
The prognostic value of the left atrial strain rate determined using cardiovascular magnetic resonance feature tracking imaging in patients with severe idiopathic dilated cardiomyopathy
Introduction
Dilated cardiomyopathy (DCM) occurs with an incidence of approximately 1:250 (1) and has a poor prognosis. About 10–50% of patients with DCM exhibit symptoms of cardiac failure within a year of the onset of DCM (2). According to current guidelines, patients with severe DCM [left ventricular ejection fraction (LVEF) <35%] are at a higher risk of cardiovascular events and should receive defibrillator implantation, resynchronization therapy, or heart transplantation (3). Therefore, the evaluation of the prognosis of dilated cardiomyopathy can provide help for the clinical management of the disease.
DCM is often associated with left atrial enlargement and impairment of left atrium (LA) reservoir function, conduit function, and pump function (4,5). The previous article reported that left atrial volume index was a prognostic factor for dilated cardiomyopathy (6). But the left atrial function cannot be fully explained by the degree of left atrial dilation in dilated cardiomyopathy (7). Strain and strain rate reflect various aspects of myocardial deformation and both measure different aspects of overall heart function, which can be used to assess risk, fatality rate, and prognosis of heart attack (8-10). Recently, Dr. Li and his colleague found that left atrial reservoir and conduit strain are independent predictors of clinical outcomes in idiopathic dilated cardiomyopathy (11). Simultaneously, left atrial strain rate (LASR) has good prognostic value in atrial fibrillation (12) and rheumatic heart disease (13). However, the prognostic value of LASR in patients with DCM is unknown, and published research articles on LASR and DCM are presently lacking.
Previously, left atrial strain and strain rate can be assessed with echocardiography. However, the echocardiography examination is limited by heavy interference of gas exchange in the lungs and a low signal-to-noise ratio. In recent years, cardiovascular magnetic resonance (CMR) has been widely used in the imaging LA of cardiomyopathy (14,15). Cardiac magnetic resonance feature tracking (CMR-FT) could provide prognostic information regardless of LA size (16) and be superior to 2-dimensional echocardiography and exhibit outstanding inter-study reliability in normal, dilated, and hypertrophic hearts (17).
Therefore, this study aimed to investigate the prognostic value of left atrial strain and strain rate in dilated cardiomyopathy using CMR imaging. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-305/rc)
Methods
Study population
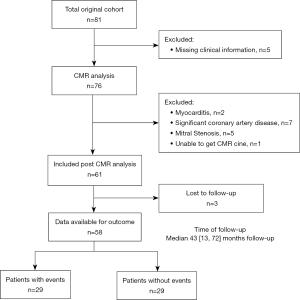
This is a single-center retrospective cohort study. The patients with severe idiopathic DCM who underwent CMR imaging at Guangdong provincial people’s hospital from March 2015 to March 2017 were selected for the analyses. The severe idiopathic DCM was diagnosed based on the World Health Organization/International Society and Federation of Cardiology criteria (18). The exclusion criteria were as follows: (I) patients with congenital heart disease (CHD); (II) patients with valvular disease; (III) patients having significant coronary artery disease (defined as showing at least 50% luminal stenosis), previous coronary revascularization, or myocardial infarction(19); (IV) patients with an estimated glomerular filtration rate less than 30 mL/min/1.73 m2 or implanted devices (20); (V) LVEF ≥35% in CMR (21). A flowchart of the study design and timelines is presented in Figure 1.
CMR image acquisition
A 3T scanner was used to perform the CMR imaging (Ingenia; Philips Medical Systems, Best, The Netherlands). Procedures and setting up parameters followed the instrument guidelines (22). The 32-element body coil for reception was adopted to transmit radiofrequency signals. A stack of short-axis single-shot balanced-standard steady-state free-precession sequence images was collected from apex to base with the two-, three-and four-chamber views.
Feature tracking (FT)
Left atrial (LA) function and volume were analyzed by QStrain, a commercial post-processing software (QStrain; Medis Suite 3.1, Leiden, the Netherlands). At left ventricular (LV) end-systole the LA maximum volume was measured, at LV diastole before LA contraction LA diastatic volume was measured, and at late LV diastole after LA contraction LA minimum volume was determined. The LA volume index (LAVi) was calculated as LAV/body surface area (BSA).
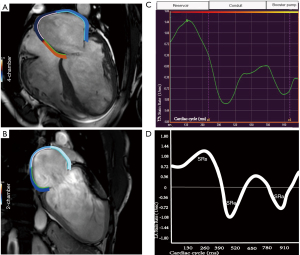
The LA endocardial border was manually delineated on two- and four- chamber cine images (avoiding pulmonary vein and atrium appendage) when the atrial volume was at its highest and lowest points (Figure 2A,2B). The shape was then replicated automatically over all frames of the whole cardiac cycle (25 frames per cardiac cycle). To ensure proper tracking, the CMR-FT was visually verified. The propagation algorithm was reapplied after the manual adjustment of the endocardial border in cases of unsatisfactory tracking. LA strain rate was calculated as the average of the values from two and four-chamber views (23). In both the two- and four-chamber views perspectives, tracking was performed three times with a blinded mode to obtain the LA volume and strain rate from the three tracking repetitions being averaged in both views. As previously stated, three characteristics of LA strain rate were analyzed (24) (Figure 2C), peak positive strain rate (referring to the function of the LA reservoir), peak late negative strain rate (referring to the function of the LA booster pump), and peak early negative strain rate (corresponding to LA conduit function). Accordingly, three strain rate (SR) parameters such as SRs, SRa, SRe were evaluated (Figure 2D). The analyze of left atrium strain rate may take 5 minutes per patients. The details of CMR-FT analysis of left ventricular global longitudinal strain (LVGLS), left ventricular global circumferential strain (LVGCS) and left ventricular global radial strain (LVGRS) are in a previous study (25).
Longitudinal follow-up and clinical event
Clinical events were defined as a combination of all-cause death, treatment with implantable cardioverter-defibrillator (ICD), hospitalization for cardiac failure, and heart transplantation. Death was adjudicated by a combination of medical record review, reports from family members, and reviewing available death certificates. Follow-up information was retrieved from clinical records, death certificates, and correspondence.
Reproducibility
Intraclass correlation coefficient as well as Bland-Altman plot were used to examine the within and between observers’ variability and reproducibility for LA strain rate in 25 randomly selected subjects. The same subject was re-analyzed within 1 month by the same observer to determine intra-observer reproducibility. A second independent observer who was blinded to the first observer’s results determined the inter-observer reproducibility.
Statistics analysis
For descriptive data, the data were reported as the means ± standard deviation or median and interquartile range for continuous variables and follow-up times, respectively. The number and percentage were used for non-missing values of categorical variables. The relationship between the two continuous variables was assessed by Pearson’s r and Spearman’s rho as appropriate.
For all survival analyses, follow-up was up to a maximum of 92 months. The Kaplan-Meier method for significance testing using log-rank statistics illustrated the cumulative probability of endpoints. Univariate and multivariate predictors of event-free patients were assessed using Cox’s proportional hazards models. The time-dependent receiver ROC was used to further assess and confirm the independent predictors’ predictive performance (26).
The variance inflation factor (vif) was assessed to avoid collinearity. A parameter with a vif greater than 4 was excluded from the multivariate analysis (27).
The primary analysis was performed using available data, including missing values. An additional sensitivity analysis was performed using multiple imputations for missing data. Multiple imputations by chained equations, an iterative imputation procedure, were used. Imputation was performed for clinical measure with any missing data, and was based on linear and logistic regression and derived for 20 imputations.
A P value (two-sided) of less than 0.05 was considered statistically significant. SPSS software [Statistical Package for the Social Sciences (SPSS) version 26.0; Armonk, NY, USA] and R version 4.0.2 were used for all statistical analyses (R Foundation, Vienna, Austria). The “survival” and “timeROC” functions were used for the analyses.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective cohort study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (No. KY-Q-2021-130-02) and written informed consent was obtained from all subjects.
Results
Baseline characteristics
Of the 61 patients who met the inclusion criteria for the study, 3 patients were not followed up on and 58 patients were followed up for a median of 43 months (IQR, 13–72 months) (Figure 1). During the follow-up period of 92 months after enrollment, adverse cardiovascular events developed in 29 (50%) patients, all-cause death occurred in 15 participants (25.8%), ICD treatment was performed in 5 participants (8.6%), hospitalization due to heart failure occurred in 8 participants (13.8%), and heart transplant was performed in 1 participant (1.7%). Regarding clinical characteristics (except age, P=0.007), hemodynamic parameters, and comorbidity parameters (except hypertension, P=0.020; diabetes, P=0.014), there were no significant differences between patients. Some clinical, hemodynamics and comorbidities characteristics of participants were missing. But each variable of interest had no missing value. The clinical, hemodynamic and comorbidity characteristics of patients are presented in Table 1.
Table 1
| Variable | All patients (n=58) | Cardiovascular events | p | |
|---|---|---|---|---|
| No (n=29) | Yes (n=29) | |||
| Clinical, hemodynamics, and comorbidities characteristics of participants with and without event | ||||
| Mean age (y) | 46.04±14.15 | 41.24±12.42 | 51.19±14.29 | 0.007# |
| No. of men | 41 [73] | 22 [76] | 19 [70] | 0.650 |
| Systolic BP (mmHg) | 111.17±16.29 | 113.3±11.47 | 109.04±20.00 | 0.342 |
| Diastolic BP (mmHg) | 74.48±16.73 | 73.74±8.47 | 75.22±22.31 | 0.748 |
| BMI (kg/m2) | 24.46±4.55 | 25.07±4.88 | 23.81±4.14 | 0.304 |
| No. of participants who smoke | 17 [34] | 7 [29] | 10 [38] | 0.498 |
| No. of participants who drink alcohol | 7 [14] | 4 [17] | 3 [12] | 0.610 |
| NYHA class ≤ II | 28 [52] | 16 [57] | 12 [46] | 0.816 |
| LBBB | 7 [14] | 2 [8] | 5 [21] | 0.188 |
| Hypertension | 9 [18] | 1 [4] | 8 [30] | 0.020# |
| Diabetes | 9 [18] | 1 [4] | 8 [31] | 0.014# |
| Serum creatinine value (mmol/L)* | 85.00 (75.75, 99.00) | 81.00 (75.75, 95.25) | 89.00 (70.25, 101.00) | 0.791 |
| Troponin T value (ng/L)* | 19.71 (8.00, 30.00) | 17.5 (1.75, 30.00) | 21.01 (8.00, 37.00) | 0.472 |
| NT-proBNP value (pg/mL)* | 1,836.00 (840.00, 3,558.00) | 1,826.00 (713.00, 2,532.00) | 2,559.00 (1,197.00, 4,861.00) | 0.264 |
| ACEI/ARB | 12 [24] | 6 [26] | 6 [23] | 0.812 |
| B-blockers | 49 [96] | 24 [100] | 25 [93] | 0.181 |
| Spironolactone | 47 [92] | 24 [100] | 23 [85] | 0.051 |
| Diuretics | 0.93±0.26 | 0.96±0.19 | 0.89±0.32 | 0.308 |
| Cardiac MRI characteristics of participants with and without event | ||||
| LVEF (%) | 19.57±8.74 | 19.84±8.42 | 19.29±9.23 | 0.817 |
| LVEDVi (mL/m2) | 167.93±55.82 | 164.19±50.86 | 171.95±61.43 | 0.608 |
| LVESVi (mL/m2) | 135.28±50.25 | 131.23±43.88 | 139.63±56.84 | 0.537 |
| LVmassi (g/m2) | 51.42±17.38 | 52.01±17.92 | 50.78±17.09 | 0.795 |
| LVGLS (%) | −9.08±3.84 | −8.85±3.06 | −9.32±4.52 | 0.641 |
| LVGCS (%) | −6.72±3.23 | −6.58±2.86 | −6.86±3.62 | 0.748 |
| LVGRS (%) | 25.08 (1.56, 48.10) | 19.57 (1.39, 37.75) | 31.71 (3.10, 60.32) | 0.123 |
| Mitral valve regurgitation | 1.94±0.99 | 1.71±1.01 | 2.21±0.91 | 0.074 |
| LGE present | 41 [75] | 22 [79] | 19 [70] | 0.494 |
| LGE extent* | 6.00 (0.00, 9.62) | 3.85 (0.14, 10.21) | 3.25 (0.00, 8.68) | 0.529 |
| LA maximum volume index (mL/m2)* | 62.07 (46.60, 100.80) | 54.28 (44.66, 76.75) | 76.70 (56.93, 110.10) | 0.051 |
| LA diastatic volume index (mL/m2)* | 54.80 (36.20, 93.95) | 47.80 (33.16, 63.28) | 68.99 (44.81, 99.21) | 0.032# |
| LA minimum volume index (mL/m2)* | 47.71 (24.50, 83.00) | 40.85 (23.55, 57.95) | 57.94 (31.11, 94.85) | 0.069 |
| SRs (sec-1)* | 0.55 (0.40, 0.71) | 0.65 (0.50, 0.98) | 0.45 (0.33, 0.63) | 0.003# |
| SRe (sec-1)* | −0.36 (−0.72, −0.17) | −0.52 (−0.73, −0.13) | −0.31 (−0.72, −0.18) | 0.458 |
| SRa (sec-1)* | −0.46 (−0.74, −0.20) | −0.59 (−0.90, −0.32) | −0.37 (−0.65, −0.15) | 0.027# |
Normally distributed continuous variables are shown as mean ± SD; non-normally distributed continuous variables are shown as median (interquartile range); categorical variables are shown as n [%]. *, data are medians; data in parentheses are the interquartile range; #, P<0.05. BP, blood pressure; BMI, body mass index; NYHA, New York Heart Association; LBBB, left bundle branch block; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricle ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index; LVmassi, left ventricular mass index; LVGLS, global longitudinal strain of left ventricle; LVGCS, global circumferential strain of left ventricle; LVGRS, global radial strain of left ventricle; LGE, late gadolinium enhancement; LA, left atrium; SRs, reservoir strain rate; SRe, conduit stain rate; SRa, booster pump stain rate.
CMR imaging
LA strain rate and other CMR imaging characteristics are shown in Table 1. In patients with severe idiopathic DCM with events, LA diastatic volume index was larger than patients without events [68.99 (44.81, 99.21) vs. 47.80 (33.16, 63.28), P=0.032] (mL/m2). LA reservoir and pump function were worse in patients with event [LASRs 0.45 (0.33, 0.63) vs. 0.65 (0.50, 0.98) (sec−1), P=0.003; LASRa −0.37 (−0.65, −0.15) vs. −0.59 (−0.90, −0.32) (sec−1), P=0.027].
Cox regression analysis
In univariate analysis, LASRs [HR 0.12; 95% CI (0.02, 0.55), P=0.007], and left atrium booster pump strain rate (LASRa) [HR 3.21; 95% CI (1.08, 9.58), P=0.036] displayed significant predictive associations with cardiovascular events (Table 2).
Table 2
| Variable | Univariable | |
|---|---|---|
| HR (95% CI) | P value | |
| Mean age (y) | 1.04 (1.01, 1.08) | 0.010* |
| No. of men | 0.68 (0.29, 1.57) | 0.364 |
| Systolic BP (mmHg) | 0.99 (0.97, 1.02) | 0.665 |
| Diastolic BP (mmHg) | 0.99 (0.98, 1.02) | 0.981 |
| BMI (kg/m2) | 0.92 (0.83, 1.01) | 0.074 |
| No. of participants who smoke | 0.97 (0.43, 2.17) | 0.941 |
| No. of participants who drink alcohol | 0.68 (0.20, 2.26) | 0.400 |
| NYHA class | 1.22 (0.65, 2.28) | 0.291 |
| LBBB | 1.23 (0.45, 3.36) | 0.683 |
| Hypertension | 3.19 (1.37, 7.44) | 0.007* |
| Diabetes | 2.74 (1.16, 6.47) | 0.021* |
| Serum creatinine value (mmol/L) | 1.00 (0.98, 1.01) | 0.620 |
| Troponin T value (ng/L) | 1.01 (0.99, 1.02) | 0.273 |
| NT-proBNP value (pg/mL) | 1.00 (1.00, 1.00) | 0.030* |
| ACEI/ARB | 0.99 (0.39, 2.49) | 0.988 |
| B-blockers | 0.43 (0.10, 1.84) | 0.259 |
| Spironolactone | 0.36 (0.12, 1.08) | 0.067 |
| Diuretics | 0.36 (0.11, 1.2) | 0.096 |
| LVEF (%) | 0.99 (0.95, 1.04) | 0.817 |
| LVEDVi (mL/m2) | 1.00 (0.99, 1.01) | 0.362 |
| LVESVi (mL/m2) | 1.00 (0.99, 1.01) | 0.316 |
| LVmassi (g/m2) | 1.00 (0.98, 1.02) | 0.817 |
| LVGLS (%) | 0.97 (0.88, 1.07) | 0.573 |
| LVGCS (%) | 0.97 (0.87, 1.09) | 0.636 |
| LVGRS (%) | 1.02 (0.99, 1.04) | 0.078 |
| Mitral valve regurgitation | 1.73 (1.09, 2.75) | 0.021* |
| LGE present | 0.63 (0.26, 1.51) | 0.301 |
| LGE extent | 1.01 (0.95, 1.08) | 0.799 |
| LA maximum volume index (mL/m2) | 1.01 (1.00, 1.01) | 0.041* |
| LA diastatic volume index (mL/m2) | 1.01 (1.00, 1.01) | 0.002* |
| LA minimum volume index (mL/m2) | 1.01 (1.00, 1.02) | 0.024* |
| SRs (sec−1) | 0.12 (0.02, 0.55) | 0.007* |
| SRe (sec−1) | 1.40 (0.52, 3.76) | 0.499 |
| SRa (sec−1) | 3.21 (1.08, 9.58) | 0.036* |
*, P<0.05. HR, hazard ratio; CI, confidence interval; BP, blood pressure; BMI, body mass index; NYHA, New York Heart Association; LBBB, left bundle branch block; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVEF, left ventricle ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index; LVmassi, left ventricular mass index; LVGLS, global longitudinal strain of left ventricle; LVGCS, global circumferential strain of left ventricle; LVGRS, global radial strain of left ventricle; LGE, late gadolinium enhancement; LA, left atrium; SRs, reservoir strain rate; SRe, conduit stain rate; SRa, booster pump stain rate.
In multivariate analysis, every significant LA strain rate (P<0.05) was put into the adjusted model individually. In adjusted model 1, after adjusting for significant traditional cardiovascular risk factors, including hypertension, diabetes, and NT-proBNP), the LASRs [HR 0.12, 95% CI (0.02, 0.58), P=0.009]; the LASRa [HR 1.69, 95% CI (0.51, 5.62), P=0.388]. After additional adjustment for significant cardiac function factors, including mitral valve regurgitation, LA maximum volume index, LA diastatic volume index, the LASRs [HR 0.13, 95% CI (0.02, 0.82), P=0.030]; the LASRa [HR 2.40, 95% CI (0.65, 8.83), P=0.189] (Table 3). The overall findings with respect to LASRs and LASRa were not substantially altered in a sensitivity analysis using multiple imputations for missing data (Table S1).
Table 3
| Model | LASRs | LASRa | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Unadjusted | 0.12 (0.02, 0.55) | 0.007* | 3.21 (1.08, 9.58) | 0.036* | |
| Adjusted1 | 0.12 (0.02, 0.58) | 0.009* | 1.69 (0.51, 5.62) | 0.388 | |
| Adjusted 2 | 0.13 (0.02, 0.82) | 0.030* | 2.40 (0.65, 8.83) | 0.189 | |
*, P<0.05. Adjusted1: the variables including age, hypertension, diabetes, NT-proBNP; Adjusted2: the variables including age, hypertension, diabetes, NT-proBNP were adjusted, as well as mitral valve regurgitation, LA maximum volume index, LA diastatic volume index. LASRs, left atrium reservoir strain rate; LASRa, left atrium booster pump strain rate; HR, hazard ratio; CI, confidence interval; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
LA strain rate and event risk
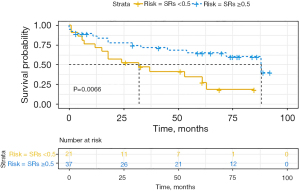
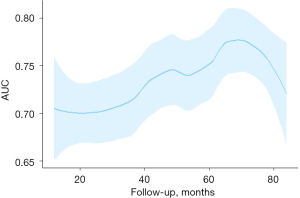
Participants with the cardiovascular event exhibited impaired LASRs (P<0.05) (Table 1). Optimal cut-off values were 0.5 for LASRs from time-dependent ROC analysis. Participants with reservoir strain rate less than 0.5 sec−1 displayed higher risks of the event by Kaplan-Meier analysis (log-rank, P=0.0066) (Figure 3). Results of time-dependent ROC analysis on LASRs against adverse cardiac events are shown in Figure 4. LASRs showed the greatest integrated areas under the ROC curve for predicting adverse cardiac events. The area under the curve (AUC) of receiver operating characteristic (ROC) curve were 0.697 (0.617, 0.777) for 1 year, 0.716 (0.643, 0.788) for 3 years, 0.716 (0.651, 0.798) for 5 years and 0.703 (0.597, 0.809) for 7 years.
Correlation between LA strain rate and other parameters
The correlation between the LA strain rate, LA volume index, and LV function is shown in Table 4. LASRs was mildy decreased with NT-proBNP value (P=0.03). LASRs was found to be moderately related to LA volume index and mildly related to LV ventricular end-diastolic volume index, left ventricular mass index, LVGLS and LVGCS (all P<0.05). The left atrium conduit strain rate (LASRe) was mildly associated with left ventricular ejection fraction (LVEF), LA diastolic volume index LVGLS and LVGCS (P<0.05). LASRa was moderately decreased with LA volume index (all P<0.001) and mild increased with LVGCS (P=0.036).
Table 4
| Parameter | LASRs | LASRe | LASRa | |||||
|---|---|---|---|---|---|---|---|---|
| r value | P value | r value | P value | r value | P value | |||
| NT-proBNP value | −0.30 | 0.030 | 0.24 | 0.093 | 0.20 | 0.149 | ||
| LVEF | 0.15 | 0.270 | −0.27 | 0.045 | −0.15 | 0.274 | ||
| LVEDVi | −0.35 | 0.008 | 0.17 | 0.198 | 0.17 | 0.200 | ||
| LVmassi | −0.30 | 0.027 | 0.17 | 0.218 | 0.12 | 0.368 | ||
| LVGLS | −0.33 | 0.012 | 0.32 | 0.013 | 0.22 | 0.102 | ||
| LVGCS | −0.38 | 0.003 | 0.30 | 0.025 | 0.28 | 0.036 | ||
| LVGRS | 0.28 | 0.077 | −0.21 | 0.194 | −0.26 | 0.100 | ||
| LA maximum volume index | −0.42 | 0.001 | 0.12 | 0.357 | 0.58 | <0.001 | ||
| LA diastatic volume index | −0.50 | <0.001 | 0.28 | 0.038 | 0.58 | <0.001 | ||
| LA minimum volume index | −0.56 | <0.001 | 0.26 | 0.052 | 0.66 | <0.001 | ||
LASRs, left atrium reservoir strain rate; LASRe, left atrium conduit strain rate; LASRa, left atrium booster pump strain rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVEF, left ventricle ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LVmassi, left ventricular mass index; LVGLS, global longitudinal strain of left ventricle; LVGCS, global circumferential strain of left ventricle; LVGRS, global radial strain of left ventricle; LA, left atrium.
Reproducibility analysis
LA strain rate parameters were reproducible both within and between observers. Bland-Altman plot analysis as well as Intraclass correlation coefficient for strain rate measurements are shown in Table S2. The analysis of intra-observer measurements revealed a high level of congruence. For SR, the minimum and maximum standard deviations were 0.2 and 0.32, respectively. Intraclass correlation for 95% of patients was 0.844 (0644 to 0.932), and 0.948 (0.883 to 0.978). Bland-Altman analysis and intraclass correlation efficient (ICC) showed no evidence of any significant difference regarding inter- and intra-observer variability. The results are in Table S2.
Discussion
The accurate role of left atrial strain rate in predicting the prognosis of severe idiopathic DCM remains unclear. Our study evaluated the LA strain rate in participants with severe idiopathic DCM in a cardiac MRI cohort with a median follow-up of 43 months (IQR, 13–72 months). One major finding of this study was that LASRs and SRa were powerful prognostic markers in univariate analysis. Moreover, the LASRs demonstrated independent prognostic value in severe idiopathic DCM after adjustment for significant basic cardiovascular risk factors and cardiac function indicators.
Left atrial volume and phasic function in DCM patients
The volume of the LA was a prognostic factor of DCM. Gulati et al. (28) reported that LAVi was a strong predictor of transplant-free survival and HF outcome in DCM. Our results demonstrated that the LA volume index also provided a significant prognostic value, which was consistent with a previous study (6). This might have related to the pathology and mechanism of dilated cardiomyopathy. Dilated cardiomyopathy is characterized by left ventricular dilation associated with systolic dysfunction. Diastolic dysfunction and impaired right ventricular function may occur (29,30). LA dilation can explain the left ventricular dilation dysfunction to a certain extent. While the degree of left atrial dilation cannot fully explain left atrial dysfunction in dilated cardiomyopathy (20). Compared with ischemic patients, LA booster pump function was inhibited in idiopathic DCM, even under the same load conditions (31). Moreover, D’Andrea et al. (32) reported that although the LA maximum volume had no difference in all analyzed atrial segments, the peak systolic myocardial atrial strain was significantly lower in patients with idiopathic DCM than in ischemic DCM. The evaluation of LA function is very significant in predicting the occurrence of cardiovascular events. Both strain rate and strain were relatively independent of holistic heart function and they reflected different aspects of myocardial deformation (33). Previous reports indicated that LASR had prognostic value for atrial fibrillation (12) and rheumatic heart disease (13). But as far as we know, no reported research were made on the prognostic value of LASR in severe idiopathic DCM patients. In our study, LASRs and LASRa were found to be powerful prognostic markers from univariate analysis. This may have been due to LA reservoir function, and pump function might be useful in estimating left ventricular filling pressure (23). LA reservoir strain to store blood during ventricular systole was an independent predictor of cardiovascular events in myocardial infarction (34) and dilated cardiomyopathy (11). And in our study, after adjustment for model 1 and model 2, LASRs exhibited independent prognostic value.
Echocardiography and CMR measurement of left atrial function
Echocardiography and CMR imaging have their advantages and disadvantages in the measurement of left atrial function. Strain imaging using 2D speckle tracking of the LA, which was less susceptible to the limitations of Doppler echocardiographic assessment, was used for the assessment of left atrial function (35). However, reports confirmed that left atrial function using CMR imaging was not related to LA size significantly, thus may have provided the prognostic information independent of LA size (16). CMR imaging had excellent inter-study reproducibility in normal, dilated, and hypertrophic hearts, and is superior to 2-dimensional echocardiography (17). In our study, LA strain rate assessment had a great reproducibility.
Limitation
First, the insufficient number of patients was one of the important limitations in our study, which might have reduced the weight of evidence. In addition, another important limitation of this study is that this study was conducted in one hospital with a single CMR imaging protocol and explanation. A multi-institutions study is required to validate our conclusions. What’s more, some clinical, hemodynamics, and comorbidities characteristics of participants were missing. However, a sensitivity analysis using multiple imputations to account for missing data showed similar findings to the primary analysis (Table S1). Finally, the cutoff of LASRs should ideally be derived from a larger healthy populations. The cutoff of LASRs (0.5 sec−1) in our study obtained from time-dependent ROC analysis of the 58 participants could not represent the true effect.
Conclusions
LA reservoir strain rate and active strain rate were powerful prognostic markers. The LASRs by MRI-FT provided independent prognostic value in patients with severe idiopathic DCM.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant Nos. 81974262 and 81970288), Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010650), and Guangdong Cardiovascular Institute Project (Grant No. 2020XXG009).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-305/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-305/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-305/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-305/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013;10:531-47. [Crossref] [PubMed]
- Deedwania PC. The key to unraveling the mystery of mortality in heart failure: an integrated approach. Circulation 2003;107:1719-21. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Abdelghani Abdelzaher M, Atteia WM. Left atrial geometry and pump function in ischemic cardiomyopathy. Int J Cardiol Heart Vasc 2014;5:45-50. [Crossref] [PubMed]
- Kurzawski J, Janion-Sadowska A, Gackowski A, et al. Left atrial longitudinal strain in dilated cardiomyopathy patients: is there a discrimination threshold for atrial fibrillation? Int J Cardiovasc Imaging 2019;35:319-25. [Crossref] [PubMed]
- Modena MG, Muia N, Sgura FA, et al. Left atrial size is the major predictor of cardiac death and overall clinical outcome in patients with dilated cardiomyopathy: a long-term follow-up study. Clin Cardiol 1997;20:553-60. [Crossref] [PubMed]
- Triposkiadis F, Pitsavos C, Boudoulas H, et al. Left atrial myopathy in idiopathic dilated cardiomyopathy. Am Heart J 1994;128:308-15. [Crossref] [PubMed]
- Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493-505. [Crossref] [PubMed]
- Ramkumar S, Yang H, Wang Y, et al. Association of the Active and Passive Components of Left Atrial Deformation with Left Ventricular Function. J Am Soc Echocardiogr 2017;30:659-66. [Crossref] [PubMed]
- Chirinos JA, Sardana M, Ansari B, et al. Left Atrial Phasic Function by Cardiac Magnetic Resonance Feature Tracking Is a Strong Predictor of Incident Cardiovascular Events. Circ Cardiovasc Imaging 2018;11:e007512. [Crossref] [PubMed]
- Li Y, Xu Y, Tang S, et al. Left Atrial Function Predicts Outcome in Dilated Cardiomyopathy: Fast Long-Axis Strain Analysis Derived from MRI. Radiology 2022;302:72-81. [Crossref] [PubMed]
- Shih JY, Tsai WC, Huang YY, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr 2011;24:513-9. [Crossref] [PubMed]
- Caso P, Ancona R, Di Salvo G, et al. Atrial reservoir function by strain rate imaging in asymptomatic mitral stenosis: prognostic value at 3 year follow-up. Eur J Echocardiogr 2009;10:753-9. [Crossref] [PubMed]
- Yang Y, Yin G, Jiang Y, et al. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson 2020;22:1. [Crossref] [PubMed]
- Walters TE, Ellims AH, Kalman JM. The role of left atrial imaging in the management of atrial fibrillation. Prog Cardiovasc Dis 2015;58:136-51. [Crossref] [PubMed]
- Kühl JT, Lønborg J, Fuchs A, et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging 2012;28:1061-71. [Crossref] [PubMed]
- Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29-34. [Crossref] [PubMed]
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841-2. [Crossref] [PubMed]
- Arenja N, Riffel JH, Fritz T, et al. Diagnostic and Prognostic Value of Long-Axis Strain and Myocardial Contraction Fraction Using Standard Cardiovascular MR Imaging in Patients with Nonischemic Dilated Cardiomyopathies. Radiology 2017;283:681-91. [Crossref] [PubMed]
- Riffel JH, Keller MG, Rost F, et al. Left ventricular long axis strain: a new prognosticator in non-ischemic dilated cardiomyopathy? J Cardiovasc Magn Reson 2016;18:36. [Crossref] [PubMed]
- Park SM, Kim YH, Ahn CM, et al. Relationship between ultrasonic tissue characterization and myocardial deformation for prediction of left ventricular reverse remodelling in non-ischaemic dilated cardiomyopathy. Eur J Echocardiogr 2011;12:887-94. [Crossref] [PubMed]
- Kramer CM, Barkhausen J, Flamm SD, et al. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [Crossref] [PubMed]
- Leng S, Tan RS, Zhao X, et al. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson 2018;20:71. [Crossref] [PubMed]
- Kowallick JT, Kutty S, Edelmann F, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 2014;16:60. [Crossref] [PubMed]
- Chen R, Wang J, Du Z, et al. The comparison of short-term prognostic value of T1 mapping with feature tracking by cardiovascular magnetic resonance in patients with severe dilated cardiomyopathy. Int J Cardiovasc Imaging 2019;35:171-8. [Crossref] [PubMed]
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337-44. [Crossref] [PubMed]
- Akinwande MO, Dikko HG, Samson A. Variance Inflation Factor: As a Condition for the Inclusion of Suppressor Variable(s) in Regression Analysis. Open Journal of Statistics 2015;5:754-67. [Crossref]
- Gulati A, Ismail TF, Jabbour A, et al. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail 2013;15:660-70. [Crossref] [PubMed]
- Schultheiss HP, Fairweather D, Caforio ALP, et al. Dilated cardiomyopathy. Nat Rev Dis Primers 2019;5:32. [Crossref] [PubMed]
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375:752-62. [Crossref] [PubMed]
- D'Andrea A, Caso P, Romano S, et al. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: a two-dimensional speckle strain study. Eur Heart J 2007;28:2738-48. [Crossref] [PubMed]
- D'Andrea A, Caso P, Romano S, et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two-dimensional speckle strain study. Int J Cardiol 2009;132:354-63. [Crossref] [PubMed]
- Wang Z, Tan H, Zhong M, et al. Strain rate imaging for noninvasive functional quantification of the left atrium in hypertensive patients with paroxysmal atrial fibrillation. Cardiology 2008;109:15-24. [Crossref] [PubMed]
- Nayyar D, Nguyen T, Pathan F, et al. Cardiac magnetic resonance derived left atrial strain after ST-elevation myocardial infarction: an independent prognostic indicator. Cardiovasc Diagn Ther 2021;11:383-93. [Crossref] [PubMed]
- Kebed KY, Addetia K, Lang RM. Importance of the Left Atrium: More Than a Bystander? Heart Fail Clin 2019;15:191-204. [Crossref] [PubMed]


