
Placing an appropriate tunneled dialysis catheter in an appropriate patient including the nonconventional sites
Introduction
The prevalence of end stage kidney disease (ESKD) requiring chronic maintenance dialysis or a kidney transplant in the U.S. has more than quadrupled from 1990 to 2018 (180,659 patients to 785,883 patients) (1). At the end of 2018 more than 0.5 million patients in the U.S. were on chronic maintenance dialysis and 0.2 million patients had a functional kidney transplant (1). Hemodialysis (HD) was the modality of choice for about 90% of the patients undergoing dialysis in the U.S. According to the Center for Disease Control (CDC), about 350 new patients start dialysis every day in the U.S. (2). Extrapolating from the CDC and United States Renal Data System (USRDS) data, 80% of the new patients starting dialysis start with a catheter, adding over 100,000 new catheter insertions per year in the U.S. alone. As per the USRDS 2019 report, 46% of patients who initiate dialysis remain catheter dependent at 6 months (1).
Why do so many patients in the U.S. start dialysis with a catheter? Why has catheter dependency in the prevalent dialysis population remained high? Epidemiological studies have identified complex interacting barriers that lead to higher catheter use in the incident and prevalent dialysis population. Some of the challenges include: (I) late referral to a nephrologist (3); (II) a knowledge gap amongst primary care physicians (4); (III) health system related challenges including insurance status (4); (IV) limited health literacy in the general population and patient-noncompliance (4); (V) socioeconomic disparity and others (4). As a result, many patients with advanced kidney disease are seen by a nephrologist for the first time when they are close to needing dialysis (3). About 25–40% of patients start dialysis within 3 months of their first referral to a nephrologist (3). Consequently, the patients are ill-prepared regarding their treatment options, expected outcomes, goals of care and often “crash land” at a hospital with an urgent need to initiate dialysis as a life-saving measure. The problem is not limited to the U.S. and has been reported from other developed countries (4). The problem is further compounded by the increasing prevalence of chronic kidney disease (CKD) and ESKD with the rising incidence of common risk factors leading to CKD development, i.e., diabetes mellitus, hypertension and obesity. About 15% of the adult U.S. population (approximately 37 million) is estimated to have chronic kidney disease stage 1–4 and 40% of the patients with advanced CKD remain unaware of their disease (2), further multiplying the problem of delayed disease recognition and late referral. These multifactorial challenges are not easily addressed, and thus unlikely to change in the near term.
When a patient with ESKD without a vascular access needs to start dialysis, the HD catheter becomes the default vascular access of choice. A HD catheter remains a key vascular access option despite the associated complications such as blood stream infection (5,6), frequent dysfunction and central vein stenosis. From acutely ill patients requiring emergent kidney replacement therapy to dialysis dependent ESKD patients without a functional vascular access, the dialysis catheter serves a key role in providing emergent/lifesaving and maintenance/life sustaining vascular access options. The HD catheter also remains the default option for ESKD patients with failed arteriovenous fistula (AVF) or arteriovenous graft (AVG) access. Similarly, for a peritoneal dialysis (PD) patient wishing to switch dialysis modality or a patient with a failing kidney transplant HD catheter becomes a default immediate vascular access option.
The revised 2019 Kidney Disease Outcome Quality Initiative (KDOQI) Clinical Practice Guidelines on vascular access recommends adopting a more patient focused approach to integrate and plan treatment options to manage ESKD, including a dialysis vascular access (7). The guidelines recommend creating a vascular access life plan for each individual patient with ESKD with a special emphasis on planning the appropriate access for the appropriate patient at the appropriate time. For example, most patients with CKD and progressive decline in renal function should be referred for vascular access evaluation when eGFR is 15–20 mL/min/1.73 m2. The penultimate goal is for each patient to have a functional vascular access such as AVF or AVG at all times that suits patient’s desires and also meets the high-quality clinical standards at the same time. The advanced planning phase could include interventions to maintain the current functional access plus future needs. The planning for the future arteriovenous (AV) access may be structured around the following questions: what is an ideal access (AVF vs. AVG) at a given moment in patient’s ESKD plan, where to place (the anatomical site of the new AV access), and when to place (timing of referral). Based on the KDOQI 2019 guidelines a 5-step approach to an AV access life-plan has been outlined in Figure 1 (8). The guidelines stress on ensuring efforts to achieving a functional AVF/AVG for all patients, and also accept the role of HD catheters towards achieving the overall ESKD life plan. The multifactorial challenges described above are part of the rationale of changing from the “fistula first” approach of the 2006 guidelines, with a new emphasis on evaluating “what’s next” for patients to anticipate future needs and thereby reduce the dependence upon HD catheters. Whether these new guidelines translate to improved outcomes is yet to be seen. In this article we focus on the crucial role of HD catheters in the AV access paradigm and answer clinical questions relevant to HD catheter use, including unconventional sites for HD catheter placement.
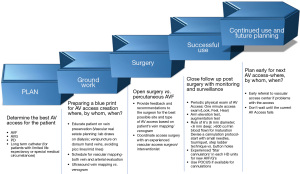
When is catheter an appropriate vascular access?
An ideal AV access is easy to place, available for immediate use, requires limited interventions for continued optimal use, and supports long-term dialysis treatments (7). The commonly used dialysis vascular access (AVF, AVG or HD catheter) has unique inherent advantages and disadvantages as summarized from author experiences and KDOQI practice guidelines (7) in Figure 2.
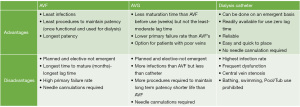
Of the three hemodialysis access options currently available, AVF is the most preferred based on the historical data suggesting an AVF has the least risk for infection, the longest patency with least number of interventions (9-12). However, an AVF has the longest maturation time from placement to first use for dialysis. Several studies have shown median maturation time for an AVF of 4 months and a high rate of primary maturation failure (9,10). This delayed timing is likely a result of half of surgically created AVFs requiring additional interventions (such as angiogram, angioplasty, balloon assisted maturation, coil embolization or ligation of accessory and collateral veins, etc.) to promote the maturation process and eventually support dialysis therapy (9,13). These patients with non-maturing AVF remain dependent on HD catheters for a variable period of few days to several months accounting for a higher incidence of catheter dependent prevalent ESKD population (14). The economic impact of multiple interventions related to catheter dysfunction remains substantial. Al-Balas et al. reported over an 8-year period, 1 in 6 patients starting dialysis at a large medical center using a HD catheter remained dependent on HD catheter (11). Patients who continued to dialyze using a HD catheter had the highest and three fold median annual overall access related cost as compared to the group who received an AVF or AVG. Further, the HD catheter group had 2–4 times mortality as compared to those with AVF and AVG.
AVG on the other hand is an acceptable AV access option for a patient with poor native veins who presents a surgical challenge to creating an AVF. The surgical procedure, time and cost for AVG placement is comparable to AVF creation (11). The incidence of primary maturation failure with AVG is significantly lower than AVF and rarely require secondary interventions prior to first successful cannulation (13). The time from creation to successful use on dialysis is much shorter (2–3 weeks) when compared to AVF (9,10,13). So, are elderly patients better off with an AVG (15)? Lee et al. evaluated five-year data of 9,458 patients aged >67-year from the United States Renal Data System who initiated hemodialysis with a catheter between 2010 to 2011 (16). Of these, 7,433 patients received an AVF and 2025 patients received an AVG within six months of dialysis. The authors concluded that amongst elderly patients 51% of new AVF were unable to support dialysis in the first six months compared to 45% of AVG, and a higher proportion of AVF (42.5%) required intervention before being successfully used for dialysis compared to 23.5% of AVG.
As compared to an AVF or AVG, a HD catheter is relatively easier to place, requires less planning and pre-operative steps (no vein mapping, no pre surgery appointment or evaluation etc.), can be placed on an emergent basis and can be used immediately for HD (17,18). The HD catheter serves as a safe, reliable and indispensable AV access option for many clinical situations, particularly in patients susceptible to the high output cardiac failure associated with AVFs and AVGs (7). The HD catheter may be the only viable option and in fact in some clinical situations may be the most suitable and preferred AV access type (19). While the clinical scenario for each patient is unique and may demand individualization of care considering individual preferences and goals, the overall plan of care should align with the best practices for safety and quality of care. The 2019 KDOQI expert committee on vascular access found it reasonably acceptable to use tunneled dialysis catheters for short term and long-term use in special circumstances as summarized in Figure 3 (7).
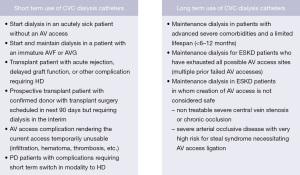
Below we discuss the common clinical scenarios where a HD catheter may serve as an appropriate vascular access:
Emergent dialysis in critically ill patients
For most emergent situations involving acutely ill and critical patient who require initiation of renal replacement therapy (RRT), placing a hemodialysis catheter is practically the quickest, easiest, and most reliable AV access to start RRT. A non-tunneled hemodialysis catheter (NTDC) can be quickly and safely placed under ultrasound guidance at bedside even in patients with sub-optimal coagulation parameters [such as thrombocytopenia or high international normalized ratio (INR)]. Patients with ESKD who have exhausted all the vascular sites for creation of an AV access are left with no option other than a long term tunneled HD catheter (TDC) to continue maintenance HD. Catheter placements at unconventional sites (trans-lumbar approach etc.) for such patients has been discussed later in this article.
Dialysis in patients without a functional AV access
Unfortunately, 8 out of 10 patients start dialysis with a catheter and includes patients without a prior AV access and those with failed or non-maturing AV access (1,2). From a functional and usability standpoint any AV access that cannot be used for HD is akin to not having an AV access and includes immature small AVF, deep AVF or AVG that are difficult to cannulate, thrombosed AVF/AVG, or AVF/AVG with severe inflammation, swelling, hematoma etc. rendering it inaccessible for cannulation.
Dialysis in elderly patients
Dialysis catheter is a reasonable choice as an AV access in elderly patients, as increased age has been associated with failure to mature for fistulas and lower primary and secondary patency rates in AVFs (20). The AV access life plan should be tailored to individual situation with a shared decision to incorporate patient’s wishes. Elderly patients who wish to pursue dialysis for a short period to complete pending life matters or get to a milestone may prefer to forego an AV access surgery and instead choose a tunneled HD catheter (16,21). In a study of veterans over 70 years of age with ESKD who chose to initiate dialysis, the cumulative survival rate at 1, 3, and 5 years was found to be 60%, 37%, and 20% respectively (21).
Palliative dialysis for short term
An AVF/AVG in patients with advanced end organ disease such as severe cardiomyopathy, terminal metastatic cancer with limited life expectancy, cirrhosis liver with chronic hepato-renal syndrome without candidacy for liver transplant, who wish to pursue dialysis for palliative reasons as a bridge to eventually transitioning to hospice. A tunneled HD catheter may be an acceptable choice, avoiding unnecessary surgeries and procedures related to creation and maturation of AV access.
Bridge dialysis to transplant
Continuing HD with a tunneled HD catheter may also be reasonable in patients with acute hepato-renal syndrome who are awaiting liver transplant, or in patients with ESKD who are expected to undergo a live donor kidney transplant in a reasonably short period of time. Indications for short term and long term use of dialysis catheters are summarized in Figure 3.
New ESKD patients opting to transition to home hemodialysis
Since the technical aspect of connecting the tunneled HD catheter ports to the extra-corporeal circuit of a dialysis machine are relatively less challenging, avoids the fear and apprehension of needle cannulation by the patient or family, a HD catheter, is more likely to be considered and pursued for home HD. Starting HD with a catheter may facilitate the transition to home HD more acceptable.
THDC for intensive care unit (ICU) patients
Most critically ill patients in the intensive care units require emergent initiation of renal RRT including intermittent hemodialysis or continuous renal replacement therapy. An NTDC is commonly placed at bedside in a central vein. A significant number of critically ill patients with AKI who initiate dialysis remain dialysis dependent for several weeks (22,23). A standard practice in the US is to replace the NTDC after <7–10 days to minimize the risk of blood stream infection. We (24) and others (22,25) have earlier proposed to place a TDC at the earliest possible opportunity to preserve central veins and possibly reduce the risk of blood stream infection. The early TDC placement in the ICU approach offers several short term and long term benefits. Short term benefits include patient safety by avoiding transporting a critically ill patients out of ICU, minimize the risk of procedural related complications and possibly reduce the healthcare cost. The early transition strategy to TDC can facilitate ICU care and transition of care to a regular nursing floor or discharge from the hospital, if the patient remains dialysis dependent. We have reported our experience of placing TDCs safely at bedside in the critical care unit patients using anatomical landmarks and under ultrasound guidance without using fluoroscopy (24). Our initiative to place TDCs and not NTDCs was driven by prior studies reporting similarly low peri-procedural complications, but higher overall complications (mechanical and positive cultures) in patients with NTDCs (relative risk 13.6) (26). Other groups have reported similar experience and safety of bedside placement of TDCs in critically ill patient using ultrasound guidance and bedside portable radiography (25). Out of 24 TDC placements, a single patient experienced complications of pneumothorax and cardiac tamponade, though no direct comparison was made to placement of non-tunneled dialysis catheters.
Patient factors for catheter selection
A temporary HD catheter's tip needs to be in a large lumen vena cava (superior or inferior) or cavoatrial junction or in the mid-right atrium, as with a tunneled HD catheter, to achieve extracorporeal blood flow of 350–400 mL/min. Central veins with diameter averaging 10–20 mm suit this purpose well. Prior to the widespread use of ultrasound guided needle cannulations for venipuncture, femoral veins were the most common site selected for an emergent insertion of non-tunneled HD catheter. Due to increased risk of infections, primarily due to close vicinity to the perineum, femoral veins are not the preferred first site for HD catheter insertion (6,7). A randomized controlled trial comparing femoral and jugular vein catheterization and risk of nosocomial events in acutely ill adults requiring acute renal replacement therapy found a significant interaction between body mass index (BMI) and the risk for catheter colonization on catheter removal. In patients with BMI of greater than 28.4 there was a significantly increased incidence of catheter colonization (50.9 vs. 24.5 per 1,000 catheter days; P<0.001) but this association was not significant for those with lower BMI (27). In the upper torso, subclavian veins are less preferred due to higher rates (up to four-fold) of vein stenosis as compared to internal jugular vein catheters (7,28). Below we discuss more specifics related to site and catheter length selection for different patients.
Site selection: right vs. left
In the upper torso, catheter insertions in right internal jugular (IJ) vein is preferred over the left internal jugular vein for several reasons. (I) Size: The right IJ vein is dominant and wider in diameter in most people (29,30). Tartière et al. looked at 190 CT scans of general outpatient adults and found that the right IJ vein diameter (median diameter 17 mm, range 13–20 mm) and cross sectional area (median 160 mm2, range 108–235 mm2) was larger as compared to the left IJ vein diameter (median diameter 13 mm, range 10–16 mm) and cross sectional area (median 102 mm2, range 63–168 mm2). (II) Anatomy: The right IJ vein follows a straight course into the right brachiocephalic and superior vena cava and hence allows for easier passage, less trauma and potentially less risk of perforation while passing the wire, dilators, and the peel away sheath (31,32). On the other hand, the left IJ vein follows a more curvilinear and longer course to the superior vena cava. As such there are potentially more chances of encountering resistance while advancing the wire, dilators, sheath and subsequently the catheter as well (31,32). (III) Catheter dysfunction: Engstrom et al., evaluated 532 IJ vein catheter insertions (398 were in right IJ vein and 194 in left IJ vein) and reported that catheters placed in left IJ vein are associated with a higher rate of catheter dysfunction (0.25 vs. 0.11 per 100-catheter days; P=0.036) as compared to catheters in the right IJ vein (30). The same study also reported higher rates of catheter related infection in the left IJ vein (0.50 vs. 0.27 per 100 catheter days’ P=0.005) compared to the right IJ vein (32).
Catheter length selection
While optimal positioning of the central venous catheter is best estimated via direct measurements such as using a J wire and repositioning under image guidance, several prospective methods have been used to estimate the correct length for appropriate catheter selection, particularly in settings where imaging guidance is not available. In our practice, these estimations are useful even in settings with fluoroscopic guidance to ensure the estimated catheter length is immediately available in the room. The ideal length has been found to correlate the most with the patient height. One of the most used methods to ensure placement of the catheter tip in the lower superior vena cava or the superior cavoatrial junction is Peres’ formula, where the ideal length of a right internal or external jugular catheter is the patient’s height in cm divided by 10 and the ideal length of a left internal or external jugular catheter is an additional 4 cm (33). The proposed ideal lengths for subclavian catheters is 2 cm less than their respective jugular venous catheter ideal lengths. Thus, for an average male patient with a height of 178cm, an 18cm length catheter may be used for right internal jugular access. Subsequent studies to validate Peres’s formula have found accuracy rates of up to 95% in adult patients of European descent (34). However, several other confounding factors have been found to reduce the accuracy of Peres’s formula, such as ethnicity. For example, in East Asian populations, a lower accuracy of 77% for Pere’s formula was found, which the authors attributed to the difference in body habitus (35). Subsequent studies have also attempted to refine the original formula via linear regression based on other correlating patient characteristics such as thoracic diameter (36). These correlating factors must be considered in the application of Peres’s formula to select appropriate catheter length.
For femoral veins, no difference in vein diameter between left side and right side has been reported in the literature. Since most people are right handed, the right femoral vein has been the first preference simply for the ease of the operator to allow for easier cannulation and subsequent dilating and tunneling. For non-tunneled temporary catheters in the groin, 20–24 cm catheters often work well as long as the venipuncture site is not too distal to the inguinal crease. For tunneled dialysis catheter in the groin, a longer 29 cm or 33 cm (tip to cuff) catheter is recommended to ensure that the catheter tip passes through the common iliac vein into the inferior vena cava (37).
Non-conventional TDC placement
With a mean initial patency of only 202 days, dialysis patients typically experience many failures associated with their tunneled dialysis catheters. Common complications such as fibrin sheaths and central venous occlusion may be salvaged via techniques such as stripping, sharp recanalization, and the recently FDA approved Surfacer Inside-Out device (38-40). However, patients on chronic dialysis who are dependent on their catheters over the span of many years often develop venous thrombosis, soft tissue infections, and central venous occlusions that lead to non-salvageable exhaustion of commonly used access sites. The National Kidney Foundation’s KDOQI guidelines for prolonged central venous catheter usage without arteriovenous fistula access recommend the use of the internal jugular veins, followed by external jugular, femoral, and subclavian veins (7). Once the common access sites have been exhausted, the most commonly used nonconventional access is a translumbar inferior vena cava (IVC) approach (41). As originally described in 1995, this approach involves the percutaneous access of the IVC at approximately the level of the L3 vertebral body under fluoroscopic or CT guidance (42). Though approach has been universally associated with low peri-procedural complications, serious complications such as retroperitoneal hematomas and iatrogenic aortic punctures have been described (43). Particular complications associated with this technique include migration of the catheter in subcutaneous soft tissues and migration of the catheter tip into the iliac veins, particularly in obese patients, making morbid obesity a relative contraindication (44). Long term device patency rates have been variable, with median initial device patency ranging from 65 days (45) to 245 days (46), most often foreshortened due to inadequate blood flow for dialysis and catheter thrombosis. IVC thrombosis is rare, exceptionally so in patients without pre-existing thrombus (47). Due to the exceptionally low risk of site infections and long term site patency, some authors prefer the lumbar site over the femoral sites (48). A hierarchy of sites most commonly used by the authors and in keeping with KDOQI guidelines is shown in Figure 4.
In the case of patients with infrarenal IVC occlusion, where the translumbar approach is infeasible, a transhepatic approach has been described (49). In this approach, middle or right hepatic venous access may be obtained via a percutaneous needle puncture in the eight intercostal space, mid axillary line. A snare is sometimes advanced into one of the hepatic veins via an alternative site to use as a fluoroscopic target. Though sample sizes and experiences are limited compared to the commonly used sites, a higher rate of thrombosis and catheter dislodgment has been reported, compared to a translumbar approach (50). This has been theorized by some authors to be a result of the relatively short catheter distance from the hepatic vein to the right atrium. Minor rare peri-procedural complications have been reported, including hematomas. Only a single case of death from hemorrhagic shock has been reported in the literature (51).
In patients with specific limited access sites, several other exotic access sites have been successfully used. In order to preserve infrarenal IVC and iliac veins in a renal transplant candidate, a percutaneous transrenal catheter placement was first described in 2002 (52). In this approach, percutaneous access was first obtained with a Chiba needle under ultrasound guidance into a segmental interpolar vein, and dilated into a tract for a catheter. In a patient with an IVC occlusion, a successful catheter placement into the azygous vein via an ascending lumbar vein has been reported (53). This catheter served the patient’s dialysis needs for seven months prior to a morbid catheter-related infection. In patients with no viable venous access sites, intentional arterial placement of dialysis catheters have been reported (54).
Key takeaways
- Dialysis vascular access selection is an integral part of ESKD life-plan;
- Despite all the drawbacks, hemodialysis catheter is an important device to provide kidney replacement therapy in acute and chronic settings due to a variety of challenges in ensuring timely execution of an ESKD life-plan;
- Catheter length selection depends on multiple patient characteristics, most commonly height and laterality;
- Preferred venous access sites for HD catheter placement are internal jugular vein, followed by external jugular, femoral, and subclavian vein;
- Non-conventional sites for HD catheter placement include trans-lumbar, trans-hepatic, and trans-renal venous access.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-426/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-426/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Available online: https://adr.usrds.org/2020
- Chronic Kidney Disease in the United States, 2021. Accessed May 31, 2021. Available online: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html
- Cass A, Cunningham J, Snelling P, et al. Late referral to a nephrologist reduces access to renal transplantation. Am J Kidney Dis 2003;42:1043-9. [Crossref] [PubMed]
- Navaneethan SD, Aloudat S, Singh S. A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol 2008;9:3. [Crossref] [PubMed]
- Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 2011;79:587-98. [Crossref] [PubMed]
- Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013;24:465-73. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]
- Sharma M, Niyyar VD. Hemodialysis Access 101. Kidney News 2020;12:16-7.
- Allon M, Imrey PB, Cheung AK, et al. Relationships Between Clinical Processes and Arteriovenous Fistula Cannulation and Maturation: A Multicenter Prospective Cohort Study. Am J Kidney Dis 2018;71:677-89. [Crossref] [PubMed]
- Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 2013;8:810-8. [Crossref] [PubMed]
- Al-Balas A, Lee T, Young CJ, et al. The Clinical and Economic Effect of Vascular Access Selection in Patients Initiating Hemodialysis with a Catheter. J Am Soc Nephrol 2017;28:3679-87. [Crossref] [PubMed]
- Polkinghorne KR, McDonald SP, Atkins RC, et al. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004;15:477-86. [Crossref] [PubMed]
- Harms JC, Rangarajan S, Young CJ, et al. Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg 2016;64:155-62. [Crossref] [PubMed]
- Dember LM. Fistulas first--but can they last?. Clin J Am Soc Nephrol 2011;6:463-4. [Crossref] [PubMed]
- Allon M. Vascular Access for Hemodialysis Patients: New Data Should Guide Decision Making. Clin J Am Soc Nephrol 2019;14:954-61. [Crossref] [PubMed]
- Lee T, Qian J, Thamer M, et al. Tradeoffs in Vascular Access Selection in Elderly Patients Initiating Hemodialysis With a Catheter. Am J Kidney Dis 2018;72:509-18. [Crossref] [PubMed]
- Vachharajani TJ, Moossavi S, Kaufman T, et al. Central vein hardware: cannot live with it, cannot live without it. Semin Dial 2009;22:588-9. [Crossref] [PubMed]
- Vachharajani TJ. Dialysis Catheter: "Love-Hate Relationship". Indian J Nephrol 2018;28:185-6. [Crossref] [PubMed]
- Vachharajani TJ, Agarwal AK, Asif A. Vascular access of last resort. Kidney Int 2018;93:797-802. [Crossref] [PubMed]
- Moist LM, Lok CE, Vachharajani TJ, et al. Optimal hemodialysis vascular access in the elderly patient. Semin Dial 2012;25:640-8. [Crossref] [PubMed]
- Vachharajani TJ, Atray NK. Aging veterans and the end-stage renal disease management dilemma in the millennium. Hemodial Int 2007;11:456-60. [Crossref] [PubMed]
- Coryell L, Lott JP, Stavropoulos SW, et al. The case for primary placement of tunneled hemodialysis catheters in acute kidney injury. J Vasc Interv Radiol 2009;20:1578-81; quiz 1582. [Crossref] [PubMed]
- VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7-20. [Crossref] [PubMed]
- Hanane T, Lane J, Mireles-Cabodevila E, et al. Safety of bedside placement of tunneled dialysis catheter in COVID-19 patients. J Vasc Access 2022;23:145-8.
- Williams AD, Qaqish M, Elnagar J, et al. Bedside Tunneled Hemodialysis Catheter Placement in Patients with COVID-19. Ann Vasc Surg 2021;73:133-8. [Crossref] [PubMed]
- Mendu ML, May MF, Kaze AD, et al. Non-tunneled versus tunneled dialysis catheters for acute kidney injury requiring renal replacement therapy: a prospective cohort study. BMC Nephrol 2017;18:351. [Crossref] [PubMed]
- Parienti JJ, Thirion M, Mégarbane B, et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA 2008;299:2413-22. [Crossref] [PubMed]
- Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant 1991;6:722-4. [Crossref] [PubMed]
- Tartière D, Seguin P, Juhel C, et al. Estimation of the diameter and cross-sectional area of the internal jugular veins in adult patients. Crit Care 2009;13:R197. [Crossref] [PubMed]
- Lichtenstein D, Saïfi R, Augarde R, et al. The Internal jugular veins are asymmetric. Usefulness of ultrasound before catheterization. Intensive Care Med 2001;27:301-5. [Crossref] [PubMed]
- Oliver MJ, Edwards LJ, Treleaven DJ, et al. Randomized study of temporary hemodialysis catheters. Int J Artif Organs 2002;25:40-4. [Crossref] [PubMed]
- Engstrom BI, Horvath JJ, Stewart JK, et al. Tunneled internal jugular hemodialysis catheters: impact of laterality and tip position on catheter dysfunction and infection rates. J Vasc Interv Radiol 2013;24:1295-302. [Crossref] [PubMed]
- Peres PW. Positioning central venous catheters--a prospective survey. Anaesth Intensive Care 1990;18:536-9. [Crossref] [PubMed]
- Czepizak CA, O'Callaghan JM, Venus B. Evaluation of formulas for optimal positioning of central venous catheters. Chest 1995;107:1662-4. [Crossref] [PubMed]
- Kim WY, Lee CW, Sohn CH, et al. Optimal insertion depth of central venous catheters--is a formula required? A prospective cohort study. Injury 2012;43:38-41. [Crossref] [PubMed]
- Park SJ, Chung HH, Lee SH, et al. New formulas to predict the length of a peripherally inserted central catheter based on anteroposterior chest radiographs. J Vasc Access 2022;23:550-7.
- Huriaux L, Costille P, Quintard H, et al. Haemodialysis catheters in the intensive care unit. Anaesth Crit Care Pain Med 2017;36:313-9. [Crossref] [PubMed]
- Benfor B, Chinnadurai P, Peden EK. Advanced intraoperative imaging guidance for inside-out central venous recanalization using a novel catheter access system. J Vasc Surg Venous Lymphat Disord 2022;10:1334-1342.e1. [Crossref] [PubMed]
- Hentschel DM, Minarsch L, Vega F, et al. The Surfacer(®) Inside-Out(®) Access System for right-sided catheter placement in dialysis patients with thoracic venous obstruction. J Vasc Access 2020;21:411-8.
- Razavi MK, Peden EK, Sorial E, et al. Efficacy and safety associated with the use of the Surfacer(®) Inside-Out(®) Access Catheter System: Results from a prospective, multicenter Food and Drug Administration-approved Investigational Device Exemption study. J Vasc Access 2021;22:141-6.
- Lund GB, Trerotola SO, Scheel PJ Jr. Percutaneous translumbar inferior vena cava cannulation for hemodialysis. Am J Kidney Dis 1995;25:732-7. [Crossref] [PubMed]
- Kariya S, Tanigawa N, Kojima H, et al. Percutaneous translumbar inferior vena cava cannulation under computed tomography guidance. Jpn J Radiol 2009;27:176-9. [Crossref] [PubMed]
- Leś J, Spaleniak S, Lubas A, et al. Early complications of translumbar cannulation of the inferior vena cava as a quick, last-chance method of gaining access for hemodialysis. Ten years of experience in one clinical center. Wideochir Inne Tech Maloinwazyjne 2021;16:282-8. [Crossref] [PubMed]
- Rajan DK, Croteau DL, Sturza SG, et al. Translumbar placement of inferior vena caval catheters: a solution for challenging hemodialysis access. Radiographics 1998;18:1155-67; discussion 1167-70. [Crossref] [PubMed]
- Liu F, Bennett S, Arrigain S, et al. Patency and Complications of Translumbar Dialysis Catheters. Semin Dial 2015;28:E41-7. [Crossref] [PubMed]
- Herscu G, Woo K, Weaver FA, et al. Use of unconventional dialysis access in patients with no viable alternative. Ann Vasc Surg 2013;27:332-6. [Crossref] [PubMed]
- Rahman S, Kuban JD. Dialysis Catheter Placement in Patients With Exhausted Access. Tech Vasc Interv Radiol 2017;20:65-74. [Crossref] [PubMed]
- Power A, Singh S, Ashby D, et al. Translumbar central venous catheters for long-term haemodialysis. Nephrol Dial Transplant 2010;25:1588-95. [Crossref] [PubMed]
- Lorenz JM, Regalado S, Navuluri R, et al. Transhepatic guidance of translumbar hemodialysis catheter placement in the setting of chronic infrarenal IVC occlusion. Cardiovasc Intervent Radiol 2010;33:635-8. [Crossref] [PubMed]
- Stavropoulos SW, Pan JJ, Clark TW, et al. Percutaneous transhepatic venous access for hemodialysis. J Vasc Interv Radiol 2003;14:1187-90. [Crossref] [PubMed]
- Smith TP, Ryan JM, Reddan DN. Transhepatic catheter access for hemodialysis. Radiology 2004;232:246-51. [Crossref] [PubMed]
- Murthy R, Arbabzadeh M, Lund G, et al. Percutaneous transrenal hemodialysis catheter insertion. J Vasc Interv Radiol 2002;13:1043-6. [Crossref] [PubMed]
- Jaber MR, Thomson MJ, Smith DC. Azygos vein dialysis catheter placement using the translumbar approach in a patient with inferior vena cava occlusion. Cardiovasc Intervent Radiol 2008;31:S206-8. [Crossref] [PubMed]
- Punzi M, Ferro F, Petrosino F, et al. Use of an intra-aortic Tesio catheter as vascular access for haemodialysis. Nephrol Dial Transplant 2003;18:830-2. [Crossref] [PubMed]



