
Cardiovascular and bleeding risks of inactive cancer in patients with acute myocardial infarction who received primary percutaneous coronary intervention using drug-eluting stent and dual/triple antithrombotic therapy
Introduction
Cancer has been considered as a high-risk disease substrate which elevates both cardiovascular and bleeding events’ risks (1-6). This is partly due to concomitant atherosclerotic and bleeding risk factors, and cancer-related abnormal hemostasis (2,7,8). Recently, the Academic Research Consortium for high bleeding risk (HBR) has proposed “active cancer” as a major criterion for HBR in subjects undergoing percutaneous coronary intervention (PCI) (9). Given that dual antiplatelet therapy (DAPT) has become a guideline-recommended anti-thrombotic therapy in patients with acute myocardial infarction (AMI) receiving drug-eluting stent (DES), management of both cardiovascular and bleeding risks is a cornerstone of antithrombotic therapy in AMI patients with active cancer. Recent chemotherapeutic agents have improved survival rate with better efficacy to induce inactive status of cancer (10), which expects that the number of inactive cancer patients with AMI will further rise in the future. Given the aforementioned cancer-related pathophysiological features, inactive cancer may still exhibit greater risks of cardiovascular and bleeding events (11-13). Therefore, the current study sought to investigate cardiovascular and bleeding risks in AMI subjects with inactive cancer who received PCI using DES. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-306/rc).
Methods
Study subjects
The current study is a retrospective cross-sectional study including 1,785 consecutive de novo AMI patients who received primary PCI from January 2007 to December 2017 at the National Cerebral and Cardiovascular Center in Suita, Japan (Figure 1). Myocardial infarction (MI) was diagnosed according to the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the Universal Definition of MI (14). Primary PCI in ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) subjects was defined as PCI performed within 48 and 72 hours of symptom onset, respectively (15).
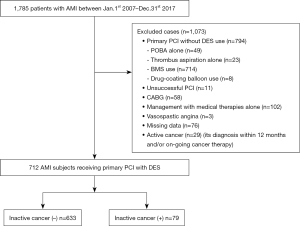
Of these, the current study excluded the following subjects: patients who treated by primary PCI without DES (n=794) [plain old balloon angioplasty alone (n=49), thrombus aspiration alone (n=23), bare metal stent use (n=714), drug-coating balloon (n=8)], patients with unsuccessful PCI (n=11), patients who received coronary artery bypass surgery for revascularization (n=58), those who were managed by medical therapies (n=102), those with AMI attributable to vasospastic angina (n=3) and missing data (n=76), and active cancer patients (= its diagnosis within 12 months prior to primary PCI and/or on-going cancer therapy) (n=29). The remaining 712 patients receiving primary PCI with DES were included into the current analysis (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised on 2013). The study was approved by the ethics committee of the National Cerebral and Cardiovascular Center (M24-055-7). Informed consent was not obtained in each subject due to the observational analysis of hospitalized patients.
Definition of inactive cancer
Inactive cancer was defined as (I) its history for more than 12 months before the index of primary PCI and (II) subjects who did not receive any on-going cancer therapies (16). Cancer diagnosis, and its types and status were determined through medical record review, and when necessary, through a questionnaire by mail or telephone follow-up.
PCI procedure and antithrombotic regimens
After identification of the culprit lesion on diagnostic coronary angiography, primary PCI was performed. All procedural decisions, including device selection, the use of mechanical support, and adjunctive pharmacotherapy were made according to the discretion of the individual PCI operator.
Loading of DAPT (200 mg aspirin + 300 mg clopidogrel or 20 mg prasugrel) was conducted prior to primary PCI. After the completion of the procedure, DAPT with its approved maintenance dose in Japan (100 mg/day aspirin + 75 mg/day clopidogrel or 3.75 mg/day prasugrel) was continued for at least 1 year. In patients with atrial fibrillation, anticoagulation agent [vitamin K antagonist (VKA) or direct oral anticoagulant (DOAC)] was added according to the Japanese Circulation Society guideline (9). The selection of P2Y12 inhibitor and anticoagulation agents was conducted by each physician’s discretion. The number of antithrombotic agents was analyzed as follows; single antiplatelet therapy (SAPT), dual antithrombotic therapy (DAT: DAPT or a combination of SAPT and DOAC/VKA) and triple antithrombotic therapy (TAT: a combination of DAPT and DOAC/VKA) (17).
Assessment of bleeding risk
Bleeding risk in the current subjects was evaluated by the Academic Research Consortium on high bleeding risk (ARC-HBR) and Japanese version of HBR criteria (Japanese-HBR). ARC-HBR defined HBR as a BARC (bleeding academic research consortium) 3 or 5 bleeding risk of ≥4% or a risk of an intracranial hemorrhage of ≥1% at 1 year after PCI. Patients are considered to be HBR in subjects who fulfilled at least 1 major or 2 minor published HBR criteria (18,19). With regard to Japanese-HBR, the Japanese Circulation Society has proposed it by considering additional clinical characteristics (low body weight, frailty, chronic kidney disease, heart failure, peripheral vascular disease) associated with bleeding events in Japanese patients (20-22). By incorporating ARC-HBR major/minor criteria and these ones, HBR in Japanese patients are defined as at least one major or 2 minor Japanese-HBR criteria (9).
Outcomes
The primary outcome was the occurrence of cardiovascular and bleeding events. The cardiovascular events consisted of a composite of all-cause death, non-fatal MI and ischemic stroke. Ischemic stroke was defined as lacunar infarction, atherothrombotic brain infarction or cardioembolic infarction. Bleeding events included major and minor ones defined by International Society on Thrombosis and Haemostasis. In brief, major bleeding was defined (I) fatal bleeding; and/or (II) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome; and/or (III) bleeding causing a fall in hemoglobin level of 2 g/dL or more, or leading to transfusion of two or more units of whole blood or red cells. All non-major bleedings were considered as minor bleeding. The secondary outcome was the occurrence of (I) cardiovascular events; (II) bleeding events and (III) each component of primary outcomes (all-cause death, non-fatal MI, ischemic stroke, major and minor bleeding). These outcomes were firstly obtained through reviewing the medical records. If needed, questionnaire was conducted by mail or telephonic follow-up. A clinical event committee consisting of 2 cardiologists (HH and YK) and another referee (MF) in case of disagreement adjudicated all events based on the aforementioned original source documents of outcomes.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation and compared using the t-test if data were normally distributed. Categorical variables were compared using the Fisher exact test or the Chi-square test as appropriate. The Kaplan-Meier method was used to estimate survival curves for primary and secondary outcomes, and the log-rank test was used to assess differences between patients with and without inactive cancer. Unadjusted hazard ratios (HR) for primary and secondary outcomes were calculated by a univariate Cox proportional hazards model. Adjusted hazard ratios were calculated by a multivariate Cox proportional hazards model with a P value <0.10. All P values <0.05 were considered statistically significant. All analyses were performed with JMP version 13.0.0 (SAS Institute, Cary, NC, USA).
Results
Clinical demographics
In the current study, 11.1% of AMI subjects (=79/712) concomitantly had inactive cancer. Of these, major types of inactive cancers were gastrointestinal one, followed by breast and kidney cancer (Figure S1). Baseline clinical demographics are shown in Table 1. Inactive cancer subjects were more likely to be older (77.0±7.5 vs. 68.7±12.8 years, P<0.001) and female (35.4% vs. 22.0%, P=0.03), and have a history of atrial fibrillation (25.3% vs. 10.0%, P<0.001) with smaller body mass index (BMI) (22.5±3.8 vs. 23.9±3.6 kg/m2, P=0.003). There were no significant differences in the proportion of STEMI and NSTEMI between two groups (P=0.952), whereas inactive cancer subjects more likely exhibited a greater frequency of AMI patients in Killip class IV (21.5% vs. 6.0%, P<0.001), accompanied by a lower hemoglobin level (12.8±1.9 vs. 13.9±1.8 g/dL, P<0.001) and estimated glomerular filtration rate (51.8±24.4 vs. 65.9±24.1 mL/min/1.73 m2, P<0.001). As a consequence, ARC-defined HBR (65.8% vs. 32.1%, P<0.001) and Japanese-HBR (77.2% vs. 48.7%, P<0.001) was significantly higher in inactive cancer subjects.
Table 1
| Inactive cancer (+), (n=79) | Inactive cancer (−), (n=633) | P value | |
|---|---|---|---|
| Age (years) | 77.0±7.5 | 68.7±12.8 | <0.001 |
| Male | 51 (64.6) | 494 (78.0) | 0.025 |
| BMI (kg/m2) | 22.5±3.8 | 23.9±3.6 | 0.003 |
| Coronary risk factors | |||
| HT | 49 (62.0) | 361 (57.0) | 0.349 |
| DLP | 50 (63.3) | 462 (73.0) | 0.073 |
| DM | 34 (43.0) | 310 (49.0) | 0.324 |
| AF | 20 (25.3) | 63 (10.0) | <0.001 |
| Active smoker | 25 (31.6) | 240 (37.9) | 0.281 |
| Characteristics of AMI | |||
| STEMI | 63 (79.7) | 513 (81.0) | 0.952 |
| NSTEMI | 16 (20.3) | 120 (19.0) | |
| peak CK (IU/L) | 1,388 (576–2,630) | 1,532 (651–3,172) | 0.459 |
| EF (%) | 46.9±17.8 | 47.7±14.3 | 0.354 |
| Killip class IV | 17 (21.5) | 38 (6.0) | <0.001 |
| Multivessel disease | 45 (57.0) | 329 (52.0) | 0.389 |
| Biochemistry data | |||
| Hemoglobin (g/dL) | 12.8±1.9 | 13.9±1.8 | <0.001 |
| Platelet (103/μL) | 201±58 | 208±63 | 0.360 |
| eGFR (mL/min/1.73 m2) | 51.8±24.4 | 65.9±24.1 | <0.001 |
| LDL-C (mg/dL) | 111.6±34.3 | 123.3±36.8 | 0.008 |
| HDL-C (mg/dL) | 43.8±13.2 | 45.3±13.2 | 0.364 |
| HBR measures | |||
| ARC-HBR | 52 (65.8) | 203 (32.1) | <0.001 |
| Japanese-HBR | 61 (77.2) | 308 (48.7) | <0.001 |
Values expressed as n (%), median and interquartile range (IQR), or mean ± SD. Values in parentheses are percentages. BMI, body mass index; HT, hypertension; DLP, dyslipidemia; DM, diabetes mellitus; AF, atrial fibrillation; AMI, acure myocardial infarction; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; CK, creatine kinase; EF, ejection fraction; eGFR, estimated glomerular filtration rate; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; ARC-HBR, The Academic Research Consortium for high bleeding risk.
The use of antithrombotic and other medical therapies is summarized in Table 2. Over 80% of current study subjects received DAT (82.3% vs. 86.7%, P=0.279), and the remains were treated with TAT (17.7% vs. 13.3%). With regard to regimen of DAT, inactive cancer subjects were less likely received a combination of aspirin and prasugrel (12.7% vs. 25.9%, P=0.01) (Table 2). There were no significant differences in the regimens of TAT, duration of DAT and TAT, and the use of other established medical therapies (Table 2). Following the use of DAT or TAT, around 77% of patients with and without inactive cancer (77.2% vs. 77.3%, P=0.994) switched to single anti-platelet therapy (460±298 vs. 538±470 days, P=0.213). A longer duration of SAPT use was observed in inactive cancer subjects [760 (356–1,300) vs. 658 (390–867) days, P=0.006].
Table 2
| Therapy | Inactive cancer (+), (n=79) | Inactive cancer (−), (n=633) | P value |
|---|---|---|---|
| Anti-thrombotic therapy | |||
| DAT | 65 (82.3) | 549 (86.7) | 0.279 |
| TAT | 14 (17.7) | 84 (13.3) | |
| Regimen of DAT | |||
| DAPT | 65 (82.3) | 547 (86.4) | 0.319 |
| Aspirin + clopidogrel | 49 (62.0) | 364 (57.5) | 0.443 |
| Aspirin + prasugrel | 10 (12.7) | 164 (25.9) | 0.010 |
| Clopidogrel + DOAC | 0 (0.0) | 2 (0.3) | 0.617 |
| Duration of DAT (days) | 673±609 | 609±510 | 0.344 |
| Regimen of TAT | |||
| DAPT + warfarin | 12 (15.2) | 62 (9.8) | 0.138 |
| DAPT + DOAC | 2 (2.5) | 22 (3.5) | 0.661 |
| Duration of TAT (days) | 534±527 | 580±505 | 0.487 |
| Switching to SAPT | |||
| Frequency | 61 (77.2) | 489 (77.3) | 0.994 |
| Its timing after DAT/TAT (days) | 460±298 | 538±470 | 0.213 |
| Aspirin alone | 36 (45.6) | 335 (52.9) | 0.217 |
| P2Y12 receptor inhibitor alone | 25 (31.6) | 154 (24.3) | 0.158 |
| Duration of SAPT (days) | 760 (356–1,300) | 658 (390–867) | 0.006 |
| Other medical therapy | |||
| ACEI/ARB | 70 (88.6) | 567 (89.6) | 0.792 |
| β-blocker | 59 (74.7) | 481 (76.0) | 0.799 |
| Statin | 72 (91.1) | 582 (91.9) | 0.806 |
Values expressed as n (%), median and interquartile range (IQR), or mean ± SD. DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy; DAPT, dual anti platelet therapy; DOAC, direct oral anticoagulant; SAPT, single anti platelet therapy; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Cardiovascular and bleeding outcomes
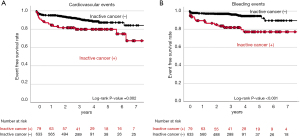
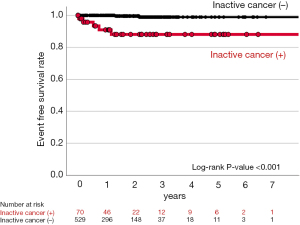
In the current study, there were 107 cardiovascular and bleeding events during the observational period (median: 1,088 days) (Table 3). As shown in Figure 2, inactive cancer was associated with 3.59-fold greater likelihood experiencing a composite of cardiovascular and bleeding events (95% CI: 2.13–6.04, P<0.001) (Table 4 and Figure 2). On multivariate analysis adjusting for covariates, inactive cancer was still an independent predictor for the occurrence of cardiovascular and bleeding events (HR: 2.22, 95% CI: 1.15–4.28, P=0.017) (Table 4). Furthermore, inactive cancer exhibited 2.93- and 5.26-fold greater risks of cardiovascular (95% CI: 1.62–5.28, P<0.001) and bleeding events (95% CI: 2.66–10.4, P<0.001), respectively (Table 5). The relationship of inactive cancer with bleeding events still existed even after adjusting covariates (HR: 3.98, 95% CI: 1.90–8.34, P<0.001), whereas adjusted P value for the relationship between inactive cancer and cardiovascular events became non-significant (HR: 1.47, 95% CI: 0.65–3.34, P=0.353) (Figure 3 and Table 5). A greater frequency of each component of composite outcomes (non-fatal MI, ischemic stroke, major and minor bleeding) was consistently observed in subjects with inactive cancer (Figure S2 and Figure S3). Further analysis was conducted to evaluate the frequency of bleeding events in subjects who switched to single anti-thrombotic therapy after DAT or TAT use (Figure 4). Despite this anti-thrombotic regimen, a continuing elevated risk of bleeding events was observed in subjects with inactive cancer (Figure 4).
Table 3
| Events | Overall, (n=712) | Inactive cancer (+), (n=79) | Inactive cancer (−), (n=633) |
|---|---|---|---|
| Cardiovascular and bleeding events | 107 (15.0) | 27 (34.2) | 80 (12.6) |
| Cardiovascular events | 76 (10.7) | 18 (22.8) | 58 (9.2) |
| All-cause death | 65 (9.1) | 15 (19.0) | 50 (7.9) |
| Nonfatal MI | 5 (0.7) | 2 (2.5) | 3 (0.5) |
| Ischemic stroke | 18 (2.5) | 6 (7.6) | 12 (1.9) |
| Bleeding events | 42 (5.9) | 15 (19.0) | 27 (4.3) |
| Major bleeding | 29 (4.1) | 8 (10.1) | 21 (3.3) |
| Minor bleeding | 13 (1.8) | 7 (8.9) | 6 (0.9) |
Categorical variables are expressed as n (%). MI, myocardial infarction.
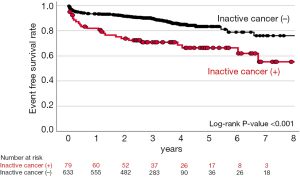
Table 4
| Cardiovascular and bleeding events | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio* (95% CI) | P value | Hazard ratio* (95% CI) | P value | ||
| Cancer | 3.59 (2.13–6.04) | <0.001 | 2.22 (1.15–4.28) | 0.017 | |
| Age | 1.04 (1.02–1.06) | <0.001 | 1.02 (0.99–1.04) | 0.196 | |
| Male gender | 1.11 (0.68–1.81) | 0.675 | – | – | |
| BMI | 0.92 (0.86–0.98) | 0.007 | 0.97 (0.90–1.05) | 0.484 | |
| Hypertension | 1.15 (0.76–1.76) | 0.512 | – | – | |
| Dyslipidemia | 1.25 (0.79–1.93) | 0.335 | – | – | |
| Type 2 DM | 0.93 (0.62–1.40) | 0.723 | – | – | |
| Smoker | 1.02 (0.66–1.56) | 0.944 | – | – | |
| CKD (eGFR <60 mL/min/1.73 m2) | 1.80 (1.18–2.75) | 0.006 | 0.93 (0.53–1.61) | 0.784 | |
| AF | 3.03 (1.80–5.08) | <0.001 | 2.01 (1.04–3.88) | 0.037 | |
| Killip class IV | 7.49 (4.19–13.4) | <0.001 | 2.18 (0.92–5.13) | 0.075 | |
| LDL-C | 0.99 (0.98–0.99) | <0.001 | 1.00 (0.99–1.01) | 0.518 | |
| β-blocker | 0.53 (0.34–0.82) | 0.004 | 0.84 (0.47–1.48) | 0.537 | |
| ACEI/ARB | 0.30 (0.18–0.50) | <0.001 | 0.40 (0.21–0.78) | 0.007 | |
| Statin | 0.41 (0.21–0.80) | 0.015 | 0.46 (0.21–1.03) | 0.060 | |
*, the effect estimate represents the change in score per 1 unit change in the parameter after adjusting for all other terms in the model. CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; CKD, chronic kidney disease; AF, atrial fibrillation; LDL-C, low density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 5
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Hazard ratio* (95% CI) | P value | Hazard ratio* (95% CI) | P value | ||
| Predictors for cardiovascular events | |||||
| Cancer | 2.93 (1.62–5.28) | <0.001 | 1.47 (0.65–3.34) | 0.353 | |
| Age | 1.05 (1.03–1.07) | <0.001 | 1.01 (0.98–1.04) | 0.199 | |
| Male gender | 1.21 (0.68–2.16) | 0.523 | – | – | |
| BMI | 0.92 (0.85–0.99) | 0.029 | 0.99 (0.90–1.08) | 0.782 | |
| Hypertension | 1.38 (0.86–2.22) | 0.187 | – | – | |
| Dyslipidemia | 1.71 (1.04–2.82) | 0.034 | 1.32 (0.69–2.53) | 0.397 | |
| Type 2 DM | 0.97 (0.62–1.56) | 0.893 | – | – | |
| Smoker | 1.10 (0.67–1.81) | 0.710 | – | – | |
| CKD (eGFR <60 mL/min/1.73 m2) | 2.05 (1.24–3.36) | 0.005 | 1.30 (0.65–2.59) | 0.461 | |
| AF | 3.77 (2.15–6.60) | <0.001 | 2.71 (1.28–5.73) | 0.009 | |
| Killip class IV | 12.0 (6.56–22.0) | <0.001 | 3.93 (1.57–9.81) | 0.003 | |
| LDL-C | 0.99 (0.98–0.99) | 0.004 | 1.00 (0.99–1.01) | 0.826 | |
| β-blocker | 0.44 (0.27–0.72) | 0.001 | 0.78 (0.40–1.54) | 0.471 | |
| ACEI/ARB | 0.25 (0.15–0.44) | <0.001 | 0.36 (0.17–0.77) | 0.009 | |
| statin | 0.43 (0.20–0.93) | 0.033 | 0.57 (0.22–1.49) | 0.252 | |
| Predictors for bleeding events | |||||
| Cancer | 5.26 (2.66–10.4) | <0.001 | 3.98 (1.90–8.34) | <0.001 | |
| Age | 1.03 (0.99–1.06) | 0.057 | 1.00 (0.98–1.04) | 0.682 | |
| Male gender | 1.01 (0.49–2.10) | 0.974 | – | – | |
| BMI | 0.96 (0.87–1.06) | 0.411 | – | – | |
| Hypertension | 1.24 (0.65–2.35) | 0.518 | – | – | |
| Dyslipidemia | 1.27 (0.61–2.64) | 0.520 | – | – | |
| Type 2 DM | 0.72 (0.38–1.37) | 0.319 | – | – | |
| Smoker | 1.02 (0.53–1.96) | 0.952 | – | – | |
| CKD (eGFR <60 mL/min/1.73 m2) | 1.30 (0.70–2.43) | 0.410 | – | – | |
| AF | 2.16 (0.99–4.70) | 0.051 | 1.39 (0.59–3.28) | 0.448 | |
| Killip class IV | 3.00 (1.31–6.85) | 0.009 | 1.75 (0.68–4.49) | 0.247 | |
| LDL-C | 0.99 (0.98–1.01) | 0.086 | 1.00 (0.99–1.01) | 0.460 | |
| β-blocker | 0.85 (0.42–1.69) | 0.634 | – | – | |
| ACEI/ARB | 0.68 (0.29–1.59) | 0.378 | – | – | |
| Statin | 0.49 (0.18–1.31) | 0.154 | – | – | |
*, the effect estimate represents the change in score per 1 unit change in the parameter after adjusting for all other terms in the model. CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; CKD, chronic kidney disease; AF, atrial fibrillation; LDL-C, low density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Discussion
Recent chemotherapeutic agents have improved to achieve inactive status of cancers (10). Whether inactive cancer affects cardiovascular and bleeding outcomes in AMI subjects has not been fully characterized yet. In the current study, 11% of AMI subjects receiving primary PCI with DES use concomitantly had inactive cancer. They exhibited severer clinical presentation of AMI with a greater frequency of HBR features. In addition, inactive cancer more likely experienced a composite of cardiovascular and bleeding events following primary PCI. These findings indicate that even inactive status of cancer still elevates cardiovascular and bleeding risks in the setting of AMI.
To date, while active cancer has been reported to worsen clinical outcomes in AMI subjects (23-25), it remains uncertain whether inactive cancer also increases cardiovascular and bleeding risks in AMI subjects receiving PCI. Some studies reported an elevated risk of cardiovascular events in inactive cancer patients (26-28). By contrast, one recent study analyzing national database in the United States showed a lower frequency of a composite of all-cause mortality, cardiac complications, and stroke in inactive cancer patients. In addition, a modestly increased risk of bleeding events was observed (29). Given that study period in this study is from 2004 to 2014, antithrombotic and antiatherosclerotic management was different from current guideline-recommended ones. Moreover, only 37.6% of study subjects received PCI. These characteristics of this study do not necessarily reflect real-world data in AMI subjects under current guideline-recommended therapies. In our study including AMI subjects receiving primary PCI with DES, AMI subjects with inactive cancer exhibited worse clinical outcomes, reflected by a substantially elevated risks of a composite of cardiovascular and bleeding events. Our current observations suggest that elevated cardiovascular and bleeding risks still continue to exist in AMI subjects with inactive cancer status.
In the current analysis, as shown in Figure 3, worse cardiovascular outcome was observed in inactive cancer patients. However, a multivariate Cox proportional hazard analysis showed that inactive cancer was not an independent predictor for cardiovascular events. Given that AMI subjects with inactive cancer concomitantly exhibited high-risk clinical characteristics including Killip-class IV and atrial fibrillation, these features may be more dominant factors associated with their cardiovascular outcomes rather than inactive cancer itself.
Currently, major criterion of ARC-HBR has included active malignancy, which is defined as its diagnosis within the past 12 months or cancer patients receiving ongoing cancer therapy, whereas cancer with its complete remission is not included (18). In the current analysis, most of inactive cancer subjects receiving primary PCI with DES were treated by DAT which included potent P2Y12 inhibitor, and the remaining subjects received TAT mainly using the combination of DAPT and VKA. Under these antithrombotic regimen, inactive cancer was associated with considerably elevated bleeding risks in AMI subjects. In particular, this greater risk in inactive cancer subjects was observed in both major and minor bleeding events. As shown in Table 1, inactive cancer subjects more frequently had anemia and chronic kidney disease. Anemia itself may associate with diminished function of platelet and more inflammatory activity which could elevate bleeding risks (30). Moreover, chronic kidney disease is accompanied by platelet dysfunction, and elevated levels of anti-Xa and additional abnormalities in the coagulation cascade (31,32). These characteristics in inactive cancer subjects may increase bleeding events after AMI. As such, our findings support inactive cancer as a potential major criterion for HBR in patients receiving PCI with DES.
Considering an elevated bleeding risk in inactive cancer subjects, physicians should consider additional actions which include to shorten duration of their DAT and/or TAT. However, we also observed that this inactive cancer-related bleeding risk continued even after switching to SAPT. In particular, Figure 4 showed a continuing occurrence of bleeding events by the first year after switching to SAPT, and then its frequency became almost plateau. The aforementioned clinical features of inactive cancer subjects may cause these on-going bleeding risks despite SAPT. More meticulous clinical follow-up is required to monitor bleeding evens after switching to SAPT in AMI subjects with inactive cancer.
Several caveats should be considered to interpret the current findings. Firstly, this is a retrospective, single-center observational study. Secondly, the selection of antithrombotic therapy and other medical therapies was conducted according to each physician’s discretion. Thirdly, due to the limited number of inactive cancer subjects, the current study does not have enough power to compare primary and secondary outcomes in those stratified according to cancer types. Fourthly, the current study’s period was from 2007 to 2017. During this period, guidelines for antithrombotic and lipid-lowering therapies has changed, which may affect cardiovascular and bleeding outcomes in study subjects. However, even after adjusting for the use of duration of antithrombotic therapy and low-density lipoprotein cholesterol (LDL-C), inactive cancer was still an independent predictor for the composite of cardiovascular and bleeding events. In particular, inactive cancer was an independent predictor for the bleeding events, but not for cardiovascular events.
In conclusion, concomitance of inactive cancer was observed in 11% of AMI subjects receiving primary PCI with DES. They more likely harboured ARC-HBR features with severer status of AMI. Furthermore, inactive cancer was associated with 2.14-fold greater likelihood experiencing cardiovascular and bleeding events. These findings suggest that inactive cancer status is not necessarily a low-risk but malignant feature which worsen their clinical outcomes, especially bleeding ones in AMI subjects receiving primary PCI with DES.
Acknowledgments
The current study was posted on the website of our institution (https://www.ncvc.go.jp/hospital/pub/clinical-research/untersuchung/untersuchung01/) to inform its detail and ensure that patients could refuse inclusion into the current analysis. When we contacted with participants by a mail or telephone, we explained the study subjects and then obtained informed consent.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-306/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-306/dss
Conflicts of Interests: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-306/coif). YK serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2021 to August 2023, and has received research support from Nipro and Abbott, and honoraria from Nipro, Abbott, Kowa, Amgen, Sanofi, Astellas, Takeda and Daiichi-Sankyo. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised on 2013). The research protocol was approved by the ethics committee of the National Cerebral and Cardiovascular Center (M24-055-7). Informed consent for publication was not obtained in each subject due to the observational analysis of hospitalized patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Milazzo V, Cosentino N, Campodonico J, et al. Characteristics, Management, and Outcomes of Acute Coronary Syndrome Patients with Cancer. J Clin Med 2020;9:3642. [Crossref] [PubMed]
- Al-Hawwas M, Tsitlakidou D, Gupta N, et al. Acute Coronary Syndrome Management in Cancer Patients. Curr Oncol Rep 2018;20:78. [Crossref] [PubMed]
- Malmborg M, Christiansen CB, Schmiegelow MD, et al. Incidence of new onset cancer in patients with a myocardial infarction - a nationwide cohort study. BMC Cardiovasc Disord 2018;18:198. [Crossref] [PubMed]
- Larsen JB, Hojbjerg JA, Hvas AM. The Role of Platelets in Cancer-Related Bleeding Risk: A Systematic Review. Semin Thromb Hemost 2020;46:328-41. [Crossref] [PubMed]
- Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract 2011;2011:394740. [Crossref] [PubMed]
- Leader A, Ten Cate V, Ten Cate-Hoek AJ, et al. Managing Anti-Platelet Therapy in Thrombocytopaenic Patients with Haematological Malignancy: A Multinational Clinical Vignette-Based Experiment. Thromb Haemost 2019;119:163-74. [Crossref] [PubMed]
- Ikushima S, Ono R, Fukuda K, et al. Trousseau's syndrome: cancer-associated thrombosis. Jpn J Clin Oncol 2016;46:204-8. [Crossref] [PubMed]
- Gervaso L, Dave H, Khorana AA. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2021;3:173-90. [Crossref] [PubMed]
- Nakamura M, Kimura K, Kimura T, et al. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients With Coronary Artery Disease. Circ J 2020;84:831-65. [Crossref] [PubMed]
- Shapiro CL. Cancer Survivorship. N Engl J Med 2018;379:2438-50. [Crossref] [PubMed]
- Potts JE, Iliescu CA, Lopez Mattei JC, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J 2019;40:1790-800. [Crossref] [PubMed]
- Shivaraju A, Patel V, Fonarow GC, et al. Temporal trends in gastrointestinal bleeding associated with percutaneous coronary intervention: analysis of the 1998-2006 Nationwide Inpatient Sample (NIS) database. Am Heart J 2011;162:1062-1068.e5. [Crossref] [PubMed]
- Nishikawa T, Morishima T, Fujii Y, et al. Prognostic Impact of Cancer Activity on Clinically Relevant Bleeding Events After Percutaneous Coronary Intervention. J Med Invest 2021;68:29-37. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Kanenawa K, Yamaji K, Morinaga T, et al. Clinical Outcomes After Percutaneous Coronary Intervention in Patients With Cancer. Circ J 2021;85:837-46. [Crossref] [PubMed]
- Eyileten C, Postula M, Jakubik D, et al. Non-Vitamin K Oral Anticoagulants (NOAC) Versus Vitamin K Antagonists (VKA) for Atrial Fibrillation with Elective or Urgent Percutaneous Coronary Intervention: A Meta-Analysis with a Particular Focus on Combination Type. J Clin Med 2020;9:1120. [Crossref] [PubMed]
- Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J 2019;40:2632-53. [Crossref] [PubMed]
- Urban P, Mehran R, Colleran R, et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019;140:240-61. [Crossref] [PubMed]
- Natsuaki M, Morimoto T, Yamaji K, et al. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J Am Heart Assoc 2018;7:008708. [Crossref] [PubMed]
- Numasawa Y, Inohara T, Ishii H, et al. Comparison of Outcomes of Women Versus Men With Non-ST-elevation Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention (from the Japanese Nationwide Registry). Am J Cardiol 2017;119:826-31. [Crossref] [PubMed]
- Nakamura M, Kozuma K, Kitazono T, et al. Prasugrel for Japanese Patients With Ischemic Heart Disease in Long-Term Clinical Practice (PRASFIT-Practice II) - A 3-Month Interim Analysis of a Postmarketing Observational Study. Circ J 2019;83:637-46. [Crossref] [PubMed]
- Itzhaki Ben Zadok O, Hasdai D, Gottlieb S, et al. Characteristics and outcomes of patients with cancer presenting with acute myocardial infarction. Coron Artery Dis 2019;30:332-8. [Crossref] [PubMed]
- Iannaccone M, D'Ascenzo F, Vadalà P, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care 2018;7:631-8. [Crossref] [PubMed]
- Velders MA, Hagström E, James SK. Temporal Trends in the Prevalence of Cancer and Its Impact on Outcome in Patients With First Myocardial Infarction: A Nationwide Study. J Am Heart Assoc 2020;9:e014383. [Crossref] [PubMed]
- Armenian SH, Xu L, Ky B, et al. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol 2016;34:1122-30. [Crossref] [PubMed]
- Schoormans D, Vissers PAJ, van Herk-Sukel MPP, et al. Incidence of cardiovascular disease up to 13 year after cancer diagnosis: A matched cohort study among 32 757 cancer survivors. Cancer Med 2018;7:4952-63. [Crossref] [PubMed]
- Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889-97. [Crossref] [PubMed]
- Bharadwaj A, Potts J, Mohamed MO, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J 2020;41:2183-93. [Crossref] [PubMed]
- Kuhn V, Diederich L, Keller TCS 4th, et al. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid Redox Signal 2017;26:718-42. [Crossref] [PubMed]
- Caracciolo A, Scalise RFM, Ceresa F, et al. Optimizing the Outcomes of Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease. J Clin Med 2022;11:2380. [Crossref] [PubMed]
- Lutz J, Menke J, Sollinger D, et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant 2014;29:29-40. [Crossref] [PubMed]


