating different types of lesions within AVFs and AVGs were included.
Table 1
| Items | Specification |
|---|---|
| Date of search | 5/15/22 |
| Databases and other sources searched | EMBASE, PubMed |
| Search terms used | Dialysis, hemodialysis, fistula, graft, angioplasty, PTA, stenosis |
| Timeframe | 1980–2022 |
| Inclusion and exclusion criteria | English language literature only |
| Selection process | Literature review performed by DMD and SOT |
PTA, percutaneous transluminal angioplasty.
The pathophysiology of AV access stenosis
Stenosis development in AVFs and AVGs is a result of the same primary process: venous NIH, defined as a fibro-muscular thickening of the vessel lumen. NIH pathogenesis is commonly described as a series of upstream events, which cause vascular damage, and downstream events, representing the subsequent biologic response, as demonstrated in Figure 1 (28-32). These events result in vascular smooth muscle cell, fibroblast, and myofibroblast proliferation and extracellular matrix deposition within the subintima, termed NIH, resulting in progressive luminal narrowing. This narrowing leads to increased hemodynamic changes, propagating further NIH, and eventually leading to a clinically significant stenosis (29,33).
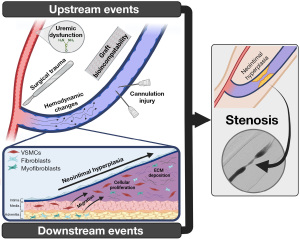
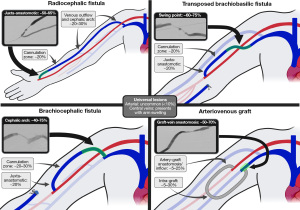
The above events do not occur uniformly across AVFs and AVGs, nor do they occur uniformly within a single access. Stenoses are known to occur at specific sites, dependent upon the particular hemodynamics and upstream/downstream events at play (29). For example, surgical manipulation of the vein during AVF creation may play a larger role in juxta-anastomotic stenosis while repeat cannulation injury plays a larger role in intra-access (cannulation zone) lesions. The patterns of stenosis within common access configurations are well described, and summarized in Figure 2. In brief, juxta-anastomotic lesions are the most common lesion of radiocephalic fistulas, while cephalic arch stenoses are most common in brachiocephalic fistulas. Swing point stenoses are unique to transposed fistulas (34). AVGs are characterized by stenosis at the graft-vein anastomosis (49). Characteristically different types of stenoses may exist as well, with varying degrees of NIH, fibrosis, and vascular constriction (7,50).
Mechanisms of plain balloon angioplasty and restenosis
Once NIH progresses to the critical point of becoming a clinically significant stenosis resulting in AV access dysfunction, current guidelines recommend plain balloon angioplasty as first-line therapy (2). Angioplasty is a purely mechanical treatment, forcefully dilating the stenotic vessel lumen and disrupting the neointimal and medial tissue layers, with the goal of promoting outward remodeling (32,51-53). Modern angioplasty has technical success rates in the 90–95% range (20,54-56). However, angioplasty has been hypothesized to result in extensive barotrauma and vascular injury related to tearing and dissection of the vessel layers, de-differentiation of vascular smooth muscle cells from a contractile to a proliferative, secretory phenotype, and fibroblast activation with release of growth factors and cytokines (51,57-59). In doing so, angioplasty-induced injury may represent an additional, and potentially more potent, event in the pathogenesis of NIH, resulting in high rates of restenosis (30,32). Angioplasty-induced injury may not only activate, but accelerate the cascade of downstream events leading to continued NIH and restenosis. This phenomenon was described by Chang et al., in their study demonstrating markedly increased cellular proliferation within the intima and media of early restenotic lesions compared to primary stenotic lesions. They concluded that, after development of an initial stenotic lesion, the most important factor predisposing to restenosis was angioplasty-induced vascular injury occurring as part of treatment (5). This cycle of stenosis, angioplasty-induced vascular injury, and restenosis is thought to make the need for repeat angioplasty almost inevitable (60). Accelerated rates of lesion recurrence after repeat angioplasty have also been observed in single studies (5,10,32,57). For example, in one study of AVGs undergoing PTA, one-year primary patency rates were 40% after initial angioplasty, 25% after second angioplasty, and 0% after third angioplasty (10). Efforts to prevent the above include pairing angioplasty with a therapy that inhibits NIH and restenosis. This is the rationale behind DCB angioplasty, covered in part 2 of this review.
Monitoring for stenosis and knowing when to treat
Given the propensity of angioplasty-induced injury to accelerate NIH, the question of whether a stenotic lesion should be treated or left alone must be answered prior to determining a treatment strategy, as it is of utmost importance that only stenotic lesions with an appropriately matched clinical indicator undergo PTA (2,61). These clinical indicators are identified either on physical exam or as part of recommended routine AV access monitoring, with the goal of detecting and treating clinically significant stenosis early in their presentation, prior to it resulting in access thrombosis, missed dialysis sessions, and/or catheter placement.
The presence of a clinical indicator should prompt referral for angiography and possible intervention. From an imaging perspective, a hemodynamically-significant stenosis is defined as a >50% reduction in vessel diameter compared to normal adjacent segments. This corresponds to an approximate 75–80% reduction in cross-sectional area (19,62). A clinically-significant stenosis requiring treatment is one that meets the above imaging criteria and has a matching clinical indicator (2). Stenosis identified on angiography without a matching clinical indicator should not be treated, as numerous studies have shown no benefit in treating these clinically silent lesions preemptively (2,63-65). Such prudent use of angioplasty avoids starting the cycle of angioplasty-induced injury, NIH, and restenosis unnecessarily, and ensures interventionists are not performing unnecessary interventions that would potentially do more harm than good. Clinical indicators and their matching lesion locations are summarized in Figure 3. Readers are referred to the articles by Salman and Beathard for review of physical examination of AV access (66,67).

Plain balloon angioplasty: technical factors
The equipment and technique of balloon angioplasty was developed Andreas Grüntzig in 1974 and first described in the treatment of AV access stenosis by Gordon et al. in 1982 (68,69). In subsequent years, numerous studies demonstrated that outcomes of transluminal angioplasty versus surgical revision for the treatment of AV access stenosis were comparable (8,70-74). Specifically, assisted primary patency rates after angioplasty were equivalent to surgical revision, noting a higher stenosis recurrence rate after angioplasty requiring repeat PTA to maintain access patency. However, given the ease at which a patient could undergo repeat angioplasty, and the added benefits of being less invasive, avoiding hospitalization, having a lower complication rate, and improving venous preservation, while still allowing for future surgical revision if needed, angioplasty would become the first-line therapy for treatment of AV access stenosis (2). Numerous equipment and technique improvements have sought to improve angioplasty outcomes. Knowledge of these technical factors may help one deliver the best angioplasty treatment and, in turn, best outcome for their patients. A general algorithm is provided in Figure 4, noting this should be viewed in the context of lesion-specific considerations, described later on.

Measurements of angioplasty success
There are several nuances to defining “success” in the treatment of AV access stenosis and there are different mechanisms of technical failure. Technical or anatomic success is defined as effacement of the stenotic lesion during angioplasty with <30% residual stenosis diameter on post-angioplasty angiography in the treated segment (16). Clinical success refers to the resumption of normal HD (16). The restoration of a thrill within the access has been shown to be the best indicator of clinical success and may be used as a technical endpoint during interventions (75). Hemodynamic success refers to the return of the clinical/physiologic indicator used to detect the stenosis to baseline or to within acceptable limits (2,16). Procedural success encompasses technical success plus an indicator of clinical or hemodynamic success (16).
Various national guidelines have suggested goals and thresholds for expected patency rates after angioplasty. While the 2006 KDOQI suggested a goal of 50% 6-month primary patency after angioplasty for both AVFs and AVGs, the 2019 KDOQI offers no such suggestion (2,76). Rather, a target of ≤3 interventions to maintain AV access per year was recommended (2). The 2016 Society of Interventional Radiology (SIR) quality improvement guidelines suggested thresholds of 30–50% 6-month primary patency for AVFs after angioplasty, dependent upon the balloon type used, and a threshold of 15% for AVGs, noting average 6-month rates of 40–60% for AVFs and 20% for AVGs (16).
There are two main PTA failure modes in AV access: resistant stenosis and elastic stenosis. Resistant stenosis is defined as the inability of angioplasty to completely efface the balloon waist, and is seen as a band-like waist in the balloon at the level of the resistant lesion (77). It is thought to be the result of dense fibrous strands within the neointima or scar tissue. This differs from elastic stenosis, in which angioplasty results in complete effacement of the balloon waist, however, the stenosis immediately recurs on post-angioplasty angiography, possibly related to elastic fibers within the vessel layers. The different ways in which resistant and elastic stenoses are treated are discussed in later sections and an algorithm is provided in Figure 4, based on the below evidence.
Balloon types and dilation pressures
AV access stenoses have been known to be difficult to dilate since the first use of Grüntzig’s polyvinyl chloride balloon in 1982, at which time many lesions were seen to be resistant to balloon dilation (69). This was also true for subsequently-developed polyethylene balloons, which boasted only a 67% technical success rate (78). The first “high-pressure” balloon, intermediate-pressure by today’s standards and capable of 10–12 atmospheres (atm) of pressure, was the Olbert balloon, achieving improved technical success rates of ~90% (78,79). Subsequent high pressure balloons (HPBs) were rated to burst pressures of ~20 atm and could achieve pressures up to 27 atm with off-label over-inflation. While not specifically defined, most authors agree that 20 atm of pressure delineates HPB angioplasty (<20 atm) from ultra-high pressure balloon (UHPB) angioplasty (>20 atm) (55,56). UHPBs are capable of producing up to 30–40 atm of pressure (56).
Studies of modern HPBs and UHPBs by Trerotola et al., Rajan et al., and Vesely et al. found that 8%, 13%, and 34% of stenoses required inflation pressures >20 atm for successful dilation, respectively, with 3–8% of lesions requiring inflation pressures >30 atm (20,54-56). The mean pressure to successfully efface the balloon waist in any stenosis was on the order of 15–17 atm (54,56). UHPB angioplasty was required more often in AVFs compared to AVGs (20% vs. 9%, P=0.02) (54). Overall, 96–100% of stenoses were successfully treated with HPBs and/or UHPBs (20,54-56). It has been postulated that UHPBs cause additional barotrauma compared to HBPs, potentially causing increased NIH and restenosis after their use, however, in their direct comparison of HBPs and UHBPs, Rajan et al. found no difference in primary patency rates to suggest this occurs (55).
Overall, HPBs are successful in treating the majority of stenotic lesions encountered in AVFs and AVGs, while UHPBs have been found to be successful in treating nearly all stenoses. There are no downsides of UHPBs compared to HPBs from a technical perspective. Given findings suggesting an UHPB may be required more often in the treatment of stenosis in AVFs compared to AVGs, one may consider initial use of an UHPB when treating AVF stenosis, as is the authors practice (54). Alternatively, one can consider angioplasty with a HPB, followed by an UHPB only when necessary. The cost effectiveness of this decision will differ from institution to institution. Either option will allow the interventionalist to achieve technical success in the treatment of the large majority of lesions. UHPB angioplasty is the treatment of choice for resistant stenotic lesions (Figure 4). It is important to note that use of HPB and UHPB angioplasty does not mean putting more pressure in the balloon than needed to efface a waist—i.e., the balloon does not always need to be brought to its maximum pressure. Rather, only the pressure needed to efface the waist is required.
Alternative options: cutting balloons
Given the high success rates seen with the UHPBs in the treatment of resistant stenoses, the role of alternative devices and techniques, including atherectomy devices, the “infiltrate and perforate” technique, parallel wire technique, and cutting balloons, is small. While such methods can be tried in the few stenoses resistant to UHBPs, such lesions should also be considered for surgical revision (79,80).
Of the above, cutting balloons warrant specific discussion. Originally developed for coronary angioplasty, cutting balloons have multiple longitudinally-mounted blades along the balloon exterior that create microsurgical incisions in the vascular wall upon balloon inflation (81). These blades score the stenotic lesion and allow for technically successful dilation of a stenosis at a lower pressure than HPBs and UHPBs. By disrupting the neointimal layer in a more controlled fashion and with less required outward force, it was theorized that cutting balloons would minimize angioplasty-induced vascular injury compared to HBPs and UHPBs, thereby decreasing restenosis and improving patency rates (21).
This has largely been shown not to be the case. While some studies have suggested cutting balloons may result in higher primary patency rates in the treatment of graft-vein anastomotic stenoses in AVGs (noting evidence now supports the use of stent-grafts over PTA for such lesions), no difference in patency rates have been found in the treatment of other lesions in AVGs or AVFs (24-26,82,83). Multiple randomized trials have been performed comparing cutting balloons and high-pressure balloons in the treat of de novo lesions in AVFs and AVGs. In their single-center RCT, Rasuli et al. demonstrated no difference in outcomes between cutting balloons and HPBs in the treatment of de novo stenoses in native AVFs, with 6- and 12-month primary patency rates for the cutting balloon group of 28% and 11% compared to 42% and 26% in the HPB group (P>0.3 in both instances) (84). Vesely and Siegel performed a multi-center RCT comparing cutting balloons and plain balloons in AVGs which also demonstrated no difference in outcomes, with 6-month primary target lesion patency rates of 48% for cutting balloons and 41% for plain balloons (P=0.37) (21). Additional non-randomized studies have yielded similar conclusions to the above RCTs (85,86). Given the similar outcomes between cutting balloons and HPBs, and the additional costs and potential increased complication rates, the use of cutting balloons in lieu of HPBs is not supported by available literature (21).
While cutting balloon use is not supported in treatment of untreated, de novo stenoses, there may be a very limited role for cutting balloons in the treatment of resistant stenoses. One must bear in mind that most studies demonstrating this were performed utilizing conventional angioplasty at moderate-high pressures, and if one is initially treating lesions with modern HPBs and in particular UHPBs (including off label inflation in excess of rated pressure), the number of resistant lesions will be exceedingly small. In their RCT comparing cutting balloons and plain balloons in the treatment of stenoses resistant to PTA up to 15 atm of pressure, Aftab et al. demonstrated 100% technical success in both arms, with improved patency rates in the cutting balloon group compared to the HPB group (77). Notably, this study did not allow oversizing of plain balloons or inflation beyond rated burst pressures and follow-up was variable. Additional non-randomized trials and retrospective studies have also demonstrated a potential role for cutting balloons for lesions resistant to angioplasty between 20–24 atm of pressure, however, none describe the use of cutting balloons in stenoses resistant to initial UHPB angioplasty >24 atm, as is easily achievable with modern UHPBs (82,87,88). This may be because the number of such lesions is small. Overall, the use of a cutting balloon in any situation must be balanced with the increased cost of these devices, up to ×4 that of plain balloons, and with potential for increased complications, again noting nearly all resistant lesions will be able to be effaced with use of an UHPB (in excess of 40 atm) (21,89).
Balloon sizing
Balloons come in a variety of diameters and lengths, chosen at the operator’s discretion. When treating stenoses in AVGs, a balloon 1 mm larger in diameter than the graft is typically chosen as the initial balloon. For AVFs, balloons are typically oversized by ~10–20% compared to the adjacent segment of normal vein. This usually corresponds to a balloon 1–2 mm larger than the adjacent normal vein, typically in the range of 7–10 mm (21,55,56,90,91). One exception to the above is treatment of arterial anastomotic lesions, in which a smaller balloon is typically chosen due to the smaller caliber of the artery and adjacent vein at this location, and to not over-dilate the surgical anastomosis (35,91). Balloons may be progressively upsized as needed when elastic stenoses are encountered. Balloon length is dependent upon the length of the stenotic lesion, with the goal of treating the entire lesion while limiting angioplasty to the smallest amount of normal vessel as possible, as to reduce unnecessary angioplasty-induced vascular injury in normal segments.
Balloon inflation times and elastic stenosis
Balloon inflation times are most often considered in the setting of residual or elastic stenosis, as longer inflation times have been associated with less residual stenosis and prolonged PTA is often used to treat elastic stenoses (56). Two studies have directly examined balloon inflation times. Forauer et al. performed a randomized trial of 1-minute versus 3-minute inflation durations, with technical success of 75% in the 1-minute group and 89% in the 3-minute group (P=0.12). After multivariate analysis, controlling for patient age, sex, and access age, this was found to be statistically-significant, with odds ratio 4.7 and P=0.03 (90). Despite differences in technical success, 1-, 3-, and 6-month primary patency did not differ between groups. One limitation of this study was that PTA was performed at 18 atm, with 60% of technical failures in the 1-minute group and 50% in the 3-minute group due to resistant, rather than elastic, stenoses. Balloon inflation time is not known to have any effect upon resistant stenoses (which require higher pressure rather than prolonged inflation), however, this was not accounted for in the analysis or determination of sample size. Elramah et al. prospectively collected data in patients undergoing 30-second and 1-minute angioplasty, based on two different operator’s practice patterns, and compared their outcomes (18). Immediate technical success and patency in the first 3 months was similar in both groups, however, there was a greater incidence of access failure in the longer 1-minute inflation group after 3 months [hazard ratio (HR) =1.74; 95% confidence interval (CI): 1.09–2.70]. It was postulated that the prolonged inflation time may have caused additional vascular trauma, inciting more aggressive NIH and restenosis, leading to decreased patency rates after 3 months. While no difference in patient age, gender, race, access type, or access age were observed, the severity of lesions was not accounted for and it is unclear what technical differences may have existed between the two operators. Based on these two studies, the ideal PTA inflation time remains elusive and it is unclear whether inflation time affects outcomes given conflicting data within the two available studies on the matter (2). In addition to prolonged angioplasty, progressive balloon upsizing has also been employed in the treatment of elastic stenosis, with anecdotal success, although without specific data regarding its effectiveness (36,92).
Rajan et al. specifically looked at elastic recoil after PTA in 76 patients and 154 angioplasties who underwent high or ultra-high pressure PTA for a minimum of 45 seconds, with fistulograms obtained at 0, 5, 10, and 15 minutes after treatment to assess for elastic recoil (17). Elastic recoil was observed in 16% of lesions, with the majority (63%) observed after 5 minutes. No additional intervention was performed if elastic recoil was observed. Six-month primary patency rates were no different in those in whom elastic recoil was observed versus those without evidence of recoil. The authors concluded that, while elastic recoil occurs, it does not seem to have an effect on primary access patency. Overall, the significance of elastic recoil on access patency is not certain, but its occurrence does appear to be decreased by prolonged angioplasty. Given the possibility its presence does not affect patency, aggressive pursual of its treatment (i.e., stent-graft placement) may not be necessary if the clinical indicator of dysfunction has normalized and there is adequate flow in the fistula after angioplasty (17). If the elastic lesion is causing persistent access dysfunction, a stent-graft should be considered.
When elastic lesions are encountered, it is the authors practice to perform prolonged PTA (with one or more cycles of 5-minute inflations) with progressive balloon oversizing (in 1 mm increments) prior to considering alternatives such as a stent-graft or surgery (Figure 4).
Plain balloon angioplasty in practice: AVFs
HD vascular access should be thought of as a complete circuit, extending from the heart and arterial system to the (arterial) anastomosis, through the fistula or graft, into the venous outflow, and finally back to the heart (2). Stenoses can occur at any point in the circuit. The first step in the treatment of a dysfunctional access is to identify the most probable location of the culprit lesion, based on patient history, access type, presenting clinical indictor, and physical exam, so that one may develop the most appropriate procedural strategy. This is followed by a well performed fistulogram, extending from the (arterial) anastomosis to the right atrium (93). Treatment strategy, equipment, and outcomes will differ based on the type of access and stenosis location, of which an overview is provided below, organized by lesion location in AVFs and AVGs.
Inflow stenosis: arterial, anastomotic, and juxta-anastomotic lesions
Inflow lesions are a broad category of stenoses with a sometimes confusing nomenclature. Arterial stenoses exist within the native artery. Arterial anastomotic stenoses exist at the exact site of surgical AV connection. Both of these lesions are uncommon compared to juxta-anastomotic lesions, which are located on the venous side of the anastomosis, within a few centimeters of the anastomosis itself, and always before the arterial cannulation zone. The term peri-anastomotic is also commonly used, and refers to stenoses that are within 1 cm of the anastomosis, whether they be arterial, anastomotic, or in the juxta-anastomotic venous segment (37).
Arterial stenosis
Remote arterial stenoses within the brachiocephalic, subclavian, brachial, or forearm arteries, proximal to the AV anastomosis, are infrequently the cause of access dysfunction, representing approximately 1–10% of clinically-significant lesions (12-15,35,38,39). These lesions are typically related to intrinsic atherosclerotic disease, a common co-morbidity in the ESRD population (94,95). While uncommon, they should be suspected and evaluated for in cases where no other lesion is identified, where there is persistent access dysfunction despite successful treatment of stenoses elsewhere in the access, or in cases of recurrent access dysfunction without known cause (35,96). If suspected intra-procedurally, at the time of fistulography, a dedicated upper extremity arteriogram from the aortic arch to the brachial or forearm arteries, depending on the access, with appropriate endovascular treatment of clinically-significant arterial lesions is indicated. If suspected pre-procedurally, the patient can go straight to arteriography or a magnetic resonance angiography (MRA) or computed tomography angiography (CTA) can be considered for diagnosis and treatment planning. The different pathophysiology of atherosclerotic arterial lesions compared to the neointimal lesions seen in AV access result in a more durable response to endovascular treatment, with lower rates of recurrence (40,94).
Arterial anastomotic stenosis
True arterial anastomotic lesions are less common than juxta-anastomotic lesions, and may represent approximately 10–20% of stenotic lesions encountered in AVFs (35,39,91,97). Such lesions may result from a combination of technical flaws in the surgical creation of the AV connection and NIH (37). Multiple studies have demonstrated that that anastomotic lesions have a high rate of recurrence after angioplasty. Long et al. found the location of stenosis at the anastomosis to be an independent risk factor for recurrence, while Manninen et al. similarly found lesions at this location to be predictive of poor long-term patency (37,41). Anastomotic stenoses that are recurrent and difficult to treat endovascularly may be considered for surgical revision (37).
Juxta-anastomotic stenosis
Juxta-anastomotic stenoses (JAS) are the culprit lesion in approximately 50–60% of dysfunctional radiocephalic fistula, by far the most common lesion in this access (12,14,34,98). They are less common in upper arm accesses, representing ~20% of stenoses in dysfunctional upper arm fistulas (34). It has been theorized that the juxta-anastomotic region is prone to NIH due to the specific hemodynamics at play in this segment, such as low shear stress and increased turbulence, while others suggest skeletonization, mobilization, and manipulation of the juxta-anastomotic segment during AV access creation make it more prone to stenosis development (14,34,35,99,100). The majority of research regarding JAS has focused on radiocephalic fistulas, given their high prevalence in this access configuration. Classically, these lesions were corrected surgically, either by creation of a more central AV anastomosis, a vein-to-vein re-anastomosis, or using a vein patch or interposition graft. Such interventions are performed at the expense of venous reserve, as the anastomosis is relocated more centrally along the vein (12,37,71,101). Numerous studies have compared PTA and surgery in the treatment of JAS. While surgery confers a higher primary patency rate, with stenosis recurrence rates up to 3 times less in those undergoing surgery compared to angioplasty, assisted primary patency rates are comparable between the two treatment methods (37,71,102-104). In their non-randomized prospective trial, Tessitore et al. followed 21 surgically-treated and 43 angioplasty-treated JAS with a median follow-up of 24 months, with no difference in failure rates at 0.11 events/AVF-year for surgery and 0.10 events/AVF-year for PTA (P=0.736) (71). Their study also found similar cost profiles between the two treatment methods, accounting for cost of repeat PTA for recurrent stenoses (71). In a retrospective review of 147 JAS interventions on 75 radiocephalic fistulas, Mortamais et al. demonstrated primary patency rates of 47% at 1 year and 26% at 3 years, with assisted primary patency rates of 81% at 1 year and 63% at 3 years. The presence of greater than 50% residual stenosis after first angioplasty was the only variable found to confer a worse outcome after PTA compared to surgery, with a relative risk of thrombosis or need for surgical revision of 2.9 (95% CI: 1.1–8.1) compared to those with less than 50% residual stenosis (105). Napoli et al. described their experience treating patients with PTA initially, with successful surgical revision after first or second repeat PTA, concluding it is best to perform PTA before surgical revision, and reserving surgery for those in whom PTA has failed (104). These studies, and others, highlight the ability of angioplasty to maintain a functional fistula over an extended period of time with close clinical monitoring and repeat angioplasty as needed. However, it remains important to recognize when an access may benefit from surgical revision (106). Alternatives to angioplasty and surgery, such as stent-graft placement, have been infrequently considered in the treatment of JAS as their use is limited due to the proximity of these lesions to the cannulation zone, with stented segments preferred not to be used for cannulation (105).
Decision making in inflow lesion treatment
While inflow lesions with matching clinical indicators warrant treatment, a more nuanced approach must be taken in the treatment of these lesions when there are additional intra-access or outflow lesions in the presence of an overlapping clinical indicator (i.e., poor flow), or when an inflow lesion is the only lesion identified on fistulography performed for a clinical indictor inconsistent with an inflow lesion (i.e., pulsatility). Intra-procedural catheter-based flow measurements have proven useful in determining whether an inflow stenosis is hemodynamically significant and requires treatment. Leontiev et al. demonstrated that the most common reason to use intra-procedural flow measurement was to determine whether to treat an inflow stenosis, and that the use of flow in the aforementioned clinical scenarios led to a decision to treat in 16% of patients, while a decision to leave the lesion alone was made in the remaining 84%, as flow was found to be adequate (91). An additional study by Leontiev et al. retrospectively reviewed patients in whom asymptomatic inflow lesions were either treated or not treated in patients with outflow clinical indicators who underwent treatment of intra-access and outflow stenoses. There was no significant difference in access patency in those who underwent PTA of the inflow lesion compared to those who did not, indicating PTA of asymptomatic inflow lesions without an appropriate clinical indicator does not improve outcomes (107). Given the potential for angioplasty to initiate a cycle of angioplasty-induced NIH and stenosis, the use of flow and clinical indicators to decide whether to treat an inflow stenosis is critically important in cases where there is no clear indication for treatment.
Cannulation zone stenosis
The cannulation or “puncture” zone exists between the juxta-anastomotic inflow and venous outflow, and is defined by the sites of arterial and venous needle placement. Despite representing up to 20–30% of stenoses identified in dysfunctional forearm and upper arm fistulas, there is little literature regarding treatment of these lesions (12,39). PTA outcomes for cannulation zone lesions may be considered within the range of overall AVF angioplasty outcomes discussed in earlier sections (8,9,12-16). One consideration in the treatment of these lesions are the options that exist when PTA fails, either due to elastic stenosis or frequent stenosis recurrence. In other areas, one may consider stent-graft placement, however, the metal interstices of stent-grafts carry risks with frequent needling, including stent fracture, pseudoaneurysm formation, and, primarily, stent-graft infection (108). While their use in the cannulation zone has been reported, such use is off-label, and in-stent restenosis is frequently seen, with 6-month primary patency rates of 30–40% (109,110). While stent-graft placement may be used as a last resort for access salvage, surgical revision or access abandonment should also be considered (108).
Venous outflow stenosis: cephalic arch and swing point lesions
While juxta-anastomotic lesions are the hallmark of radiocephalic fistulas venous outflow lesions are the characteristic lesions of upper arm brachiocephalic and transposed brachiobasilic fistulas. The prevalence of these difficult to treat lesions are one reason why upper arm fistulas fare worse than those in the forearm (12). Given the above, their treatment has been extensively studied.
Cephalic arch stenosis (CAS)
The cephalic arch represents the terminal portion of the cephalic vein as it traverses the deltopectoral groove and claviculopectoral fascia prior to joining the axillary vein, which it does at a nearly perpendicular angle (42,111). CAS is the culprit lesion in approximately 40–75% of dysfunctional brachiocephalic fistulas, depending on the study, and less commonly seen in dysfunctional radiocephalic fistulas, where it has been found to represent 2–20% of lesions (12,34,43,44,112). Turbulent flow resulting from the perpendicular insertion of the cephalic arch make it prone to NIH and stenosis, and the lesion is more common in brachiocephalic fistulas due to the higher flow rates inherent of the brachial compared to the radial artery, with higher access flows demonstrating the greatest association with the development of CAS in a study by Jaberi et al. (113). Additionally, the lack of alternate venous drainage in brachiocephalic fistulas may also result in increased flow, noting the cephalic vein is the singular outflow in this access configuration, while a radiocephalic fistula may have additional outflow via the median cubital vein to the basilic vein and other venous communications (15,34,39). External compressive forces from the surrounding fascia and the high prevalence of venous valves in the terminal cephalic vein, present in greater than 90% of individuals, may also play a role and contribute to the highly resistant and elastic nature of these lesions (17,34,114,115).
CAS are known to be highly recalcitrant, difficult to treat lesions associated with lower technical success rates, lower patency rates, and increased complications, specifically venous rupture. Pooled data in two meta-analyses has demonstrated 6-month primary patency rates of 23–42% and 12-month patency rates of 10–23%, with 12-month assisted primary patency rates of 68–75% (42,111). The largest series, a retrospective study including 106 patients undergoing PTA of CAS in which PTA was primarily performed with high-pressure balloons, demonstrated a technical success rate of 70%, and need for frequent reintervention, with secondary intervention rates of 3.5 per person-year (116). Venous rupture as a complication of PTA has been reported in ~6% of cases, higher than most lesions, but still less than the rupture rate seen in swing point lesions of transposed fistulas (44,117). In the only dedicated study of cutting balloons in CAS, Heerwagen et al. (118) demonstrated 6- and 12-month primary patency rates of 81% and 22%, respectively, and assisted primary patency rates of 63% at 12 months, concluding that cutting balloons did not improve patency rates compared to conventional PTA results in the literature, as the study had no conventional PTA arm for comparison. It was suggested that cutting balloons required fewer interventions per-patient year (0.9 compared to 1.6–3.5 in the literature).
While patency rates of cephalic arch stenoses are modest at best following PTA, it remains first line treatment for these lesions, although alternatives, such as stent-graft placement, surgical alternatives including flow-reduction, outflow relocation, and outflow bypass may be considered, especially in cases of recurrent lesions. Shemesh et al., in their prospective randomized trial comparing stent-grafts versus bare metal stents in the treatment of CAS, demonstrated increased patency rates of stent-grafts compared to bare-metal stents (82% versus 39% at 6 months, 32% versus 0% at one year, P=0.002), resulting in early termination for ethical reasons (108). Rajan et al. performed a small prospective randomized trial comparing VIABAHN stent-graft placement versus balloon angioplasty in CAS, noting it was terminated early due to poor enrollment (n=14), with results in this small cohort demonstrating mean patency intervals of 100 days for PTA and 300 days for stent-grafts, with primary patency rates of 0% at 6 months for PTA and 67% for stent-grafts (P<0.01) (119). Subsequent non-randomized studies demonstrated improved patency rates of stent-grafts, on the order of 75–80% at 6 months and 60–70% at one year, with assisted patency rates up to 80% at 5 years (120,121). While stent-grafts demonstrate superior outcomes compared to PTA, the placement of a stent-graft carries risks of occluding venous collaterals, including jailing the basilic vein for a potential brachiobasilic fistula, stent-graft infection, and in-stent restenosis, and should be considered only after PTA fails to maintain access patency, with a potential threshold of two or more recurrences of the same lesions within 4 months or less (111,120). Stent-grafts may also be considered in the setting of elastic CAS with continued access dysfunction.
Access flow reduction via banding has been described in those with rapid cephalic arch restenosis after PTA and high flows within the fistula (>1,200 mL/min has been described) (43,122). Banding seeks to limit pathologic high flow, turbulence, and associated NIH within the cephalic arch by decreasing the luminal diameter in the juxta-anastomotic segment of the fistula, thereby decreasing inflow, and, subsequently, outflow. Miller et al. originally described the technique in a retrospective review of 33 patients with an average cephalic arch intervention rate of 3.3 per access-year prior to banding. Following banding, the intervention rate was reduced to 0.9 per access-year and 6- and 12-month primary lesion patency rates were 76% and 57%, respectively (122). No subsequent studies have been performed assessing flow reduction for the treatment of these lesions. However, given the low restenosis rates reported in this study, flow reduction may be considered a reasonable alternative to repeat PTA in those with recurrent CAS and high access flow rates, necessitating flow measurement when performing PTA of recurrent CAS (43). Newer algorithms propose measuring flow as the first step of every case when a CAS is encountered so that a decision regarding primary treatment with PTA or flow-reduction can be made (43). Surgical revision is typically reserved for when other options, including PTA +/− stent placement, have failed to produce durable outcomes and when flow is not high enough to consider flow reduction. The most commonly employed surgical technique is outflow relocation via cephalic vein transposition, in which the healthy cephalic vein is incised proximal to the stenosis and re-anastomosed to the upper basilic or axillary vein (43,123,124).
The treatment of CAS is complex and demanding given the recurrent nature of these lesions. A recent white paper by Beathard et al. describes a CAS treatment algorithm for these lesions focusing on an individualized approach and taking into account flow measurement in decision making, to which readers are directed for further information (43).
Swing point stenosis
While CAS is characteristic of brachiocephalic fistulas, “swing point” stenoses are the equivalent characteristic lesion of brachial artery-basilic vein transposition fistulas (BVTs). BVTs are the third preferred option for fistulas, behind forearm and upper arm cephalic fistulas, due to their more technically challenging creation. The basilic vein is located on the medial aspect of the upper arm, next to the brachial artery and median nerve, and deeper than the cephalic vein. If left in its native location, the vein may be difficult to cannulate due to its depth and surrounding structures would be at risk of puncture (45). To address this, the vein is transposed laterally and superficialized to enable easier, safer access. This mobilization can result in a significant angle at the point of transposition, also referred to as the “swing point”, which occurs in the central aspect of the vein, near the axilla, at what will be the distal venous outflow. Note that the term “swing point” has also been applied to juxta-anastomotic lesions by some authors, however as used here it refers only to the outflow segment of a transposed fistula where the transposed and in situ portions meet. Tendency to develop stenosis at this location is multi-factorial, like due to unfavorable hemodynamics at the angle of transposition, surgical trauma and devascularization of the vein during transposition, and external compression from the brachial fascia (46,47).
Swing point stenoses have an incidence of 60–75% in dysfunctional BVTs, and may account for up to 90% of interventions on this access (46,47,125). In their study of 93 brachiobasilic fistulas, Beaulieu et al. reported assisted patency rates of 68% at one year after angioplasty. Swing point stenoses recurred frequently, with approximately 50% of lesions requiring ≥3 repeat angioplasties at a median of 75 days between interventions (47). Other studies have reported transposed fistulas require more interventions to maintain patency than non-transposed fistulas, although this study was not limited to basilic vein transpositions (126). One study has reported on the use of stent grafts for swing point stenoses. Nassar et al. demonstrated a positive impact of stent grafts on both target lesion and access patency in comparison to PTA, with 6- and 12-month pre-stent assisted patency rates of 36% and 10%, respectively, compared to post-stent assisted patency rates of 82% and 75%, respectively (127). While the majority of stents required angioplasty of in-stent stenosis, overall access intervention rates decreased from 0.5/month to 0.15/month after stenting. There have been few reports of surgical revision of swing point stenoses in BVTs, although vein patch angioplasty has been reported (128).
Plain balloon angioplasty: AVGs
AV grafts are the less preferred compared to AVFs for a number of reasons. They are associated with higher morbidity, including increased infection rates, higher rates of steal, and increased risk of symptomatic central venous stenosis, as well as an increased risk of mortality, measured as high as 20% (13,45). Most important in the context of this review, however, is their increased rates of stenosis requiring intervention compared to AVFs (13,45). In addition to the pathophysiologic mechanisms that lead to NIH and stenosis in AVFs, which also apply to grafts, additional factors may contribute stenosis development. Primarily, the polytetrafluoroethylene (PTFE) grafts used in AVGs have been shown to attract macrophages, which line the graft material and perigraft region. These macrophages and their associated cytokine expression may result in more aggressive NIH (4). AVGs have one-year PTA-assisted primary patency rates of 20–40% and lesions often recur quickly (9,11-13,16). The characteristic stenotic lesion of the AVG is seen at the graft-native vein anastomosis.
Stenosis of the native artery-graft anastomosis
Correct identification of an arterial anastomotic lesion in a graft can be difficult, as there is an inherent size mismatch between a native artery, which is typically 3–5 mm in diameter, and the fixed caliber of the PTFE graft (usually 4–6 mm) (96). Intra-access flow measurement is useful to determine whether an apparent stenosis in this location is hemodynamically significant and requires treatment (88,107,129). Arterial anastomotic lesions in grafts were historically thought to be uncommon, reported in <5% of dysfunctional AVGs (10,13). A subsequent retrospective review by Khan and Vesely found arterial anastomotic lesions in nearly 30% of patients with AVGs, however, this was in a group of patients who were informally selected for arteriography based on factors such as diabetes, hypertension, and age, in addition to poor access blood flow identified during access monitoring (96). In a prospective study by Asif et al. in which 122 consecutive patients with dysfunctional grafts underwent evaluation of the arterial inflow, 24% were found to have arterial anastomotic stenoses (35). All arterial lesions were associated with concurrent venous stenoses, implying these stenoses are rarely seen in isolation. No studies have specifically evaluated the effectiveness of PTA solely at this location, and outcomes may be considered within the range of those in pooled studies.
Intragraft stenosis
Stenoses that develop within a PTFE graft, remote from anastomoses, are thought to develop via a slightly different pathophysiologic mechanism. Rather than the typical NIH, intragraft lesions may be related to perigraft scar formation and ingrowth or “invasion” of fibroblastic tissue through needle tracts in the graft material at cannulation sites (130,131). The reported incidence of these lesions in dysfunctional grafts ranges from 2–28% (8,10). In the largest study of intragraft stenoses, Bautista et al. described angioplasty outcomes in 229 lesions in 183 grafts. Angioplasty was technically successful in 85% of cases, with elastic recoil of the stenotic lesion seen in all cases of failure. These were all subsequently treated with a stent or stent-graft. Primary patency rates at 6- and 12-month were 40% and 23% and assisted primary patency rates were 77% and 67%, respectively. Graft thrombosis and concurrent lesions elsewhere in the access (present in 76% of patients) were negative predictors of patency, while stent or stent-graft deployment was a positive predictor of patency. As implied, when an elastic stenosis is encountered and PTA fails, placement of an intragraft stent or stent-graft is an option, however, this must be done with caution, for reasons discussed earlier including risk of stent-graft infection, stent fracture, and difficulty with cannulation (132-134).
Stenosis of the graft-native vein anastomosis
Graft-native vein anastomotic stenosis is the characteristic lesions of AV grafts, accounting for 45–85% of lesions resulting in graft dysfunction (10,12,19,34,48). These lesions are notoriously difficult to treat (55). As a result, extensive efforts have been made to improve treatment outcomes. These efforts have most recently focused on the use of stent-grafts, and the graft-vein anastomosis is one of the few locations in which a stent-graft may be considered as a primary treatment, supplanting PTA as first line therapy in amenable lesions (24).
Whether treating a lesion with angioplasty alone or a stent-graft, a balloon must be used that is capable of overcoming these highly resistant lesions. Studies have demonstrated that between 9–13% of stenoses at this location require an inflation pressure >20 atm to efface the waist of these lesions (55,56). Routine use of an UHP balloon should therefore be considered. As mentioned earlier, the use of UHP has been theorized to cause increased vascular damage, potentially exacerbating the intimal hyperplasia response and leading to increased lesion recurrence. However, in their comparison of high pressure and ultrahigh pressure angioplasty of graft-vein anastomotic lesions, Rajan et al. demonstrated no difference in patency rates between the two groups (median 4.6 months for UHP, 5.4 months for HP), supporting the use of UHP balloons based on the improved probability of technical success (55).
Graft-vein anastomotic lesions are one of the few areas where cutting balloons have demonstrated increased patency rates compared to plain balloon angioplasty, although not as durable as stent-grafts. In their comparison of cutting and plain balloon angioplasty in different types of AV access stenosis, Kariya et al. found that primary patency rates for graft-vein anastomotic lesions were significantly higher when a cutting balloon was used, with 6- and 12-month patency rates of 71% and 57% for cutting balloons, and 38% and 7% for plain balloons (P=0.04) (82). However, other studies, including randomized trials, demonstrated no benefit to use of cutting balloons for similar lesions (21).
Multiple studies, including large randomized trials, have shown stent-grafts to have superior outcomes in terms of patency in the treatment graft-vein anastomoses compared to PTA. Stent-grafts prevent elastic recoil and inhibit trans-stent growth of neointimal tissue, thereby decreasing recurrent stenosis caused by NIH, and are thought to provide more in-line laminar flow by converting the end-to-side graft-vein anastomosis into a more end-to-end configuration, thereby decreasing hemodynamic turbulence and other factors that may promote NIH (24). The prospective, multicenter, randomized FLAIR trial demonstrated significantly greater target-lesion and access patency rates at 6 months in the primary treatment of graft-vein anastomotic lesions, with 6-month target-lesions patency rates of 51% in the stent-graft group compared to 23% in the PTA group (P<0.01), as well as decreased need for repeat interventions in the stent-graft group (24). The follow-up 2-year results reported in the RENOVA study demonstrated sustained advantages of stent-grafts compared to PTA for these lesions, with approximately two-fold greater target-lesion and access circuit patency rates at 24 months (25). The REVISE trial demonstrated similar results using the ViaBahn stent-graft, with similar improvements in patency rates at 6 months and increased time from index procedure to next intervention in the stent-graft group compared to the PTA group (26). A 2-year follow-up study demonstrated similar overall treatment costs between those treated with stent-grafts compared to PTA, supporting early use of a stent-graft despite the higher upfront cases of the device compared to PTA (135). Overall, one should consider early, if not initial, use of a stent-graft to treat graft-vein anastomotic lesions, as there is now a large body of literature demonstrating superior outcomes to PTA.
In-stent restenosis
As mentioned throughout this review, there are times in which a stent may be placed to treat elastic stenosis or early/frequent lesion recurrence after PTA. These stents are themselves prone to stenosis, although this risk is decreased when a stent-graft is used. The multi-center, randomized RESCUE trial evaluated the used of PTFE stent-grafts compared to PTA in the treatment of in-stent restenosis. Six-month target lesion patency rates were significantly higher in the stent-graft group (66%) compared to the PTA group (12%) (P<0.01), with improved 6-month access circuit patency rates as well, although overall low in both groups (18% in stent-graft group, 5% in PTA group, P<0.01) (136).
Conclusions
High-quality plain balloon angioplasty, performed utilizing the available evidence-basis regarding technique and considerations for specific lesion locations, is successful in treating the large majority of AV access stenoses. While initially successful, patency rates remain non-durable. Additional interventions, such as stent-graft placement, have proven successful in extending patency rates for certain types of lesions, however, a more broadly applicable solution that can inhibit the post-angioplasty neointimal response and decrease the rate of restenosis is desirable. This is the goal of DCBs, which seek to prolong primary patency rates after successful PTA. The extensive research performed regarding DCBs, including a number of high-quality RCTs, is covered in part 2 of this review.
Acknowledgments
All figures were created with Biorender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee Kirksey and Sasan Partovi) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-375/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-375/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. SOT has received consultant fees from Medcomp, BD, and Cook and royalty fees from Teleflex. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019;34:1803-5. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-164. [Crossref] [PubMed]
- Johansen KL, Chertow GM, Gilbertson DT, et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2022;79:A8-12. [Crossref] [PubMed]
- Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int 2001;59:2325-34. [Crossref] [PubMed]
- Chang CJ, Ko PJ, Hsu LA, et al. Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: implication in prevention of restenosis. Am J Kidney Dis 2004;43:74-84. [Crossref] [PubMed]
- Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol 2013;9:348-57. [Crossref] [PubMed]
- Steiner KA. Pathophysiology of stenosis within AV fistulas and mechanisms of PTA. Endovasc Today 2016;15:28-32.
- Beathard GA. Percutaneous transvenous angioplasty in the treatment of vascular access stenosis. Kidney Int 1992;42:1390-7. [Crossref] [PubMed]
- Turmel-Rodrigues L, Pengloan J, Blanchier D, et al. Insufficient dialysis shunts: improved long-term patency rates with close hemodynamic monitoring, repeated percutaneous balloon angioplasty, and stent placement. Radiology 1993;187:273-8. [Crossref] [PubMed]
- Kanterman RY, Vesely TM, Pilgram TK, et al. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology 1995;195:135-9. [Crossref] [PubMed]
- Safa AA, Valji K, Roberts AC, et al. Detection and treatment of dysfunctional hemodialysis access grafts: effect of a surveillance program on graft patency and the incidence of thrombosis. Radiology 1996;199:653-7. [Crossref] [PubMed]
- Turmel-Rodrigues L, Pengloan J, Baudin S, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant 2000;15:2029-36. [Crossref] [PubMed]
- Lilly RZ, Carlton D, Barker J, et al. Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis 2001;37:945-53. [Crossref] [PubMed]
- Clark TW, Hirsch DA, Jindal KJ, et al. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol 2002;13:51-9. [Crossref] [PubMed]
- Rajan DK, Bunston S, Misra S, et al. Dysfunctional autogenous hemodialysis fistulas: outcomes after angioplasty–are there clinical predictors of patency? Radiology 2004;232:508-15. [Crossref] [PubMed]
- Dariushnia SR, Walker TG, Silberzweig JE, et al. Quality Improvement Guidelines for Percutaneous Image-Guided Management of the Thrombosed or Dysfunctional Dialysis Circuit. J Vasc Interv Radiol 2016;27:1518-30. [Crossref] [PubMed]
- Rajan DK, Sidhu A, Noel-Lamy M, et al. Elastic Recoil after Balloon Angioplasty in Hemodialysis Accesses: Does It Actually Occur and Is It Clinically Relevant? Radiology 2016;279:961-7. [Crossref] [PubMed]
- Elramah M, Boujelbane L, Yevzlin AS, et al. Dialysis access venous stenosis: treatment with balloon angioplasty 30-second vs. 1-minute inflation times. Hemodial Int 2015;19:108-14. [Crossref] [PubMed]
- Vesely TM. Percutaneous transluminal angioplasty in the treatment of failing hemodialysis grafts and fistulae. In: Seminars in dialysis. Waverly Press Inc., 1998:351-9.
- Trerotola SO, Stavropoulos SW, Shlansky-Goldberg R, et al. Hemodialysis-related venous stenosis: treatment with ultrahigh-pressure angioplasty balloons. Radiology 2004;231:259-62. [Crossref] [PubMed]
- Vesely TM, Siegel JB. Use of the peripheral cutting balloon to treat hemodialysis-related stenoses. J Vasc Interv Radiol 2005;16:1593-603. [Crossref] [PubMed]
- Kariya S, Tanigawa N, Kojima H, et al. Primary patency with cutting and conventional balloon angioplasty for different types of hemodialysis access stenosis. Radiology 2007;243:578-87. [Crossref] [PubMed]
- Bhat R, McBride K, Chakraverty S, et al. Primary cutting balloon angioplasty for treatment of venous stenoses in native hemodialysis fistulas: long-term results from three centers. Cardiovasc Intervent Radiol 2007;30:1166-70; discussion 1171-2. [Crossref] [PubMed]
- Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 2010;362:494-503. [Crossref] [PubMed]
- Haskal ZJ, Saad TF, Hoggard JG, et al. Prospective, Randomized, Concurrently-Controlled Study of a Stent Graft versus Balloon Angioplasty for Treatment of Arteriovenous Access Graft Stenosis: 2-Year Results of the RENOVA Study. J Vasc Interv Radiol 2016;27:1105-14.e3. [Crossref] [PubMed]
- Vesely T, DaVanzo W, Behrend T, et al. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. J Vasc Surg 2016;64:1400-10.e1. [Crossref] [PubMed]
- Vorwerk D. Cutting balloon angioplasty in dialysis fistulas: Let us start to ask the right questions. CardioVascular and Interventional Radiology 2007;30:1171-2. [Crossref]
- Lee T, Ul Haq N. New Developments in Our Understanding of Neointimal Hyperplasia. Adv Chronic Kidney Dis 2015;22:431-7. [Crossref] [PubMed]
- Remuzzi A, Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clin J Am Soc Nephrol 2013;8:2186-93. [Crossref] [PubMed]
- Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006;17:1112-27. [Crossref] [PubMed]
- Roy-Chaudhury P, Wang Y, Krishnamoorthy M, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant 2009;24:2786-91. [Crossref] [PubMed]
- Lee T. Novel paradigms for dialysis vascular access: downstream vascular biology–is there a final common pathway? Clin J Am Soc Nephrol 2013;8:2194-201. [Crossref] [PubMed]
- Boghosian M, Cassel K, Hammes M, et al. Hemodynamics in the cephalic arch of a brachiocephalic fistula. Med Eng Phys 2014;36:822-30. [Crossref] [PubMed]
- Quencer KB, Kidd J, Kinney T. Preprocedure Evaluation of a Dysfunctional Dialysis Access. Tech Vasc Interv Radiol 2017;20:20-30. [Crossref] [PubMed]
- Asif A, Gadalean FN, Merrill D, et al. Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int 2005;67:1986-92. [Crossref] [PubMed]
- Trerotola SO. Technical and Long-Term Considerations in AV Shunt Angioplasty (Lecture). J Vasc Access 2005;6:109-11.
- Long B, Brichart N, Lermusiaux P, et al. Management of perianastomotic stenosis of direct wrist autogenous radial-cephalic arteriovenous accesses for dialysis. J Vasc Surg 2011;53:108-14. [Crossref] [PubMed]
- Neuen BL, Gunnarsson R, Baer RA, et al. Factors associated with patency following angioplasty of hemodialysis fistulae. J Vasc Interv Radiol 2014;25:1419-26. [Crossref] [PubMed]
- Badero OJ, Salifu MO, Wasse H, et al. Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. Am J Kidney Dis 2008;51:93-8. [Crossref] [PubMed]
- Patel SN, White CJ, Collins TJ, et al. Catheter-based treatment of the subclavian and innominate arteries. Catheter Cardiovasc Interv 2008;71:963-8. [Crossref] [PubMed]
- Manninen HI, Kaukanen ET, Ikäheimo R, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas–initial success and long-term results. Radiology 2001;218:711-8. [Crossref] [PubMed]
- D’cruz RT, Leong SW, Syn N, et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: A systematic review and meta-analysis. J Vasc Access 2019;20:345-55.
- Beathard GA, Jennings WC, Wasse H, et al. ASDIN white paper: Management of cephalic arch stenosis endorsed by the American Society of Diagnostic and Interventional Nephrology. J Vasc Access 2021. [Epub ahead of print]. doi:
10.1177/11297298211033519 .10.1177/11297298211033519 - Rajan DK, Clark TW, Patel NK, et al. Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol 2003;14:567-73. [Crossref] [PubMed]
- Quencer KB, Arici M. Arteriovenous Fistulas and Their Characteristic Sites of Stenosis. AJR Am J Roentgenol 2015;205:726-34. [Crossref] [PubMed]
- Beathard GA. Swing Point Stenosis. In: Dialysis Access Management. Cham: Springer, 2021:179-207.
- Beaulieu MC, Gabana C, Rose C, et al. Stenosis at the area of transposition – an under-recognized complication of transposed brachiobasilic fistulas. J Vasc Access 2007;8:268-74.
- Bountouris I, Kristmundsson T, Dias N, et al. Is Repeat PTA of a Failing Hemodialysis Fistula Durable? Int J Vasc Med 2014;2014:369687. [Crossref] [PubMed]
- Li L, Terry CM, Shiu YT, et al. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int 2008;74:1247-61. [Crossref] [PubMed]
- Yamamoto Y, Nakamura J, Nakayama Y, et al. Relationship between the outcomes of stent placement and the properties of arteriovenous graft outflow vein stenotic lesions. J Vasc Access 2012;13:426-31.
- Davidson CJ, Newman GE, Sheikh KH, et al. Mechanisms of angioplasty in hemodialysis fistula stenoses evaluated by intravascular ultrasound. Kidney Int 1991;40:91-5. [Crossref] [PubMed]
- Khawaja AZ, Cassidy DB, Al Shakarchi J, et al. Systematic review of drug eluting balloon angioplasty for arteriovenous haemodialysis access stenosis. J Vasc Access 2016;17:103-10.
- Liao MT, Lee CP, Lin TT, et al. A randomized controlled trial of drug-coated balloon angioplasty in venous anastomotic stenosis of dialysis arteriovenous grafts. J Vasc Surg 2020;71:1994-2003. [Crossref] [PubMed]
- Vesely TM, Pilgram TK. Angioplasty balloon inflation pressures during treatment of hemodialysis graft-related stenoses. J Vasc Interv Radiol 2006;17:623-8. [Crossref] [PubMed]
- Rajan DK, Platzker T, Lok CE, et al. Ultrahigh-pressure versus high-pressure angioplasty for treatment of venous anastomotic stenosis in hemodialysis grafts: is there a difference in patency? J Vasc Interv Radiol 2007;18:709-14. [Crossref] [PubMed]
- Trerotola SO, Kwak A, Clark TW, et al. Prospective study of balloon inflation pressures and other technical aspects of hemodialysis access angioplasty. J Vasc Interv Radiol 2005;16:1613-8. [Crossref] [PubMed]
- Faxon DP, Sanborn TA, Haudenschild CC. Mechanism of angioplasty and its relation to restenosis. Am J Cardiol 1987;60:5B-9B. [Crossref] [PubMed]
- Çildağ MB, Çildağ S, Köseoğlu ÖF. The Relationship Between Neutrophil-Lymphocyte Ratio and Primary Patency of Percutaneous Transluminal Angioplasty in Hemodialysis Arteriovenous Fistula Stenosis When Using Conventional and Drug-Eluting Balloons. Cardiovasc Intervent Radiol 2016;39:1702-7. [Crossref] [PubMed]
- Portugaller RH, Kalmar PI, Deutschmann H. The eternal tale of dialysis access vessels and restenosis: are drug-eluting balloons the solution? J Vasc Access 2014;15:439-47.
- Funaki B. Cutting balloon angioplasty in arteriovenous fistulas. J Vasc Interv Radiol 2005;16:5-7. [Crossref] [PubMed]
- Ponce P, Mateus A, Santos L. Anatomical correlation of a well-functioning access graft for haemodialysis. Nephrol Dial Transplant 2009;24:535-8. [Crossref] [PubMed]
- Berguer R, Hwang NH. Critical arterial stenosis: a theoretical and experimental solution. Ann Surg 1974;180:39-50. [Crossref] [PubMed]
- Lumsden AB, MacDonald MJ, Kikeri D, et al. Prophylactic balloon angioplasty fails to prolong the patency of expanded polytetrafluoroethylene arteriovenous grafts: results of a prospective randomized study. J Vasc Surg 1997;26:382-90; discussion 390-2. [Crossref] [PubMed]
- Caro Monroig A, Reddy SN, Chick JFB, et al. Fistulography of a Patent Hemodialysis Access: When Not to Treat and Implications for Establishing a Nontreatment Rate. J Vasc Interv Radiol 2018;29:376-82. [Crossref] [PubMed]
- Shahin H, Reddy G, Sharafuddin M, et al. Monthly access flow monitoring with increased prophylactic angioplasty did not improve fistula patency. Kidney Int 2005;68:2352-61. [Crossref] [PubMed]
- Salman L, Beathard G. Interventional nephrology: Physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clin J Am Soc Nephrol 2013;8:1220-7. [Crossref] [PubMed]
- Beathard GA. Physical examination of the dialysis vascular access. In: Seminars in dialysis. Oxford: Blackwell Publishing Ltd., 1998:231-6.
- Barton M, Grüntzig J, Husmann M, et al. Balloon Angioplasty – The Legacy of Andreas Grüntzig, M.D. (1939-1985). Front Cardiovasc Med 2014;1:15. [Crossref] [PubMed]
- Gordon DH, Glanz S, Butt KM, et al. Treatment of stenotic lesions in dialysis access fistulas and shunts by transluminal angioplasty. Radiology 1982;143:53-8. [Crossref] [PubMed]
- Glanz S, Gordon DH, Butt KM, et al. The role of percutaneous angioplasty in the management of chronic hemodialysis fistulas. Ann Surg 1987;206:777-81. [Crossref] [PubMed]
- Tessitore N, Mansueto G, Lipari G, et al. Endovascular versus surgical preemptive repair of forearm arteriovenous fistula juxta-anastomotic stenosis: analysis of data collected prospectively from 1999 to 2004. Clin J Am Soc Nephrol 2006;1:448-54. [Crossref] [PubMed]
- Kovalik EC, Schwab SJ. A comparison of percutaneous transluminal angioplasty versus surgical correction of various access complications. In: Seminars in dialysis. Oxford: Blackwell Publishing Ltd., 1995:171-4.
- Kumpe DA, Cohen MA. Angioplasty/thrombolytic treatment of failing and failed hemodialysis access sites: comparison with surgical treatment. Prog Cardiovasc Dis 1992;34:263-78. [Crossref] [PubMed]
- Dapunt O, Feurstein M, Rendl KH, et al. Transluminal angioplasty versus conventional operation in the treatment of haemodialysis fistula stenosis: results from a 5-year study. Br J Surg 1987;74:1004-5. [Crossref] [PubMed]
- Trerotola SO, Ponce P, Stavropoulos SW, et al. Physical examination versus normalized pressure ratio for predicting outcomes of hemodialysis access interventions. J Vasc Interv Radiol 2003;14:1387-94. [Crossref] [PubMed]
- Gilmore J. KDOQI clinical practice guidelines and clinical practice recommendations–2006 updates. Nephrol Nurs J 2006;33:487-8. [PubMed]
- Aftab SA, Tay KH, Irani FG, et al. Randomized clinical trial of cutting balloon angioplasty versus high-pressure balloon angioplasty in hemodialysis arteriovenous fistula stenoses resistant to conventional balloon angioplasty. J Vasc Interv Radiol 2014;25:190-8. [Crossref] [PubMed]
- Glanz S, Gordon DH, Butt KM, et al. Stenotic lesions in dialysis-access fistulas: treatment by transluminal angioplasty using high-pressure balloons. Radiology 1985;156:236. [Crossref] [PubMed]
- Mori Y, Horikawa K, Sato K, et al. Stenotic lesions in vascular access: treatment with transluminal angioplasty using high-pressure balloons. Intern Med 1994;33:284-7. [Crossref] [PubMed]
- Forsythe RO, Chemla ES. Surgical Options in the Problematic Arteriovenous Haemodialysis Access. Cardiovasc Intervent Radiol 2015;38:1405-15. [Crossref] [PubMed]
- Vorwerk D, Günther RW, Schürmann K, et al. Use of a cutting balloon for dilatation of a resistant venous stenosis of a hemodialysis fistula. Cardiovasc Intervent Radiol 1995;18:62-4. [Crossref] [PubMed]
- Kariya S, Tanigawa N, Kojima H, et al. Percutaneous transluminal cutting-balloon angioplasty for hemodialysis access stenoses resistant to conventional balloon angioplasty. Acta Radiol 2006;47:1017-21. [Crossref] [PubMed]
- Saleh HM, Gabr AK, Tawfik MM, et al. Prospective, randomized study of cutting balloon angioplasty versus conventional balloon angioplasty for the treatment of hemodialysis access stenoses. J Vasc Surg 2014;60:735-40. [Crossref] [PubMed]
- Rasuli P, Chennur VS, Connolly MJ, et al. Randomized Trial Comparing the Primary Patency following Cutting Versus High-Pressure Balloon Angioplasty for Treatment of de Novo Venous Stenoses in Hemodialysis Arteriovenous Fistulae. J Vasc Interv Radiol 2015;26:1840-6.e1. [Crossref] [PubMed]
- Kundu S, Clemens R, Aziza J, et al. Ultrahigh-pressure angioplasty versus the Peripheral Cutting Balloon™ for treatment of stenoses in autogenous fistulas: comparison of immediate results. J Vasc Access 2010;11:303-11.
- Singer-Jordan J, Papura S. Cutting balloon angioplasty for primary treatment of hemodialysis fistula venous stenoses: preliminary results. J Vasc Interv Radiol 2005;16:25-9. [Crossref] [PubMed]
- Bittl JA, Feldman RL. Cutting balloon angioplasty for undilatable venous stenoses causing dialysis graft failure. Catheter Cardiovasc Interv 2003;58:524-6. [Crossref] [PubMed]
- Wu CC, Wen SC. Cutting balloon angioplasty for resistant venous stenoses of dialysis access: immediate and patency results. Catheter Cardiovasc Interv 2008;71:250-4. [Crossref] [PubMed]
- Chakraverty S, Meier MA, Aarts JC, et al. Cutting-balloon-associated vascular rupture after failed standard balloon angioplasty. Cardiovasc Intervent Radiol 2005;28:661-4. [Crossref] [PubMed]
- Forauer AR, Hoffer EK, Homa K. Dialysis access venous stenoses: treatment with balloon angioplasty–1- versus 3-minute inflation times. Radiology 2008;249:375-81. [Crossref] [PubMed]
- Leontiev O, Shlansky-Goldberg RD, Stavropoulos SW, et al. Should all inflow stenoses be treated in failing autogenous hemodialysis fistulae? J Vasc Interv Radiol 2014;25:542-7. [Crossref] [PubMed]
- Patel AA, Tuite CM, Trerotola SO. Mechanical thrombectomy of hemodialysis fistulae and grafts. Cardiovasc Intervent Radiol 2005;28:704-13. [Crossref] [PubMed]
- Moresco KP, Treotola SC. Treatment of the thrombosed hemodialysis graft. In: Venous Interventional Radiology with Clinical Perspectives. 2nd edition. New York, NY, USA: Thieme, 2000:262-79.
- Przewlocki T, Kablak-Ziembicka A, Pieniazek P, et al. Determinants of immediate and long-term results of subclavian and innominate artery angioplasty. Catheter Cardiovasc Interv 2006;67:519-26. [Crossref] [PubMed]
- Duijm LE, Liem YS, van der Rijt RH, et al. Inflow stenoses in dysfunctional hemodialysis access fistulae and grafts. Am J Kidney Dis 2006;48:98-105. [Crossref] [PubMed]
- Khan FA, Vesely TM. Arterial problems associated with dysfunctional hemodialysis grafts: evaluation of patients at high risk for arterial disease. J Vasc Interv Radiol 2002;13:1109-14. [Crossref] [PubMed]
- Shamimi-Noori S, Sheng M, Mantell MP, et al. Diagnosis and Treatment of Nonmaturing Fistulae for Hemodialysis Access via Transradial Approach: A Case-Control Study. J Vasc Interv Radiol 2020;31:993-99.e1. [Crossref] [PubMed]
- Cui J, Xu D, Ma J, et al. Stenoses in the surgically manipulated segment have better angioplasty response compared to the surgically I segment in fistulas. J Vasc Access 2017;18:192-9.
- Hofstra L, Tordoir JH, Kitslaar PJ, et al. Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation 1996;94:1283-90. [Crossref] [PubMed]
- Ojha M, Ethier CR, Johnston KW, et al. Steady and pulsatile flow fields in an end-to-side arterial anastomosis model. J Vasc Surg 1990;12:747-53. [Crossref] [PubMed]
- Lay JP, Ashleigh RJ, Tranconi L, et al. Result of angioplasty of rescia-cimino haemodialysis fistulae: medium-term follow-up. Clin Radiol 1998;53:608-11. [Crossref] [PubMed]
- Kwon H, Choi JY, Ko HK, et al. Comparison of surgical and endovascular salvage procedures for juxta-anastomotic stenosis in autogenous wrist radiocephalic arteriovenous fistula. Ann Vasc Surg 2014;28:1840-6. [Crossref] [PubMed]
- Argyriou C, Schoretsanitis N, Georgakarakos EI, et al. Preemptive open surgical vs. endovascular repair for juxta-anastomotic stenoses of autogenous AV fistulae: a meta-analysis. J Vasc Access 2015;16:454-8.
- Cohen A, Korzets A, Neyman H, et al. Endovascular interventions of juxtaanastomotic stenoses and thromboses of hemodialysis arteriovenous fistulas. J Vasc Interv Radiol 2009;20:66-70. [Crossref] [PubMed]
- Mortamais J, Papillard M, Girouin N, et al. Endovascular treatment of juxta-anastomotic venous stenoses of forearm radiocephalic fistulas: long-term results and prognostic factors. J Vasc Interv Radiol 2013;24:558-64; quiz 565. [Crossref] [PubMed]
- Napoli M, Prudenzano R, Russo F, et al. Juxta-anastomotic stenosis of native arteriovenous fistulas: surgical treatment versus percutaneous transluminal angioplasty. J Vasc Access 2010;11:346-51.
- Leontiev O, Mondschein JI, Dagli MS, et al. Catheter-based intraaccess blood flow measurement as a problem-solving tool in hemodialysis access intervention. J Vasc Interv Radiol 2013;24:717-21. [Crossref] [PubMed]
- Shemesh D, Goldin I, Olsha O. Stent grafts for treatment of cannulation zone stenosis and arteriovenous graft venous anastomosis. J Vasc Access 2017;18:47-52.
- Webb KM, Cull DL, Carsten CG 3rd, et al. Outcome of the use of stent grafts to salvage failed arteriovenous accesses. Ann Vasc Surg 2010;24:34-8. [Crossref] [PubMed]
- Schmelter C, Raab U, Lazarus F, et al. Outcomes of AV Fistulas and AV Grafts after Interventional Stent-Graft Deployment in Haemodialysis Patients. Cardiovasc Intervent Radiol 2015;38:878-86. [Crossref] [PubMed]
- Sivananthan G, Menashe L, Halin NJ. Cephalic arch stenosis in dialysis patients: review of clinical relevance, anatomy, current theories on etiology and management. J Vasc Access 2014;15:157-62.
- Hammes M, Funaki B, Coe FL. Cephalic arch stenosis in patients with fistula access for hemodialysis: relationship to diabetes and thrombosis. Hemodial Int 2008;12:85-9. [Crossref] [PubMed]
- Jaberi A, Schwartz D, Marticorena R, et al. Risk factors for the development of cephalic arch stenosis. J Vasc Access 2007;8:287-95.
- Vasanthamohan L, Gopee-Ramanan P, Athreya S. The Management of Cephalic Arch Stenosis in Arteriovenous Fistulas for Hemodialysis: A Systematic Review. Cardiovasc Intervent Radiol 2015;38:1179-85. [Crossref] [PubMed]
- Iimura A, Nakamura Y, Itoh M. Anatomical study of distribution of valves of the cutaneous veins of adult’s limbs. Ann Anat 2003;185:91-5. [Crossref] [PubMed]
- Davies MG, Hicks TD, Haidar GM, et al. Outcomes of intervention for cephalic arch stenosis in brachiocephalic arteriovenous fistulas. J Vasc Surg 2017;66:1504-10. [Crossref] [PubMed]
- Kornfield ZN, Kwak A, Soulen MC, et al. Incidence and management of percutaneous transluminal angioplasty-induced venous rupture in the “fistula first” era. J Vasc Interv Radiol 2009;20:744-51. [Crossref] [PubMed]
- Heerwagen ST, Lönn L, Schroeder TV, et al. Cephalic arch stenosis in autogenous brachiocephalic hemodialysis fistulas: results of cutting balloon angioplasty. J Vasc Access 2010;11:41-5.
- Rajan DK, Falk A. A Randomized Prospective Study Comparing Outcomes of Angioplasty versus VIABAHN Stent-Graft Placement for Cephalic Arch Stenosis in Dysfunctional Hemodialysis Accesses. J Vasc Interv Radiol 2015;26:1355-61. [Crossref] [PubMed]
- Jones RG, Willis AP, Jones C, et al. Long-term results of stent-graft placement to treat central venous stenosis and occlusion in hemodialysis patients with arteriovenous fistulas. J Vasc Interv Radiol 2011;22:1240-5. [Crossref] [PubMed]
- Miller GA, Preddie DC, Savransky Y, et al. Use of the Viabahn stent graft for the treatment of recurrent cephalic arch stenosis in hemodialysis accesses. J Vasc Surg 2018;67:522-8. [Crossref] [PubMed]
- Miller GA, Friedman A, Khariton A, et al. Access flow reduction and recurrent symptomatic cephalic arch stenosis in brachiocephalic hemodialysis arteriovenous fistulas. J Vasc Access 2010;11:281-7.
- Wang S, Almehmi A, Asif A. Surgical management of cephalic arch occlusive lesions: are there predictors for outcomes? Semin Dial 2013;26:E33-41. [Crossref] [PubMed]
- Kim SM, Yoon KW, Woo SY, et al. Treatment Strategies for Cephalic Arch Stenosis in Patients with Brachiocephalic Arteriovenous Fistula. Ann Vasc Surg 2019;54:248-53. [Crossref] [PubMed]
- Falk A, Teodorescu V, Lou WY, et al. Treatment of “swing point stenoses” in hemodialysis arteriovenous fistulae. Clin Nephrol 2003;60:35-41. [Crossref] [PubMed]
- Yan Y, Clark TW, Mondschein JI, et al. Outcomes of percutaneous interventions in transposed hemodialysis fistulas compared with nontransposed fistulas and grafts. J Vasc Interv Radiol 2013;24:1765-72; quiz 1773. [Crossref] [PubMed]
- Nassar GM, Beathard G, Rhee E, et al. Management of transposed arteriovenous fistula swing point stenosis at the basilic vein angle of transposition by stent grafts. J Vasc Access 2017;18:482-7.
- Kakkos SK, Kouri AK, Tsolakis IA, et al. Surgical and endovascular revision of brachio-basilic vein fistula. J Vasc Access 2016;17 Suppl 1:S6-11.
- May RE, Himmelfarb J, Yenicesu M, et al. Predictive measures of vascular access thrombosis: a prospective study. Kidney Int 1997;52:1656-62. [Crossref] [PubMed]
- Carty GA, Davis VH. Mid-graft stenosis in expanded polytetrafluoroethylene hemodialysis conduits. Dialysis & transplantation 1990;19:486-9.
- Bautista AB, Suhocki PV, Pabon-Ramos WM, et al. Postintervention Patency Rates and Predictors of Patency after Percutaneous Interventions on Intragraft Stenoses within Failing Prosthetic Arteriovenous Grafts. J Vasc Interv Radiol 2015;26:1673-9. [Crossref] [PubMed]
- Zaleski GX, Funaki B, Rosenblum J, et al. Metallic stents deployed in synthetic arteriovenous hemodialysis grafts. AJR Am J Roentgenol 2001;176:1515-9. [Crossref] [PubMed]
- Asif A, Gadalean F, Eid N, et al. Stent graft infection and protrusion through the skin: Clinical considerations and potential medico‐legal ramifications. In: Seminars in dialysis. Oxford: Blackwell Publishing Ltd., 2010:540-2.
- Kim CY, Guevara CJ, Engstrom BI, et al. Analysis of infection risk following covered stent exclusion of pseudoaneurysms in prosthetic arteriovenous hemodialysis access grafts. J Vasc Interv Radiol 2012;23:69-74. [Crossref] [PubMed]
- Mohr BA, Sheen AL, Roy-Chaudhury P, et al. Clinical and Economic Benefits of Stent Grafts in Dysfunctional and Thrombosed Hemodialysis Access Graft Circuits in the REVISE Randomized Trial. J Vasc Interv Radiol 2019;30:203-11.e4. [Crossref] [PubMed]
- Falk A, Maya ID, Yevzlin AS, et al. A Prospective, Randomized Study of an Expanded Polytetrafluoroethylene Stent Graft versus Balloon Angioplasty for In-Stent Restenosis in Arteriovenous Grafts and Fistulae: Two-Year Results of the RESCUE Study. J Vasc Interv Radiol 2016;27:1465-76. [Crossref] [PubMed]


