
Insights and perspectives into clinical biomarker discovery in pediatric heart failure and congenital heart disease—a narrative review
Introduction
Background and history of pediatric congenital heart disease
Heart failure (HF) in pediatric congenital patients is a multi-factorial process with a wide spectrum of etiologies and clinical manifestations that are very distinct from the adult HF population (1-4). With the effective disappearance of native rheumatic heart disease in the US due to improved hygiene and the application of antibiotics with symptoms, the most common cause of pediatric HF has become congenital heart disease (CHD) (5-7). Worldwide, CHD still has a very high mortality with an increasing prevalence with nearly 60% developing HF in the first 12 months of life (8-10). Hence, early discovery and diagnosis of CHD in neonates is pivotal. For infants as well as children with heart conditions, HF is the most common indication to receive medical treatment and 50% of the referrals with significant failure eventually leading to the evaluation for heart transplantation (11). Children with CHD who eventually receive heart transplantation, have a different in outcome from those with cardiomyopathy (12). The overall mortality is similar, but patients with cardiomyopathy may have fewer morbidities. Nevertheless, the incidence as well as the prevalence of pediatric HF is still not known, due to the many types and etiologies, without an accepted universal classification (5-7).
However, a simple, reliable, and quantifiable systematic test to classify and standardize pediatric HF patients, especially in CHD, has been lacking. There are still many patients who have mal-adaptive anatomical substrate or residual lesions that may lead to HF over time. Clinical trials involving invasive studies can be difficult due to the sensitive research issues and barriers involving children. The potential need to corroborate findings with invasive studies such as frequent blood tests or invasive testing such as indwelling catheters can decrease parental acceptance for research studies. Consequently, most diagnosis and therapy in pediatric HF patients is still based on subjective observations along with standard testing. Due to the intense variability with CHD, classification schema are complicated and a method to standardize and quantitatively gauge HF is difficult. Translating conclusions from adult studies into the pediatric realm has also become commonplace.
Several biomarkers for HF including the extensively investigated atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), C-reactive protein (CRP) and tumor necrosis factor alpha (TNF-α) have been identified and are applied clinically in the adult population to assess the diagnosis, severity and management of the HF condition before and after treatment (13-17). BNP as a clinical biomarker in the assessment of pediatric HF and CHD is straightforward and can be a significant factor in the diagnosis and treatment (7,12,18). More recently, additional reports have indicated that plasma BNP, sometimes in parallel to Troponin-I (19), can be used as suitable diagnostic and prognostic biomarkers in pediatric HF (15,16,20). However, in contrast to adult HF, plasma BNP as clinical biomarker is not yet included in the pediatric HF guidelines and there is no standardized reference cut-off value yet (16,21). Furthermore, in a wide variety of (multi)-Omics studies, several constituents are noteworthy and becoming increasingly popular such as Galectin-3 (22,23), Lipids (24) and Free Fatty Acids (25,26). We searched for biomarkers with respect to diagnosis and monitoring in specific anatomical types of CHD in the pediatric population, together with a concise description of our own experience in applying plasma BNP as a clinical biomarker in pediatric HF and CHD in the setting of surgical correction. We present the following article in accordance with the Narrative Review reporting checklist (available at: https://cdt.amegroups.com/article/view/10.21037/cdt-22-386/rc).
Methods
A query of the literature using the online search engines PubMed and Google scholar was conducted for pediatric HF and CHD (Table 1). Accordingly, no data restrictions were incorporated (from origin to July 2022), and only publications in English were considered. We queried a broad search using definitions and key words such as “children”, “heart failure”, “congenital heart disease”, “biomarkers”, “genes”, “proteins”, and “metabolites”. Lastly, we also combined key words “BNP and multi-omics and metabolomics” to search for BNP studies integrated with multi-omics. We encountered many manuscripts reporting biomarkers in pediatric patients also with CHD. As part of a narrative review, we focused on the most notable proteins and metabolites reported over the last 5 years. We will discuss further experiences and developments of the use of the known biomarkers BNP and N-terminal ProBNP (NT-ProBNP) in pediatric HF observing an increasing number of reports with positive application in diagnosis and management, especially in recent years.
Table 1
| ltems | Specification |
|---|---|
| Date of search | 07/1/2022–07/16/2022 |
| Databases and other sources searched | PubMed was the primary database used, Google Scholar was also used but far less extensive than PubMed |
| Search terms used | “Children” [All Fields] AND “Pediatric” [All Fields] AND “Heart Failure” [All Fields] |
| “Children” [All Fields] AND “Heart Failure” [All Fields] AND “Congenital” | |
| “Children” [All Fields] AND “Heart Failure” [All Fields] AND “Biomarkers” [All Fields] | |
| “Children” [All Fields] AND “Heart Failure” [All Fields] AND “Proteins” [All Fields] AND “Metabolites” [All Fields] AND “Genes” [All Fields] | |
| “Children” [All Fields] AND “Heart Failure” [All Fields] AND “B-type natriuretic peptide” [All Fields] AND “BNP” [All Fields] | |
| Timeframe | All published manuscripts up to July 2022 were considered in our basic query |
| There was a focus on “recent” review studies (published from 2010 onwards) that directly addressed Pediatric Heart Failure | |
| Inclusion and exclusion criteria | Focus was placed on published original papers and reviews in English. Non English manuscript not translated were excluded |
| Manuscripts were excluded that were not relevant for the scope of the paper, or that addressed adult heart failure | |
| Selection progress | The queries were conducted independently by DL, MC, SS |
In a pilot study performed in Long Beach, we conducted Metabolic Profiling of Blood Plasma before and after surgical correction of CHD in neonates in HF: Employing a multiple-reaction-monitoring (MRM) based mass spectrometry (MS) quantitative methodology, we examined 9 quantification panels of plasma metabolites [P180, Energy Metabolism, Free Fatty Acids, Eicosanoids and other oxidation products of polyunsaturated fatty acids (PUFAs), Lipids, Steroids, Neurotransmitters, Bile acids, Oxidative status assays] using service offered by Biocrates Life Science AG (Innsbruck, Austria). We aimed for a total of 610 plasma metabolites; the absolute abundance levels of plasma metabolites were quantified for following computational analyses. All children were between 3–6 months old. For statistical analysis of metabolites, we conducted a differential analysis. A multiple testing correction was applied to the P values calculated by the t-tests, using the Benjamin-Hochberg method to reduce the false positive rate. A threshold of false discovery rate (FDR) <0.05 and ±1.5 Fold-change was applied to detect metabolites with significant changes. The figure displaying neonatal metabolomics pilot data was made in r-programming. Plasma BNP levels before and after surgery were compared with paired t-test. The study on the infants is approved by the Institutional Review Board (IRB) of the Miller’s Children Hospital in Long Beach (IRB No. 086-12). The IRB is overseeing all ethical and safety aspects of the patients. Dr. Setty has had annual meetings with the IRB to discuss and report safety and privacy aspects. All blood withdrawals as part of the pilot study were conducted during routine clinical care of the patients via their already present central line. Accordingly, the patients were not subjected to any additional risk or harm during the study. For each patient, parental informed consent was received before inclusion into the study. The study carried out conforms to the Declaration of Helsinki (as revised in 2013), available at: https://www.wma.net/wp-content/uploads/2016/11/DoH-Oct2013-JAMA.pdf.
We conducted a text mining algorithm for 8,325 heart related proteins over 33M publications on PubMed to quantify these according to their correlation in CHD and created a knowledge graph. Detailed description and methods are described previously (27,28).
Pathophysiology of pediatric congenital HF
HF is a complex interplay of neuro-hormonal, molecular-cellular, and circulatory hemodynamic factors rather than a simple dysfunction of the heart (29,30). Although the majority of CHD is based on a variety of factors, some cases, such as specific cardiomyopathies, are only associated with a chromosomal abnormality, a single gene defect, teratogens, or maternal metabolic syndrome. In contrast to adult HF, CHDs in pediatric HF patients, especially based on structural heart defects, are extremely heterogeneous with a wide variety of types and subclasses each with distinct pathophysiology (31). The structural heart defect affects the pressures in all four heart chambers and the blood flow containing oxygen and carbon dioxide. The heterogenic situation depends on whether an obstruction is present in the left or right heart which subsequently determines dependency on duct/shunt. A very important element in this complex pathophysiology is the occurrence of pulmonary overflow or underflow of blood which affects its oxygen levels. It is very likely that all known clinical biomarkers such as BNP, CRP, TNF-α and any other potential multi-omics markers are strongly affected by the heterogenic condition in CHDs.
Structural defects in CHD can be separated into three general main pathophysiological groups: (I) left-to-right shunts, (II) right-to-left shunts, and (III) obstructive stenotic lesions on both the right and left sides (Table 2). Acyanotic CHD includes the left-to-right shunts with increased pulmonary blood flow but normal saturations. Right-to-left lesions can decrease pulmonary blood flow and desaturate blood on the systemic side, while obstructive lesions can cause a combination of the two depending on the specific anatomy.
Table 2
| Pathophysiology | Incidence (% of all CHD) | Shunt | Obstructive |
|---|---|---|---|
| Acyanotic | |||
| VSD | 25 | L→R | |
| ASD | 10 | L→R | |
| PDA | 10 | L→R | |
| AVSD | 5 | Mixing | |
| Coarctation of the aorta | 10 | √ | |
| Aortic stenosis | 10 | √ | |
| Pulmonary valve stenosis | 10 | √ | |
| Cyanotic | |||
| TOF | 10 | R→L | √ |
| Transposition great vessels | 5 | R→L | |
| Tricuspid atresia | 1-2 | R→L | √ |
| Truncus arteriosus | 1 | Mixing | |
| Hypoplastic left ventricle | 1 | Mixing | |
| Pulmonary atresia | 1 | √ | |
| TAPVR | 1 | Mixing | √ |
Basic overview of structural defects, acyanotic and cyanotic with a sample representation of some anatomical configurations, incidence, shunt type (either from left-to-right, from right-to-left, or mixed), and obstructive nature. VSD, ventricle septal defect; ASD, atrial septal defect; PDA, patent ductus arteriosus; AVSD, atrial ventricle septal defect; TOF, tetralogy of fallot; TAPVR, total anomalous pulmonary venous return; CHD, congenital heart disease.
Definition and classification of pediatric HF
HF can be defined as a clinical and pathophysiologic syndrome resulting from ventricular dysfunction, volume or pressure overload or a mixture of these pathologies. According to The International Society for Heart and Lung Transplantation, pediatric HF can be defined as both a clinical and pathophysiological syndrome initiated by insufficiency of the ventricle and volume or pressure overload, either alone, or a mixture of both (32). Since pediatric HF has several different triggers, it has a variety of clinical presentations and symptoms that also depend on the age of the patient (10,33,34). In infants, the earliest clinical manifestations are usually subtle and typically present with feeding difficulties due to a baseline dyspnea (35). Eventually, infants that are affected by HF will classically present with failure to thrive mainly observed by poor weight gain by following growth curves. Additional findings during the physical examination in infants who have HF include mild-to-severe retractions with respirations, tachypnea, or dyspnea with grunting (a form of positive end-expiratory pressure), tachycardia, a gallop rhythm (S3, S4), cardiomegaly and hepatomegaly. Toddlers and older may present with exercise intolerance, somnolence, anorexia, or with other signs & symptoms such a shortness of breath, crackles or wheezing, and cough. In both infants and past the toddler age, the physical examination may present with a gallop-rhythm and hepatomegaly as well as peripheral edema and jugular venous distention.
To better understand the specific patient-based management and response to pediatric HF therapy (medical and/or surgical) as well as its complex pathophysiology, a comprehensive knowledge supported by evident laboratory and clinical tests are crucial. Accordingly, pulse oximetry is beneficial in determining cyanosis, electrocardiogram (EKG) is important to assess arrhythmias that can induce cardiac dysfunction, the chest X-ray is used to determine cardiomegaly indicated by the cardiothoracic index, venous congestion and pulmonary edema. Lastly, echocardiography is pivotal for determining CHD anatomy, pressure gradients, cardiac function and identifying chamber dimensions. BNP is one of several biomarkers for HF that have been identified to assess the diagnosis, severity, and management of the cardiac condition before and after treatment in adult HF. Both TNF-α and CRP are sensitive markers for systemic inflammation in adult HF and myocardial infarction (36). In the majority of studies, volume and pressure loading and cardiac dysfunction show elevated levels of BNP regardless of the presence of a congenital heart condition as the underlying etiology which indicates that BNP alterations are more related to the physiologic state (e.g., hemodynamics) rather than the condition or type of treatment. Troponin-I and T can be used to discern ongoing myocardial damage or stress in this setting especially with the heightened sensitivity of the newer assays (18). There have been studies assessing potential biomarker parameters for pediatric CHD readmissions, but these are not routinely used clinically (15,20).
In the adult HF population, the New York Heart Association Classification (NYHA I-IV) is applied as a standard gradation method, but this has never been validated in the pediatric HF population, although it was applied to pediatric HF until 1987. The NYHA classification does not measure the HF severity but rather indicates the functional capacity of a patient. Since the functional capacity during a state of HF is profoundly different in children as in comparison to adults, the use of the NYHA classification did not translate well into children, particularly to infants. Moreover, pediatric HF patients have a significantly different clinical presentation, pathophysiology, and compensatory mechanism (37), and most importantly, a very different etiology of HF with structural CHD as the most common cause. Consequently, Ross et al. (38), developed a symptom-based numerical classification comparable to the NYHA classification, applying more age-related variables such as feeding difficulties, growth problems (failure to thrive) and exercise intolerance. The ROSS classification was initially developed to indicate an overall assessment of the HF severity in infants, but has been updated several times to pertain to all pediatric ages, and to incorporate heart rate and respiratory rate and the size of the liver (39). Assignments with the original ROSS classification have been updated with either no symptoms in class I, and continuous symptoms such as tachypnea, retractions and diaphoresis during rest in class IV (38). However, ROSS class II and III are more ambiguous and subjective and often overlap, with mild tachypnea or diaphoresis during feeding in class II and profound tachypnea and diaphoresis during feeding in class III. Additionally, the classification of failure to thrive is subjective to the judgment by the clinician much like the NYHA classification in adults and is often the only manifestation observed in pediatric HF. Furthermore, failure to thrive may also have a non-cardiac etiology such as gastrointestinal, genetic, and/or metabolic causes. This is where a specific HF biomarker in the pediatric population would be able to differentiate between the various clinical etiologies. Ideally, a specific pediatric HF classification should accurately predict the disease risk, so that clinical managing can be custom-made to the HF condition. Pediatric patients who are classified as “No risk” would not need immediate treatment, “Mild risk” would require close observation with medical treatment, “Moderate risk” would provoke rigorous management, while “Severe risk” would prompt utmost therapy and consideration for organ transplantation.
More recently, Connolly et al. (37) developed and introduced a novel grading-system named the New York University Pediatric Heart Failure Index for children (NYU-PHFI) and adolescents based on many features during the physical examination in the ROSS classification, but also incorporating a balanced score with medical therapy for HF treatment, and the presence of a single left ventricle (LV). The NYU-PHFI is a weighted, linear combination of scores centered around physical signs & symptoms, as well as the medications. With this index, healthy children have a very low score (0−2) while the maximum score of 30 indicates severe HF (37). The physical signs and symptoms are mainly HF markers that can be identified through routine history, physical exam and diagnostic tests such as chest X-ray, EKG and echocardiogram. Because an underlying disease state can present without symptoms, the index also includes current medical therapy items. The addition of HF medication increases the NYU-PHFI index, which compensates for the reduction in signs and symptoms, to account for patients whose symptoms are relieved by HF medical therapy. The HF medication list include digoxin, diuretics, angiotensin-converting enzyme (ACE) inhibitors, β-blockers, anticoagulants, and anti-arrhythmics. Drug therapies associated with severe HF such as anticoagulants and higher anti-HF medication dosages are correlated with higher index scores. As a physiologic indicator, a single ventricle anatomical configuration was also included as a separate constituent because single ventricle patients with progressive changes may not be apparent by clinical observations as well as technical measurements. For example, pediatric patients with single ventricle Fontan palliation may not have symptoms with normal aerobic capacity according to their age and body size and would not get higher index scores.
BNP as a clinical biomarker in pediatric HF and CHD
BNP is a member of the natriuretic peptide hormone family and is mainly secreted by cardiomyocytes from the ventricles in response to pressure overload, volume expansion and increased ventricular wall stress. Upon release, BNP induces diuresis, natriuresis and vasodilation to the circulatory system. The precursor hormone, NT-ProBNP is stored together with BNP in atrial granules and they are released simultaneously (40). In adult HF, BNP as well as NT-proBNP are established guideline biomarkers for diagnosis and monitoring of management (41,42), and a high plasma BNP level has been associated with a more severe condition of the disease, while a normal BNP level (i.e., <100 pg/mL) excludes HF (13,43). In pediatric patients, trends may be valuable in this capacity but also requires multiple visits and phlebotomy draws. As most biomarkers are considered individually, a multi-marker strategy may be valuable in refining HF risk stratification and HF classification. For example, the use of BNP in conjunction with Troponin-I in the adult patient population results in a better risk stratification in acute coronary syndromes than with either marker individually (13,43). The accuracy of risk prediction in HF was amplified when the ANP level was combined with additional biomarkers such as CRP and myeloperoxidase (13,43). Moreover, when the four biomarkers BNP, Troponin-I, CRP and cystatin C were coupled together, the risk stratification for mortality from cardiovascular causes among older men was significantly enhanced (13,43).
Studies reporting that plasma BNP is a suitable diagnostic and prognostic biomarker in pediatric patients with CHD have increased in recent years. However, in contrast to adult HF, plasma BNP as a clinical biomarker is not yet included in the pediatric HF guidelines, including CHD. As aforementioned, this is likely due to the heterogeneity and complex pathophysiology of CHDs. Despite growing studies on the ability of BNP to diagnose HF and monitor treatment algorithms, there are also contrasting reports pointing to hemodynamic changes in the pediatric and neonatal population that may be affecting BNP levels. An early literature study by Eindhoven et al. (44) indicated that plasma levels of BNP and NT-proBNP don’t necessarily correlate with the severity of the disease, and are not only an expression of hemodynamic overload, but also of non-cardiac confounders such as the neuro-hormonal system or organ function. Most of the literature is from retrospective single center studies including several neonatal conditions including CHD (16,45,46).
The number one cause of HF in neonates is CHD in which the type of cardiac malformation affects the risk and degree of occurrence (47). Consequently, earlier discovery and diagnosis of CHD in neonates is pivotal. BNP as a clinical biomarker in the assessment of the degree of HF is important in treatment (18). Moreover, to diagnose neonatal CHD, the age of the newborns is very important (45,46,48-50). In healthy full-term neonates, the BNP levels are increased immediately after birth, peaking at 2 days, and then slowly decreasing to reach a stable level at 1 month (51). It is not clear why BNP levels sharply increase after birth, and this is likely due to multiple factors (16). For instance, the perinatal circulation and redistribution of blood from the placenta to the lungs have a very significant effect on the volumes in the ventricles and pressure (52,53). Moreover, during the neonatal period, both lung maturation and renal development can affect hemodynamics and volume distribution resulting in BNP plasma level changes (54). Plasma BNP levels may also be affected by the growth and development of fetus, gestational age, and preterm or full-term status (16).
Overall, the plasma BNP concentrations are elevated during CHDs in the pediatric population, with the majority of studies indicating a correlation between plasma BNP levels with RV dilation and the severity of pulmonary regurgitation. In tetralogy of fallot (TOF), the BNP values were significantly higher compared to age and gender matched control patients (44,55), although the studies only take patients into account at an age between 4 to 30 years and did not include neonates. Most studies in TOF patients exhibited a positive correlation between the BNP levels and the severity of pulmonary valve regurgitation (56) and the RV end-diastolic volume (57), while a negative correlation to exercise capacity and peak oxygen uptake was reported (58). None of the studies reported a link between BNP and LV function or end-diastolic volume (56,57). It also serves to ask the question of whether elevated BNP values mean different things in uncorrected or corrected CHD states. Biomarkers, such as BNP, have been reported to be used to help stratify risk for readmission. They have also been used as an additional variable in grading volume overload in certain populations, but the overall utility is not defined and often used as an adjunct tool along with subjective clinical assessment. Although the clinical value of plasma BNP in pediatric HF is clear, there is still no standardized cut-off value for diagnosis and for therapy monitoring in the pediatric guidelines as is the case in adult HF. Moreover, neonates undergo many different phases in their development with hemodynamic and even structural changes that can strongly affect BNP levels. Plasma BNP in combination with other detection methods such as Chest-X ray, EKG, and echocardiography, or in parallel with other blood plasma (multi) omics panels (e.g., genomics, proteomics, metabolomics) may greatly increase its clinical biomarker value.
Perspectives on novel biomarker development in pediatric HF and CHD
We have witnessed several revisions in our understanding of the syndrome of HF both in the adult as well as in the pediatric population, and it is clear that the development and progression of HF does not only result from simple changes in cardiac function, but is rather a complex interface of hemodynamic, neuro-hormonal, molecular cellular and genetic constituents (7,59). The clinical evaluation of the severity and course of HF in the pediatric population is often concealed by the subjective nature of the diagnostic parameters, leading to diverse opinions and perceptions by the physicians. Currently, several enzymes, hormones, and other markers of cardiac stress, injury and dysfunction have emerged as clinically important biomarkers to support in the diagnosis, decision making, prognosis and classification in cardiovascular diseases. Biomarkers in HF can be divided into six categories: (I) markers of cardiomyocyte stress; (II) markers of inflammation; (III) markers of neurohormonal activation; (IV) markers of cardiomyocyte injury; (V) markers of extracellular matrix remodeling; (VI) markers of metabolism and nutrition. Three criteria for the assessment of biomarkers in clinical application can be outlined (60). First, biomarkers must exhibit reliable repeated measurements at reasonable costs. Second, the marker must offer additional information that is not yet acquired from a cautious clinical evaluation. Third, knowing the measured level of the marker ought to help with the clinical management and decisions (60).
The assets of biomarkers may be the following (18): (I) they are objectively quantified as numerical values; (II) they are reproducible; (III) they can be repeatedly measured over time and follow-up; (IV) they are less invasive and safe; and (V) they straightforward with relatively low costs. The application of biomarkers in pediatric HF and CHD is likely to become more common. Considering defined clinical needs, sample collection and recruitment, statistical evaluation, and the selection of an analytical platform, the workflow in biomarker development can be divided into three main phases: (I) discovery, (II) verification and (III) validation. With regards to these three phases, plasma BNP in pediatric HF and CHD may still require more validation in different strata and underlying etiologies of pediatric HF including those with CHD. Accordingly, additional (multi) Omics panels integrated with plasma BNP levels should be considered to aid in diagnosis and monitoring of management.
Omics studies for biomarker discovery in CHD
In the pediatric population, human samples for biomarker studies and validation can be retrieved as whole blood, saliva, urine or as cardiac biopsies during surgical procedures (61). Multi-Omics studies in human samples may uncover new biomarkers (in addition to BNP) for clinical stratification and outcome prediction (62-65). Accordingly, we consider several venues that may advance the development of novel potential biomarkers in CHD: (I) Genomic and transcriptomic analyses to identify specific genes and DNA sequence variants correlated to CHD (14,66-68). (II) Proteomic analyses because cardiac dysfunction in CHD and HF are likely to result from underlying gene alterations and protein expression levels (69-71), protein-interactions and protein post-translational modifications. Surprisingly, relatively few proteomic studies have been reported on CHD. (III) Metabolic analyses (72-75).
Within these 3 venues we encountered several noteworthy proteomic constituents that are becoming increasingly popular in adult HF as well as the pediatric population. For instance, the mild-regional proadrenomedullin which is a neuro-hormone responsive to myocardial stretch similar to BNP, was evaluated on admission in children (average age 24 months) presenting with HF. The study reported a high diagnostic value of plasma BNP, MR-proADM, and Troponin-I levels as a triple-panel upon admission showing a correlation between the clinical severity and disease progression of pediatric HF (76). Furthermore, Galactin-3 which is a carbohydrate-binding lectin that is released by macrophages to regulate inflammatory functions in immunity, and cancer, has been proposed as a suitable tool for the diagnosis and risk stratification of chronic HF in children (40), however it has not yet been established as in adult HF. Interestingly, Galectin-3 is secreted by many cell types, including macrophages and is involved in a variety of processes such as inflammation, apoptosis, fibrosis and inhibition of antioxidation (23). In 12–18-month-old children diagnosed with CHD and HF, plasma levels of Growth Differentiation Factor 15 (aka, GDF-15) and beta2-microglobulin (aka, beta2-MG) were positively correlated to the severity of their cardiac function comparing HF vs. healthy controls (77). As connective tissue growth factors play an important role in several biological such as cell proliferation, angiogenesis and extracellular matrix production, the plasma-N-terminal growth factor was found significantly higher in children with HF compared to healthy volunteers and may be a promising biomarker (78). Another noteworthy protein, the cardiac-myosin-binding-protein-c (aka cMyBP-C), which is a thick-filament associated protein and a specific cardiomyocyte determinating factor of the structure and integrity of sacromeres, as well as contractile function. Plasma concentrations of cardiac-myosin-binding-protein-c may be a good clinical biomarker for diagnosis of HF in children (average age of 16 months) with a sensitivity of 100% and a specificity of 96% at 45 ng/mL as a cutoff value. Furthermore, the value for the prediction of adverse outcomes in HF patients was acquired by a ROC curve with a sensitivity of 90% and specificity of 93% at a cutoff value of 152 ng/mL cMyBP-C at admittance (79). Other proteins that are known for their association to cardiac tissue injury such as neutrophil gelatinase-associated lipocalin (79), and Heart-Type Fatty Acid Binding Protein (aka, H-FABP) (26), both show increased plasma levels in children with HF as compared to healthy controls.
Metabolomic studies, including those in adult HF (2,80), are a way of highlighting biochemical pathways and signatures correlated to the underlying pathophysiology that may aid in the diagnosis or management of pediatric HF and CHD (81,82). A wide range of metabolites in a variety of CHDs have been reported (83-85), notably acylcarnitines, triacylglycerides, amino-acid related compounds, lipids, and free fatty acids. Accordingly, lipids play an important role in a wide range of cardiovascular diseases, inflammatory processes, atherosclerosis, myocardial infarction, cardiac function, ventricular remodeling, and metabolic diseases that can start at a juvenile age (24). Lipidomic analyses is an expanding field and should also be explored in pediatric HF and CHD (25). The role of free fatty acids in atherosclerosis, myocardial infarction and HF has been described without clear consensus (86), and may also be considered in the pediatric patient population.
BNP plasma levels integrated with other potential omics biomarkers for clinical guidance or monitoring
Clinically interesting multi-omics panels integrated with plasma BNP levels may aid in diagnosis and monitoring of management in CHD (as well as in adult HF). Nevertheless, clinical studies integrating plasma BNP with multi-omics are still very scarce and are currently more described in animal models and not always in the setting of HF, and not in CHDs. A study in a mouse model deficient of natriuretic peptide receptor (for the binding of BNP) showed significant metabolic differences with the control group (87). This suggests that BNP is indeed correlated to other metabolic panels and may be integrated with a panel of metabolomics. Moreover, a mouse model of alcoholic cardiomyopathy (88) revealed a correlation between increased BNP levels and 297 differentially expressed metabolites were identified which were involved in KEGG pathways related to the biosynthesis of unsaturated fatty acids, vitamin digestion and absorption, oxidative phosphorylation, pentose phosphate, and purine and pyrimidine metabolism. A clinical study in 136 HF patients over 4 stages (A-D) conducted untargeted metabolomics and lipidomics on their blood plasma (89). The investigators reported that a set of specific molecules, such as lysophosphatidylcholine 18:2, cholesteryl ester 18:1, alanine, choline, and Fructose, were associated with BNP or left ventricular ejection fractions. Lastly, a machine learning (ML) approach to analyze multi-omics in HF patients and their EKGs and echocardiography was performed. However, the pilot-study is still descriptive and concluded that multi-omics pathways still need to be revealed in future studies (88).
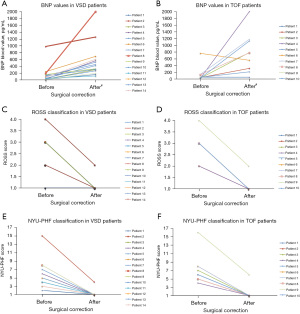
In an attempt to simplify CHD, we divided Pediatric CHD and HF into cyanotic and acyanotic groups which both may leave distinct metabolomic signatures that can be explored to predict clinical outcomes or therapeutic efficacy (90). This led us to explore metabolomics pilot studies in cyanotic CHD (TOF) vs. acyanotic CHD (VSD). Preliminary data performed and collected from a pilot study at Miller Children’s Hospital in Long Beach demonstrated interesting results. Perioperative data collected in the pilot study demonstrated that 10 TOF and 15 VSD infants aged less than 6 months had plasma BNP and Troponin-I analyzed at two-time intervals (pre-op and 2 days post-surgery) along with two different clinical evaluations for pediatric HF, ROSS and NYU-PHFI. On average, BNP levels were normal or slightly elevated in both groups prior to surgery (mean plasma BNP: 145.4±122 pg/mL in VSD as indicated in Figure 1A, and 132.0±138 pg/mL in TOF as indicated in Figure 1B). All patients in both groups showed an increase of their BNP levels 2 days post-operatively (mean plasma BNP 545.9±247 pg/mL in VSD and 779.4±371 pg/mL in TOF). All patients in both groups showed a further increase of their BNP levels, 2 days after surgical correction, but in parallel, every patient was clinically improved from HF symptoms and both the ROSS classification (Figure 1C for VSD patients, Figure 1D for TOF patients) and NYU-PHFI scores (Figure 1E for VSD patients, Figure 1F for TOF patients) were normalized in the majority of patients off inotropic support. Before surgical correction, the patients were exhibiting early signs of HF as evidenced by their clinical ROSS and NYU-PHFI evaluations. Since BNP mainly relies on pressure and stretch of the heart chambers, a hypothesis is that the acute ventricular wall stress of closing shunts and increasing volume loading on ventricles caused an acute rise in the BNP. However, with the different types of CHD, this could signify that the BNP value is just a physiological parameter responding to pressure and stretch of the LV rather than a clinical marker with diagnostic and gradation abilities as observed in the adult population. The role of BNP (in conjunction with or without Troponin-I, CRP or TNF-α) in pediatric CHD is not completely clear and not yet defined in the pediatric HF guidelines (14). Even though this was a limited pilot study with a relatively small number of neonatal patients, it highlights the question how to interpret BNP in this population before and after surgical correction and if there are better avenues in which to study potential biomarkers for HF in CHD. To better illuminate the importance of BNP as a diagnostic or prognostic marker, or as a mean to stratify the HF status in CHD, larger and well-designed prospective studies are warranted.
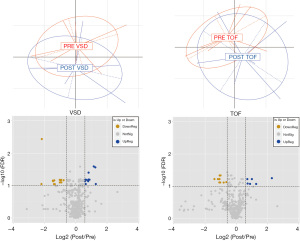
At the same time periods in the same patients, preliminary perioperative data of targeted metabolites demonstrated that these infants exhibited disparate metabolic signatures before and after surgical correction (Figure 2). An MRM-based MS spectrometry method distinguished and measured the absolute abundances of a total 610 metabolites each allocated to 20 biological classes. The TOF patients displayed 16 metabolites (out of 610) that changed 2 days after surgery, with 10 metabolites upregulated post-surgery and 6 metabolites downregulated (Figure 2). The upregulated metabolites were 2 Ceramides, 2 dihydroceramides, and 6 phosphatidylethanolamines. The 6 downregulated metabolites were 2 biogenic amines, 3 phosphatidylcholines and 1 phosphatidylethanolamine. The VSD patients displayed 28 metabolites that changed 2 days after surgical correction, with 16 metabolites upregulated and 12 metabolites downregulated. The upregulated metabolites were 1 glycerophospholipid, 11 phosphatidylethanolamines, 2 ceramides, and 2 dihydroceramides. The downregulated metabolites were 4 amino acids, 3 biogenic amines, 3 glycerophospholipids, and 2 phosphatidylethanolamines. Interestingly, in both subgroups the majority of metabolites remained unchanged, suggesting that the changed metabolites were not solely related to the impact of the surgical procedure (e.g., release of inflammatory cytokines) and may be potential biomarkers with clinical value.
Bioinformatics, data mining & ML in the age of information technology
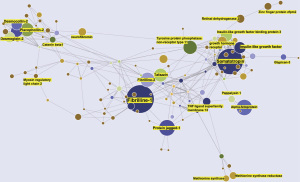
Most CHDs remain poorly understood and appear to have multiple causal factors, with genetic or environmental factors. There are several knowledge gaps in our understanding of CHD, and traditional means of statistical analyses are often not sufficient to estimate the wide spectrum of genetic and environmental factors leading to CHD. Hence, to study the roles of a wide range of multiple factors in the prediction and prevention CHDs in the age of Information Technology Machine Learning is an efficient venue to increase our understanding and treatment of CHD (91,92). Compared to traditional models, ML algorithms often have significantly better performance for prediction and are robust in handling collinearity problems and outliers (93,94). ML is growing into a popular method to discover unseen patterns in sizeable datasets of heart sounds, images (95), EKGs, and omics data for early and more accurate predictions of CHD (96,97). ML has already been applied to enable early detection and discovery of CHD and to successfully identify risk factors followed by optimum management plans. By analyzing over 44,000 medical records for 10,019 patients, Diller et al. devised a Deep Learning algorithm to predict primary clinical diagnostics, the complexity of the disease, and the most optimal treatment regimens (98). In parallel, ML algorithms can also be applied to study large Omics data from animal models or patients, or text data from patient notes and clinical reports (99-101). To address the knowledge gaps above, our group conducted a study applying a ML based Text Mining algorithm to computationally quantify 8,325 proteins known to be related to the heart according to their frequency, integrity, and distinctiveness in CHD. Hence, a vast amount of unstructured text data in over 33M publications (PubMed) was mined (~400 manuscripts/sec) according to a Text Mining algorithm to calculate protein-disease relationships developed by our collaborator (27,28,64). Out of 8,325 heart related protein, 937 proteins associated to CHD were identified. A Knowledge Graph (Figure 3) of the proteins associated to CHD indicated that fibrilline-1 had the highest association with CHD (largest radius in the KG) followed by somatotropin, alpha-fetoprotein, protein-jagged-1, and insulin-like growth factor. Both fibrilline-1 and somatotropin had the most cooccurrences with other proteins (i.e., these proteins were often described together in CHD) as indicated by the number of edges to each protein node. Altogether, we believe that our ML based Text Mining was able to unveil protein-CHD associations (in over 33M publications) that may be suitable biomarker candidates. Targeted proteomic investigations in patient’s blood and tissue samples will shed more light on these protein candidates in CHD biomarker discovery.
Conclusions
Pediatric HF, including those with CHD, do not have a standard guideline for any clinical biomarker as a diagnostic or therapeutic mean. Even BNP and NT-ProBNP, although very popular in both adult and less often is pediatric clinical practice, has not been included in the standard guidelines for pediatric HF. New biomarker discovery (that may be integrated with BNP) to enhance diagnosis and therapy monitoring in pediatric HF and CHD can be unveiled from whole blood or from myocardial biopsies. In the current age of Information Technology and large data sets, we consider (multi) Omics studies from patient samples as well as Data Mining and ML studies for the discovery of potential biomarkers in pediatric HF and CHD. Hence, future research should focus on validation and defining evidence-based cut-offs and reference ranges for specific indications using the most up-to-date assays.
Acknowledgments
Funding: This work was supported by the Larry and Helen Hoag Foundation.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at: https://cdt.amegroups.com/article/view/10.21037/cdt-22-386/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: https://cdt.amegroups.com/article/view/10.21037/cdt-22-386/coif). All authors report that this work is supported by the Larry and Helen Hoag Foundation. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study on the neonatal patients was approved by the IRB of the Miller’s Children Hospital at Memorial Care (IRB No. 086-12) and for each patient, parental informed consent was received before inclusion into the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amdani S, Marino BS, Rossano J, et al. Burden of Pediatric Heart Failure in the United States. J Am Coll Cardiol 2022;79:1917-28. [Crossref] [PubMed]
- Hill MC, Kadow ZA, Long H, et al. Integrated multi-omic characterization of congenital heart disease. Nature 2022;608:181-91. [Crossref] [PubMed]
- Daly KP, Zuckerman WA. The Burden of Pediatric Heart Failure That Lies Just Under the Surface. J Am Coll Cardiol 2022;79:1929-31. [Crossref] [PubMed]
- Ahmed H, VanderPluym C. Medical management of pediatric heart failure. Cardiovasc Diagn Ther 2021;11:323-35. [Crossref] [PubMed]
- Hsu DT, Pearson GD. Heart failure in children: part II: diagnosis, treatment, and future directions. Circ Heart Fail 2009;2:490-8. [Crossref] [PubMed]
- Hsu DT, Pearson GD. Heart failure in children: part I: history, etiology, and pathophysiology. Circ Heart Fail 2009;2:63-70. [Crossref] [PubMed]
- Madriago E, Silberbach M. Heart failure in infants and children. Pediatr Rev 2010;31:4-12. [Crossref] [PubMed]
- Su Z, Zou Z, Hay SI, et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990-2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. EClinicalMedicine 2022;43:101249. [Crossref] [PubMed]
- Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health 2020;4:185-200. [Crossref] [PubMed]
- Hinton RB, Ware SM. Heart Failure in Pediatric Patients With Congenital Heart Disease. Circ Res 2017;120:978-94. [Crossref] [PubMed]
- Boucek MM, Edwards LB, Keck BM, et al. Registry for the International Society for Heart and Lung Transplantation: seventh official pediatric report--2004. J Heart Lung Transplant 2004;23:933-47. [Crossref] [PubMed]
- Rosenthal DN, Dubin AM, Chin C, et al. Outcome while awaiting heart transplantation in children: a comparison of congenital heart disease and cardiomyopathy. J Heart Lung Transplant 2000;19:751-5. [Crossref] [PubMed]
- Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148-59. [Crossref] [PubMed]
- Davis GK, Bamforth F, Sarpal A, et al. B-type natriuretic peptide in pediatrics. Clin Biochem 2006;39:600-5. [Crossref] [PubMed]
- Green MD, Parker DM, Everett AD, et al. Cardiac Biomarkers Associated With Hospital Length of Stay After Pediatric Congenital Heart Surgery. Ann Thorac Surg 2021;112:632-7. [Crossref] [PubMed]
- Xie H, Huo Y, Chen Q, et al. Application of B-Type Natriuretic Peptide in Neonatal Diseases. Front Pediatr 2021;9:767173. [Crossref] [PubMed]
- Ploegstra MJ, Berger RMF. Prognostic biomarkers in pediatric pulmonary arterial hypertension. Cardiovasc Diagn Ther 2021;11:1089-101. [Crossref] [PubMed]
- Sugimoto M, Kuwata S, Kurishima C, et al. Cardiac biomarkers in children with congenital heart disease. World J Pediatr 2015;11:309-15. [Crossref] [PubMed]
- Lam E, Higgins V, Zhang L, et al. Normative Values of High-Sensitivity Cardiac Troponin T and N-Terminal pro-B-Type Natriuretic Peptide in Children and Adolescents: A Study from the CALIPER Cohort. J Appl Lab Med 2021;6:344-53. [Crossref] [PubMed]
- Parker DM, Everett AD, Stabler ME, et al. Novel Biomarkers Improve Prediction of 365-Day Readmission After Pediatric Congenital Heart Surgery. Ann Thorac Surg 2020;109:164-70. [Crossref] [PubMed]
- Bohn MK, Steele S, Hall A, et al. Cardiac Biomarkers in Pediatrics: An Undervalued Resource. Clin Chem 2021;67:947-58. [Crossref] [PubMed]
- Kotby AA, Youssef OI, Elmaraghy MO, et al. Galectin-3 in Children with Chronic Heart Failure with Normal and Reduced Ejection Fraction: Relationship to Disease Severity. Pediatr Cardiol 2017;38:95-102. [Crossref] [PubMed]
- Smereczyńska-Wierzbicka E, Pietrzak R, Werner B. A Scoping Review of Galectin-3 as a Biomarker of Cardiovascular Diseases in Pediatric Populations. Int J Environ Res Public Health 2022; [Crossref] [PubMed]
- Shah AS, Sadayappan S, Urbina EM. Lipids: a Potential Molecular Pathway Towards Diastolic Dysfunction in Youth-Onset Type 2 Diabetes. Curr Atheroscler Rep 2022;24:109-17. [Crossref] [PubMed]
- Tomczyk MM, Dolinsky VW. The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites 2020; [Crossref] [PubMed]
- Zoair A, Mawlana W, Abo-Elenin A, et al. Serum Level of Heart-Type Fatty Acid Binding Protein (H-FABP) Before and After Treatment of Congestive Heart Failure in Children. Pediatr Cardiol 2015;36:1722-7. [Crossref] [PubMed]
- Liem DA, Murali S, Sigdel D, et al. Phrase mining of textual data to analyze extracellular matrix protein patterns across cardiovascular disease. Am J Physiol Heart Circ Physiol 2018;315:H910-24. [Crossref] [PubMed]
- Sigdel D, Kyi V, Zhang A, et al. Cloud-Based Phrase Mining and Analysis of User-Defined Phrase-Category Association in Biomedical Publications. J Vis Exp 2019; [Crossref] [PubMed]
- Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther 2021;11:263-76. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Esmaeili A, et al. Heart failure therapy based on interventricular mechanics and cardio-vascular communications. Cardiovasc Diagn Ther 2021;11:1080-8. [Crossref] [PubMed]
- Saef JM, Ghobrial J. Valvular heart disease in congenital heart disease: a narrative review. Cardiovasc Diagn Ther 2021;11:818-39. [Crossref] [PubMed]
- Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. J Heart Lung Transplant 2014;33:888-909. [Corrected]. [Crossref] [PubMed]
- Spaziani G, Bennati E, Marrone C, et al. Pathophysiology and clinical presentation of paediatric heart failure related to congenital heart disease. Acta Paediatr 2021;110:2336-43. [Crossref] [PubMed]
- Reardon L, Lin J. Advanced heart failure and transplant in congenital heart disease. Heart 2021;107:245-53. [Crossref] [PubMed]
- Mangili G, Garzoli E, Sadou Y. Feeding dysfunctions and failure to thrive in neonates with congenital heart diseases. Pediatr Med Chir 2018; [Crossref] [PubMed]
- Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci 2014;51:13-29. [Crossref] [PubMed]
- Connolly D, Rutkowski M, Auslender M, et al. The New York University Pediatric Heart Failure Index: a new method of quantifying chronic heart failure severity in children. J Pediatr 2001;138:644-8. [Crossref] [PubMed]
- Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol 1992;13:72-5. [Crossref] [PubMed]
- Läer S, Mir TS, Behn F, et al. Carvedilol therapy in pediatric patients with congestive heart failure: a study investigating clinical and pharmacokinetic parameters. Am Heart J 2002;143:916-22. [Crossref] [PubMed]
- Goetze JP, Bruneau BG, Ramos HR, et al. Cardiac natriuretic peptides. Nat Rev Cardiol 2020;17:698-717. [Crossref] [PubMed]
- Don-Wauchope AC, McKelvie RS. Evidence based application of BNP/NT-proBNP testing in heart failure. Clin Biochem 2015;48:236-46. [Crossref] [PubMed]
- Castiglione V, Aimo A, Vergaro G, et al. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev 2022;27:625-43. [Crossref] [PubMed]
- Liquori ME, Christenson RH, Collinson PO, et al. Cardiac biomarkers in heart failure. Clin Biochem 2014;47:327-37. [Crossref] [PubMed]
- Eindhoven JA, van den Bosch AE, Jansen PR, et al. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol 2012;60:2140-9. [Crossref] [PubMed]
- Cantinotti M, Walters HL, Crocetti M, et al. BNP in children with congenital cardiac disease: is there now sufficient evidence for its routine use? Cardiol Young 2015;25:424-37. [Crossref] [PubMed]
- Cantinotti M, Giordano R, Scalese M, et al. Prognostic role of BNP in children undergoing surgery for congenital heart disease: analysis of prediction models incorporating standard risk factors. Clin Chem Lab Med 2015;53:1839-46. [Crossref] [PubMed]
- Levy PT, Tissot C, Horsberg Eriksen B, et al. Application of Neonatologist Performed Echocardiography in the Assessment and Management of Neonatal Heart Failure unrelated to Congenital Heart Disease. Pediatr Res 2018;84:78-88. [Crossref] [PubMed]
- Davlouros PA, Karatza AA, Xanthopoulou I, et al. Diagnostic role of plasma BNP levels in neonates with signs of congenital heart disease. Int J Cardiol 2011;147:42-6. [Crossref] [PubMed]
- Cantinotti M, Storti S, Crocetti M, et al. Decision levels for plasma B-type natriuretic peptide assay to diagnose significant cardiovascular disease in children. J Am Coll Cardiol 2010;55:1166-7; author reply 1167. [Crossref] [PubMed]
- Cantinotti M, Storti S, Ripoli A, et al. Diagnostic accuracy of B-type natriuretic hormone for congenital heart disease in the first month of life. Clin Chem Lab Med 2010;48:1333-8. [Crossref] [PubMed]
- Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart 2003;89:875-8. [Crossref] [PubMed]
- Nir A, Nasser N. Clinical value of NT-ProBNP and BNP in pediatric cardiology. J Card Fail 2005;11:S76-80. [Crossref] [PubMed]
- Blohm ME, Arndt F, Fröschle GM, et al. Cardiovascular Biomarkers in Amniotic Fluid, Umbilical Arterial Blood, Umbilical Venous Blood, and Maternal Blood at Delivery, and Their Reference Values for Full-Term, Singleton, Cesarean Deliveries. Front Pediatr 2019;7:271. [Crossref] [PubMed]
- Vijlbrief DC, Benders MJ, Kemperman H, et al. Use of cardiac biomarkers in neonatology. Pediatr Res 2012;72:337-43. [Crossref] [PubMed]
- Westerlind A, Wåhlander H, Lindstedt G, et al. Clinical signs of heart failure are associated with increased levels of natriuretic peptide types B and A in children with congenital heart defects or cardiomyopathy. Acta Paediatr 2004;93:340-5. [Crossref] [PubMed]
- Norozi K, Buchhorn R, Kaiser C, et al. Plasma N-terminal pro-brain natriuretic peptide as a marker of right ventricular dysfunction in patients with tetralogy of Fallot after surgical repair. Chest 2005;128:2563-70. [Crossref] [PubMed]
- Tatani SB, Carvalho AC, Andriolo A, et al. Echocardiographic parameters and brain natriuretic peptide in patients after surgical repair of tetralogy of Fallot. Echocardiography 2010;27:442-7. [Crossref] [PubMed]
- Ishii H, Harada K, Toyono M, et al. Usefulness of exercise-induced changes in plasma levels of brain natriuretic peptide in predicting right ventricular contractile reserve after repair of tetralogy of Fallot. Am J Cardiol 2005;95:1338-43. [Crossref] [PubMed]
- Molina KM, Shrader P, Colan SD, et al. Predictors of disease progression in pediatric dilated cardiomyopathy. Circ Heart Fail 2013;6:1214-22. [Crossref] [PubMed]
- Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007;115:949-52. [Crossref] [PubMed]
- Gangnus T, Burckhardt BB. Potential and Limitations of Atrial Natriuretic Peptide as Biomarker in Pediatric Heart Failure-A Comparative Review. Front Pediatr 2019;6:420. [Crossref] [PubMed]
- Bakir M, Jackson NJ, Han SX, et al. Clinical phenomapping and outcomes after heart transplantation. J Heart Lung Transplant 2018;37:956-66. [Crossref] [PubMed]
- Lam MP, Wang D, Lau E, et al. Protein kinetic signatures of the remodeling heart following isoproterenol stimulation. J Clin Invest 2014;124:1734-44. [Crossref] [PubMed]
- Liem DA, Nsair A, Setty SP, et al. Molecular- and organelle-based predictive paradigm underlying recovery by left ventricular assist device support. Circ Heart Fail 2014;7:359-66. [Crossref] [PubMed]
- Hauser JA, Demyanets S, Rusai K, et al. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart 2016;102:1633-9. [Crossref] [PubMed]
- Williams K, Carson J, Lo C. Genetics of Congenital Heart Disease. Biomolecules 2019; [Crossref] [PubMed]
- Bruneau BG. The developing heart Development. 2020;147: [Crossref] [PubMed]
- Turton N, Swan R, Mahenthiralingam T, et al. The Functions of Long Non-Coding RNA during Embryonic Cardiovascular Development and Its Potential for Diagnosis and Treatment of Congenital Heart Disease. J Cardiovasc Dev Dis 2019; [Crossref] [PubMed]
- Willinger L, Brudy L, Meyer M, et al. Prognostic value of non-acute high sensitive troponin-T for cardiovascular morbidity and mortality in adults with congenital heart disease: A systematic review. J Cardiol 2021;78:206-12. [Crossref] [PubMed]
- Biró O, Rigó J Jr, Nagy B. Noninvasive prenatal testing for congenital heart disease - cell-free nucleic acid and protein biomarkers in maternal blood. J Matern Fetal Neonatal Med 2020;33:1044-50. [Crossref] [PubMed]
- Yuan C, Chen HX, Hou HT, et al. Protein biomarkers and risk scores in pulmonary arterial hypertension associated with ventricular septal defect: integration of multi-omics and validation. Am J Physiol Lung Cell Mol Physiol 2020;319:L810-22. [Crossref] [PubMed]
- Pagano E, Frank B, Jaggers J, et al. Alterations in Metabolites Associated with Hypoxemia in Neonates and Infants with Congenital Heart Disease. Congenit Heart Dis 2020;15:251-65. [Crossref] [PubMed]
- Dong S, Wu L, Duan Y, et al. Metabolic profile of heart tissue in cyanotic congenital heart disease. Am J Transl Res 2021;13:4224-32. [PubMed]
- Li Y, Sun Y, Yang L, et al. Analysis of Biomarkers for Congenital Heart Disease Based on Maternal Amniotic Fluid Metabolomics. Front Cardiovasc Med 2021;8:671191. [Crossref] [PubMed]
- He YY, Yan Y, Chen JW, et al. Plasma metabolomics in the perioperative period of defect repair in patients with pulmonary arterial hypertension associated with congenital heart disease. Acta Pharmacol Sin 2022;43:1710-20. [Crossref] [PubMed]
- Salem SS, Saleh NY, Soliman SE, et al. On-admission plasma levels of BNP, MR-proADM, and cTnI in pediatric heart failure: contributions to diagnosis, prognosis, and outcome. Ir J Med Sci 2022;191:263-70. [Crossref] [PubMed]
- Zhou XJ, Zhang X, Zhang J, et al. Diagnostic value of growth differentiation factor-15 and β2-microglobulin in children with congenital heart disease combined with chronic heart failure and its relationship with cardiac function. Eur Rev Med Pharmacol Sci 2020;24:8096-103. [PubMed]
- Li G, Song X, Xia J, et al. The diagnostic value of plasma N-terminal connective tissue growth factor levels in children with heart failure. Cardiol Young 2017;27:101-8. [Crossref] [PubMed]
- El Amrousy D, Hodeib H, Suliman G, et al. Diagnostic and Prognostic Value of Plasma Levels of Cardiac Myosin Binding Protein-C as a Novel Biomarker in Heart Failure. Pediatr Cardiol 2017;38:418-24. [Crossref] [PubMed]
- Murashige D, Jang C, Neinast M, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364-8. [Crossref] [PubMed]
- Correia GD, Wooi Ng K, Wijeyesekera A, et al. Metabolic Profiling of Children Undergoing Surgery for Congenital Heart Disease. Crit Care Med 2015;43:1467-76. [Crossref] [PubMed]
- Gangnus T, Burckhardt BBCARS consortium. Low-volume LC-MS/MS method for the pharmacokinetic investigation of carvedilol, enalapril and their metabolites in whole blood and plasma: Application to a paediatric clinical trial. Drug Test Anal 2021;13:694-708. [Crossref] [PubMed]
- O'Connell TM, Logsdon DL, Mitscher G, et al. Metabolic profiles identify circulating biomarkers associated with heart failure in young single ventricle patients. Metabolomics 2021;17:95. [Crossref] [PubMed]
- Bentley RET, Hindmarch CCT, Dunham-Snary KJ, et al. The molecular mechanisms of oxygen-sensing in human ductus arteriosus smooth muscle cells: A comprehensive transcriptome profile reveals a central role for mitochondria. Genomics 2021;113:3128-40. [Crossref] [PubMed]
- Razavi AC, Bazzano LA, He J, et al. Novel Findings From a Metabolomics Study of Left Ventricular Diastolic Function: The Bogalusa Heart Study. J Am Heart Assoc 2020;9:e015118. [Crossref] [PubMed]
- Nomura SO, Karger AB, Weir NL, et al. Free fatty acids and heart failure in the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Lipidol 2021;15:608-17. [Crossref] [PubMed]
- Chang P, Niu Y, Zhang X, et al. Integrative Proteomic and Metabolomic Analysis Reveals Metabolic Phenotype in Mice With Cardiac-Specific Deletion of Natriuretic Peptide Receptor A. Mol Cell Proteomics 2021;20:100072. [Crossref] [PubMed]
- Cao Z, Wang T, Xia W, et al. A Pilot Metabolomic Study on Myocardial Injury Caused by Chronic Alcohol Consumption-Alcoholic Cardiomyopathy. Molecules 2021; [Crossref] [PubMed]
- Zhou J, Chen X, Chen W, et al. Comprehensive plasma metabolomic and lipidomic analyses reveal potential biomarkers for heart failure. Mol Cell Biochem 2021;476:3449-60. [Crossref] [PubMed]
- Vimal S, Ranjan R, Yadav S, et al. Differences in the serum metabolic profile to identify potential biomarkers for cyanotic versus acyanotic heart disease. Perfusion 2021; Epub ahead of print. [Crossref] [PubMed]
- Morris SA, Lopez KN. Deep learning for detecting congenital heart disease in the fetus. Nat Med 2021;27:764-5. [Crossref] [PubMed]
- Krittanawong C, Rogers AJ, Johnson KW, et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol 2021;18:75-91. [Crossref] [PubMed]
- Krittanawong C, Johnson KW, Rosenson RS, et al. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J 2019;40:2058-73. [Crossref] [PubMed]
- Krittanawong C, Virk HUH, Kumar A, et al. Machine learning and deep learning to predict mortality in patients with spontaneous coronary artery dissection. Sci Rep 2021;11:8992. [Crossref] [PubMed]
- Diller GP, Vahle J, Radke R, et al. Utility of deep learning networks for the generation of artificial cardiac magnetic resonance images in congenital heart disease. BMC Med Imaging 2020;20:113. [Crossref] [PubMed]
- Qu Y, Deng X, Lin S, et al. Using Innovative Machine Learning Methods to Screen and Identify Predictors of Congenital Heart Diseases. Front Cardiovasc Med 2022;8:797002. [Crossref] [PubMed]
- Bertsimas D, Zhuo D, Dunn J, et al. Adverse Outcomes Prediction for Congenital Heart Surgery: A Machine Learning Approach. World J Pediatr Congenit Heart Surg 2021;12:453-60. [Crossref] [PubMed]
- Diller GP, Kempny A, Babu-Narayan SV, et al. Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: data from a single tertiary centre including 10 019 patients. Eur Heart J 2019;40:1069-77. [Crossref] [PubMed]
- Mullen M, Zhang A, Lui GK, et al. Race and Genetics in Congenital Heart Disease: Application of iPSCs, Omics, and Machine Learning Technologies. Front Cardiovasc Med 2021;8:635280. [Crossref] [PubMed]
- Thomford NE, Bope CD, Agamah FE, et al. Implementing Artificial Intelligence and Digital Health in Resource-Limited Settings? Top 10 Lessons We Learned in Congenital Heart Defects and Cardiology. OMICS 2020;24:264-77. [Crossref] [PubMed]
- Perez-Riverol Y, Kuhn M, Vizcaíno JA, et al. Accurate and fast feature selection workflow for high-dimensional omics data. PLoS One 2017;12:e0189875. [Crossref] [PubMed]


