
Diagnostic performance of contemporary transesophageal echocardiography with modern imaging for infective endocarditis
Highlight box
Key findings
• Modern TEE imaging has improved diagnostic performance for endocarditis
What is known and what is new?
• TEE imaging is paramount for diagnosing endocarditis
• Improvements in TEE imaging include modern probes and increased use of 3D imaging
• Contemporary imaging has improved detection rates of infective endocarditis
What is the implication, and what should change now?
• Clinicians should note that improved diagnostic performance of modern TEE imaging have led to improved diagnosis of infective endocarditis, particularly prosthetic valve endocarditis
Introduction
Background
Infective endocarditis (IE) accounted for nearly 600,000 hospitalizations between 2003 and 2016 in the United States (1). Early detection is paramount, because IE is the fourth leading life-threatening infection, with mortality rates of 15% to 40% (2-5). Its presentation varies widely, from rapid progression with embolic phenomena in 25% to 30% of cases, to clinically indolent course over weeks to months (6,7). The heterogeneity of this disease led to the development of the Duke criteria in 1994, which emphasized the role of echocardiography for diagnosis of IE (8). These criteria have since been modified, and are now considered the clinical standard for diagnosis (9).
The diagnostic sensitivity and specificity of a transthoracic echocardiogram (TTE) ranges between 50–90% and 90%, respectively, compared to two dimensional (2D)-TEE, which has superior diagnostic accuracy, with a reported sensitivity of 90% to 100% and a specificity of >90% for native valve IE (10,11). In prosthetic valve infective endocarditis (PVIE), the sensitivities of both TTE and 2D-TEE are lower, estimated to be 40% to 70%, and 85%, respectively (10,12).
Rationale and knowledge gap
Over the recent decade, improvements in TEE imaging technology, including higher frame rates and three dimensional (3D) imaging, have improved visualization of cardiac chamber and valvular anatomy (13). TEE has been shown to be superior to cardiac CT when diagnosing valvular lesions related to IE (14). In particular, 3D TEE imaging has proven to be critical for assessment of complex valvular pathologies, as well as guiding interventional procedures, such as the percutaneous mitral valve edge-to-edge repair (15-18). The impact of contemporary TEE imaging on the diagnosis of IE and its subtypes has not been well studied (19,20). The reported sensitivity of TEE for IE has varied between 92% to 96%, and a second TEE is recommended if clinical suspicion remains high after a negative examination (21-23). However, these recommendations for repeat TEE are based on older generations of TEE imaging probes and echocardiographic storage and analysis systems from the late 1990s (21).
Objective
Our study sought to evaluate the diagnostic performance of modern TEE imaging for IE and its subtypes, and to evaluate the role of repeat imaging for patients with suspected endocarditis, despite a negative/equivocal TEE with the advent of contemporary TEE imaging with3D technology. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-431/rc).
Methods
This is a single-center retrospective cohort study performed at a high-volume tertiary center. This study was approved by the Institutional Review Board of Cleveland Clinic [19-792], and the need for informed consent was waived due to the retrospective analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients who underwent two or more TEEs, with a subsequent confirmed diagnosis of IE, within a 6-month period, in 2011 and 2019 respectively, were included. Out of 1,141 patients who had two or more TEEs within six months for suspicion of IE in 2011 and 2019, 242 patients met modified Duke criteria and were included: 70 in 2011 and 172 in 2019 TEE findings were reviewed by experienced National Board of Echocardiography (NBE) certified echocardiologists at our center in the two years compared. Those who did not meet the modified Duke criteria, based on meticulous chart review, were excluded (Figure 1) (9). We chose to compare contemporary patients in 2019 compared to 2011. Year 2011 was chosen because TEE studies performed during that year could be individually retrieved and reviewed on the echocardiographic imaging database. Prior to 2011, the images for some TEE studies could not be fully examined for review. A six month interval was used, to capture appropriate patients needing serial TEE studies, and achieve adequate sample sizes for each cohort. We aimed for a sample similar in size to those reported in previous literature examining the utility of serial TEEs for detection of IE (21). Baseline characteristics, microbiology of organisms, echocardiographic features, including left ventricular ejection fraction, right ventricular systolic pressure, and the presence of moderate or greater valvular stenosis/regurgitation were obtained from manual chart review.
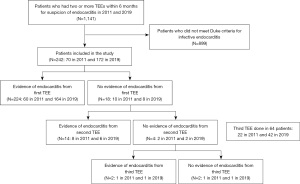
The primary endpoint was the sensitivity of TEE to detect IE in 2011 versus 2019. This was assessed by comparing the percentage of the initial TEEs with positive findings for IE (evidence of vegetations, abscesses, fistulas, or perforations). TEE sensitivity was also compared among the different subtypes of IE (native valve, prosthetic valve, cardiovascular implantable electronic device-related, central line-related, and aortic prosthetic graft-related infection). Other outcomes included the need for surgical or procedural management of IE (valve replacement, valve repair, device extraction, central line removal, and ascending aorta graft replacement), time from index admission to the diagnosis of IE, length of stay, and the rate of endocarditis-related complications (stroke, brain abscess, mycotic aneurysms, acute kidney injury, and seeding of the infection). Limitations/bias were addressed at the end of the manuscript.
Statistical analysis
Chi-squared and Fisher exact tests, when appropriate, were used to compare categorical variables, which were expressed in percentages. Continuous variables were described as median and interquartile ranges (IQRs) and analyzed using the Mann-Whitney U test. To account for the differences in baseline characteristics, a multivariable regression analysis was performed to assess the relationship between baseline characteristics and the sensitivity of TEE for IE, reported as odds ratios (ORs) for the percentage of the initial TEEs with positive findings for IE. Kaplan-Meier curves and log-rank tests were used to compare the need for a procedure or surgery for IE. Charts were reviewed manually and if there was no mention of a baseline characteristic anywhere in the chart, it was presumed negative. A P value of less than 0.05 was considered significant. SPSS version 25.0 (IBM, New York, USA) was used for statistical analysis.
Results
Among the two patient groups, there were similar baseline rates, prior to diagnosis of IE, of congenital heart disease, cardiac and dental procedures/surgeries in the past year, central lines, history of intravenous drug use, chronic kidney disease, cirrhosis, previous mechanical/bioprosthetic valve replacements, valve repairs, and previous episodes of endocarditis (P=0.06 to 0.95) (Table 1). In 2011, patients were more likely to have chronic obstructive pulmonary disease (COPD) (n=22, 31% vs. n=33, 19%, P=0.04), with higher prevalence of smoking (n=49, 70% vs. n=92, 54%, P=0.02), and a history of osteomyelitis (n=14, 20% vs. n=15, 9%, P=0.01), but were less likely to have an intracardiac device prior to diagnosis (n=11, 16% vs. n=49, 29%, P=0.04) (Table 1).
Table 1
| Baseline characteristics | 2011 (N=70) | 2019 (N=172) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 67 [50–79] | 61 [46–70] | 0.059 |
| Male, n [%] | 50 [71] | 110 [64] | 0.27 |
| Caucasian, n [%] | 14 [20] | 19 [11] | 0.07 |
| BMI (kg/m2), median [IQR] | 28 [24–31] | 27 [24–33] | 0.89 |
| Coronary artery disease n [%] | 33 [47] | 77 [45] | 0.74 |
| HTN, n [%] | 41 [59] | 105 [61] | 0.72 |
| HLD, n [%] | 30 [43] | 95 [55] | 0.08 |
| DM, n [%] | 26 [37] | 45 [26] | 0.09 |
| Chronic kidney disease, n [%] | 29 [41] | 50 [29] | 0.06 |
| COPD, n [%] | 22 [31]* | 33 [19]* | 0.04* |
| Cirrhosis, n [%] | 2 [3] | 9 [5] | 0.52 |
| History of heart failure, n [%] | 29 [41] | 59 [34] | 0.30 |
| Smoking history, n [%] | 49 [70]* | 92 [54]* | 0.02* |
| Intravenous drug use, n [%] | 10 [14] | 31 [18] | 0.48 |
| History of endocarditis, n [%] | 12 [17] | 44 [26] | 0.16 |
| History of osteomyelitis, n [%] | 14 [20]* | 15 [9]* | 0.01* |
| CRP (mg/dL), median [IQR] | 5.7 [2.8–13.6] | 7.6 [3.2–15.1] | 0.28 |
| ESR (mm/hour), median [IQR] | 38 [19–89.3] | 43 [26–74] | 0.65 |
| Creatinine (mg/dL), median [IQR] | 0.97 [0.7–1.8] | 0.99 [0.8–1.3] | 0.89 |
| Recent procedures (within 1 year), n [%] | 0.50 | ||
| Cardiac procedure† | 20 [29] | 34 [20] | |
| Dental procedure‡ | 5 [7] | 12 [7] | |
| Other surgeries | 8 [11] | 25 [14] | |
| Congenital heart disease, n [%] | 9 [13] | 36 [21] | 0.14 |
| Intracardiac device, n [%] | 11 [16]* | 49 [29]* | 0.04* |
| Central line, n [%] | 9 [13] | 38 [22] | 0.10 |
| History of valve replacement, n [%] | |||
| Mechanical valve | 6 [9] | 10 [6] | 0.41 |
| Bioprosthetic valve | 20 [29] | 54 [31] | 0.67 |
| History of valve repair, n [%] | 10 [14] | 24 [14] | 0.95 |
| Microbiology, n [%] | 0.049* | ||
| Staphylococcus aureus | 24 [34] | 42 [24] | 0.12 |
| Staphylococcus epidermidis | 6 [9] | 14 [8] | 0.91 |
| Other Staphylococcus species | 2 [3] | 3 [2] | 0.62 |
| Streptococcus species | 8 [11] | 38 [22] | 0.055 |
| Enterococcus | 4 [6] | 2 [1] | 0.25 |
| Candida/aspergillus | 13 [19] | 22 [13] | 0.06 |
| Other bacteria | 10 [14] | 28 [16] | 0.70 |
| Multi-bacterial | 0 [0] | 11 [6] | 0.04* |
| Culture negative | 3 [4] | 12 [7] | 0.56 |
| Left ventricular ejection fraction (%), median [IQR] | 55 [45–62] | 60 [53–64] | 0.03* |
| Right ventricular systolic pressure (mmHg), median [IQR] | 38 [28–50] | 36 [29–46] | 0.50 |
| Pericardial effusion, n [%] | 3 [4] | 10 [6] | 0.76 |
| Moderate or severe valvular regurgitation, n [%] | 39 [56] | 91 [53] | 0.69 |
| Moderate or severe valvular stenosis, n [%] | 4 [6] | 14 [8] | 0.51 |
*, statistically significant; †, cardiac procedures: included LVAD implant, cardiac ablation, valve repair/replacement, coronary artery bypass graft, pacemaker/intracardiac device placement/removal, aortic repair/replacement; ‡, dental procedures: root canal, tooth extraction or other procedure involving gingival manipulation (excluding routine dental cleaning). IQR, interquartile range; BMI, body mass index; HTN, hypertension; HLD, hyperlipidemia; DM, diabetes; COPD, chronic obstructive pulmonary disease; LVAD, left ventricular assist device; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
The median left ventricular ejection fraction was lower in the 2011 group [55% (45% to 62%) vs. 60% (53% to 64%), P=0.03] (Table 1). In the 2011 group, polymicrobial blood stream infections (n=0, 0% vs. n=11, 6.4%, P=0.04) were less common. The distribution of pathogens detected between the two groups showed statistically significant differences (P=0.049) (Table 1).
The sensitivities of TEE in 2011 and 2019, calculated by the percentage of the initial TEEs with positive findings for IE, were 85.7% and 95.3%, respectively (P=0.01). When analyzing by subtypes of IE, the improved diagnostic performance was driven by the increased sensitivity for the detection of prosthetic valve endocarditis (PVIE) (n=17, 70.8% vs. 59, 93.7%, P=0.009). There were no significant differences in TEE sensitivity for native valve, cardiovascular implantable electronic device-related, central line-related, and aortic prosthetic graft-related IE between 2011 and 2019 (Table 2). The median vegetation size on the initial TEE was not significantly different between the two groups: median length: 1.2 (0.7 to 2.0) cm in 2011 vs. 1.2 (0.8 to 1.9) cm in 2019, P=0.82; median width: 0.6 (0.1 to 1.0) cm in 2011 vs. 0.7 (0.4 to 1.0) cm in 2019 (P=0.059) (Table 2). On multivariable regression analysis, patients in 2019 had higher odds of endocarditis being detected from the first TEE [OR: 4.06, 95% confidence intervals (CIs): 1.41–11.71, P=0.01] (Table 3). More patients in the 2011 group had endocarditis detected on the second TEE (n=8, 11.4% vs. 6, 3.5%, P=0.03) when compared to 2019 (Table 2).
Table 2
| TEE findings and associated outcomes | 2011 (N=70), n (%) | 2019 (N=172), n (%) | P value |
|---|---|---|---|
| Evidence of endocarditis from first TEE based on lesion identified | 60 (85.7)* | 164 (95.3)* | 0.01* |
| Vegetations | 57 (81.4) | 155 (90.1) | 0.06 |
| Abscess | 6 (8.6) | 29 (16.9) | 0.1 |
| Perforation | 13 (18.6) | 22 (12.8) | 0.25 |
| Fistula | 0 (0.0) | 2 (1.2) | >0.99 |
| Evidence of endocarditis from first TEE based on subtype of endocarditis | |||
| Prosthetic valve endocarditis | 17 (70.8)* | 59 (93.7)* | 0.009* |
| Native valve endocarditis | 31 (93.9) | 75 (96.2) | 0.63 |
| Aortic prosthetic graft-related | 1 (100.0) | 1 (100.0) | – |
| CIED-related | 8 (88.9) | 24 (96.0) | 0.47 |
| Central line-related | 3 (100.0) | 5 (100.0) | – |
| New evidence of endocarditis one second TEE with initial negative TEE | 8 (11.4)* | 6 (3.5)* | 0.03* |
| Vegetations on second TEE | 47 (67.1) | 108 (62.8) | |
| Abscess on second TEE | 8 (11.4) | 25 (14.5) | |
| Perforation on second TEE | 13 (18.6) | 21 (12.2) | |
| Fistula on second TEE | 1 (1.4) | 2 (1.2) | |
| New evidence of endocarditis on third TEE with two prior negative TEEs | 1 (1.4) | 1 (0.6) | 0.5 |
| Vegetations on third TEE | 9 (40.9) | 18 (42.9) | 0.88 |
| Abscess on third TEE | 1 (4.5) | 6 (14.3) | 0.41 |
| Perforation on third TEE | 2 (9.1) | 6 (14.3) | 0.7 |
| Fistula on third TEE | 0 (0.0) | 0 (0.0) | – |
| Indications for second TEE | |||
| Intra-operative | 25 (35.7) | 106 (61.6) | <0.001* |
| Monitoring of lesions found on prior TEE | 33 (47.1) | 58 (33.7) | <0.001* |
| Clinical suspicion of endocarditis despite negative or equivocal first TEE | 7 (10.0) | 7 (4.1) | 0.051 |
| Assessment prior to cardioversion | 5 (7.1) | 1 (0.6) | 0.073 |
| Indications for third TEE (if applicable) | 0.66 | ||
| Intra-operative | 3 (13.6) | 11 (26.2) | 0.35 |
| Monitoring of lesions found on prior TEE | 13 (59.1) | 23 (54.8) | 0.74 |
| Clinical suspicion of endocarditis despite negative or equivocal TEE | 1 (4.5) | 1 (2.4) | – |
| Assessment of valvular/vascular disease or prior to cardioversion | 5 (22.7) | 7 (16.7) | 0.74 |
| Vegetation size on initial TEE (cm), median (IQR) | |||
| Length | 1.2 (0.7–2) | 1.2 (0.8–1.9) | 0.82 |
| Width | 0.6 (0.1–1) | 0.7 (0.4–1) | 0.059 |
*, statistically significant. IQR, interquartile range; TEE, transesophageal echocardiogram; CIED, cardiovascular implantable electronic device.
Table 3
| Characteristics | Bivariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, per 10-year increase | 1 (0.74–1.34) | 0.99 | – | – | |
| Male sex | 0.23 (0.05–1) | 0.051 | 0.27 (0.01–1.27) | 0.10 | |
| BMI, per 1-unit increase | 1 (0.99–1.01) | 0.83 | – | – | |
| Coronary artery disease | 1.05 (0.4–2.75) | 0.93 | – | – | |
| Hypertension | 1.24 (0.47–3.25) | 0.67 | – | – | |
| Hyperlipidemia | 1.75 (0.65–4.68) | 0.27 | – | – | |
| Diabetes mellitus | 0.82 (0.29–2.27) | 0.7 | – | – | |
| Chronic kidney disease | 4.19 (0.94–18.7) | 0.06 | 4.22 (0.88–20.16) | 0.07 | |
| COPD | 0.75 (0.25–2.2) | 0.6 | – | – | |
| Cirrhosis | 0.79 (0.1–6.58) | 0.83 | – | – | |
| Heart failure | 0.89 (0.33–2.39) | 0.82 | – | – | |
| Smoking | 1.44 (0.55–3.75) | 0.46 | – | – | |
| Intravenous drug use | 1.02 (0.28–3.7) | 0.97 | – | – | |
| Congenital heart disease | 0.42 (0.15–1.19) | 0.1 | 0.5 (0.15–1.66) | 0.26 | |
| Intracardiac device | 0.64 (0.23–1.77) | 0.39 | – | – | |
| Central line | 2.01 (0.45–9.07) | 0.36 | – | – | |
| Prosthetic valve | 0.35 (0.13–0.93) | 0.04* | 0.46 (0.16–1.35) | 0.16 | |
| Valve repair | 0.8 (0.22–2.94) | 0.74 | – | – | |
| LVEF <40% | 0.36 (0.11–1.2) | 0.1 | 0.48 (0.12–1.84) | 0.28 | |
| Year group | |||||
| 2011 | Reference | 0.01* | Reference | 0.01* | |
| 2019 | 3.42 (1.29–9.06) | 4.06 (1.41–11.71) | |||
*, statistically significant. TEE, transesophageal echocardiogram; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; OR, odds ratio; CI, confidence interval.
TEE imaging technology varied greatly between 2011 and 2019. The majority of TEE studies (1st TEE, 2nd TEE, and 3rd TEE) performed in 2011 used the X7-2T (2–7 MHz) (62.9%, 102/162) (Philips Healthcare, Andover, MA, USA), and Sequoia TE-V5M (3.5–7 MHz) (22.2%, 36/162) probes (Siemens Medical Solutions, Malvern, PA, USA) (Table 4). In 2019, 49.4% (190/385) and 31.2% (120/385) of TEEs were performed using the Philips X8-2T (2–8 MHz) and X7-2T probes, respectively (Philips Healthcare, Andover, MA, USA). When looking at the initial TEE each patient underwent, X7-2T probes (n=51/70, 72.9%, P<0.001) were used in the majority of cases. In 2019, significantly more initial TEEs were conducted using X8-2T probes than X7-2T probes, 99/172 (57.6%) versus 44/172 (25.6%), respectively (P<0.001) (Table 4).
Table 4
| TEE imaging probe details | 2011 (N=70), n (%) | 2019 (N=172), n (%) | P value |
|---|---|---|---|
| Use of 3-dimensional TEE imaging on first TEE | 43 (70.5) | 140 (97.2) | <0.001* |
| Use of 3-dimensional TEE imaging on second TEE | 32 (50.0) | 144 (86.2) | <0.001* |
| Use of 3-dimensional TEE imaging on third TEE | 12 (60.0) | 35 (87.5) | 0.02* |
| Probe used during first TEE | |||
| X7-2T | 51 (72.9) | 44 (25.6) | <0.001* |
| X8-2T | 0 (0.0) | 99 (57.6) | <0.001* |
| TE-V5M | 5 (7.14) | 0 (0.0) | 0.002* |
| S7-2 Omni | 3 (4.3) | 0 (0.0) | 0.02* |
| Z6M | 0 (0.0) | 0 (0.0) | – |
| Unavailable | 11 (15.7) | 29 (16.9) | 0.83 |
| Probe used during second TEE | N=70 | N=172 | |
| X7-2T | 37 (52.9) | 60 (34.9) | 0.01* |
| X8-2T | 0 (0.0) | 73 (42.4) | <0.001* |
| TE-V5M | 26 (37.1) | 0 (0.0) | <0.001* |
| S7-2 Omni | 1 (1.4) | 0 (0.0) | 0.29 |
| Z6M | 0 (0.0) | 2 (1.2) | >0.99 |
| Unavailable | 6 (8.6) | 37 (21.5) | 0.02* |
| Probe used during third TEE | N=22 | N=41 | |
| X7-2T | 14 (63.6) | 16 (39.0) | 0.11 |
| X8-2T | 0 (0.0) | 18 (43.9) | <0.001* |
| TE-V5M | 5 (22.7) | 0 (0.0) | 0.003* |
| S7-2 Omni | 1 (4.5) | 0 (0.0) | 0.33 |
| Z6M | 0 (0.0) | 1 (2.4) | >0.99 |
| Unavailable | 2 (9.1) | 6 (14.6) | 0.71 |
*, statistically significant. X7-2T/X8-2T (Phillips), TE-V5M (Siemens), S7-2 Omni (Phillips), Z6M (Siemens). TEE, transesophageal echocardiogram.
3D technology was utilized in 97.2% of initial TEEs in 2019, compared to 70.5% of the studies in 2011 (P<0.001). In the second and third TEEs, the use of 3D in 2011 was 50% (n=32) and 60% (n=12) (P<0.001), respectively, versus 86.2% (n=144) and 87.5% (n=35) in 2019 (P=0.02) (Table 4). The indications for the second TEE between the groups were significantly different. Those in the 2019 group were more likely to have the second TEE as an intraoperative study (n=106, 61.6% vs. n=25, 35.7%, P<0.001), whereas in the 2011 group, monitoring of lesions found on the initial TEE (n=33, 47.1% vs. n=58, 33.7%, P<0.001) and ongoing clinical suspicion of endocarditis despite negative or equivocal first TEE (n=7, 10% vs. n=7, 4.1%, P=0.051) were common indications (Table 2).
There were no significant differences in the rate of complications or lengths of stay between 2011 and 2019 (Table 5). Patients in the 2019 group had a higher rate of IE diagnosis within fifteen days of index admission; however, this did not reach statistical significance (n=161, 93.6% in 2019 vs. n=62, 88.6% in 2011, P=0.19).
Table 5
| Clinical outcomes in the study cohorts | 2011 (N=70), n (%) | 2019 (N=172), n (%) | P value |
|---|---|---|---|
| Time from first to second TEE (days), median [IQR] | 9.5 [5–30.75] | 10 [5–37.8] | 0.91 |
| Time from second to third TEE (days), median [IQR] | 42 [12–66.8] | 26 [8.8–93.5] | 0.79 |
| Neurologic complications | 0.91 | ||
| Stroke | 15 (21.4) | 33 (19.2) | |
| Brain abscess | 1 (1.4) | 2 (1.2) | |
| Acute kidney injury | 21 (30.0) | 51 (29.7) | 0.96 |
| Seeding of infection | 14 (20.0) | 56 (32.6) | 0.051 |
| Length of stay (days), median [IQR] | 16 [10–23] | 18 [10.5–28] | 0.49 |
| Diagnosis of IE in 15 days of index admission | 62 (88.6) | 161 (93.6) | 0.19 |
| Need for procedures (valve replacement or repair or device extraction) | 39 (55.7)* | 120 (69.8)* | 0.04* |
*, statistically significant. IQR, interquartile range; TEE, transesophageal echocardiogram; IE, infective endocarditis.
Discussion
Key findings
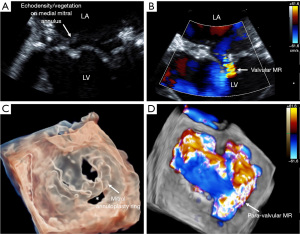
The present study was performed to investigate the diagnostic performance of contemporary TEE imaging for IE and evaluate the additive value of repeat TEE studies in patients with suspected IE. Our main finding was that the sensitivity of the initial TEE to detect IE improved from 85.7% in 2011 to 95.3% in 2019, mainly driven by the improved detection of PVIE. This remained true after multivariable analysis, accounting for various baseline characteristics, including intravenous drug use. This finding may reflect increased use of 3D imaging in the more recent cohort (97.2% in 2019 versus 70.5% in 2011, P<0.001) (Figure 2). Additionally, this may reflect the increased use of modern TEE probes with enhanced imaging capabilities during the index TEE in 2019 versus 2011 (72.9% versus 25.6%, respectively, P<0.001, Figure 2). Of note, vegetation size was not significantly different between the groups, and therefore was unlikely to account for the differences in diagnostic performance.
Strengths/limitations
Being a tertiary referral center, our echocardiographers are very well experienced in reviewing images in a very complex cohort of patients with IE. There are several limitations to be acknowledged in the present study. First, the results reported in this retrospective study came from a single center with a relatively small sample size, which limits the generalizability of our findings. In part of our cohort, specific baseline characteristics were unavailable during manual chart review and ultimately could not be accounted for in our analysis. Secondly, there was a potential for referral bias, as data from this study were more representative of tertiary and quaternary care environments, and published data has demonstrated that patients with IE detected in the community may differ in their characteristics and complexity (6). Third, selection of patients in a non-randomized fashion is another limitation in our study that may impact our study’s external validity. This selection bias in only looking at individuals who had more than one TEE within six months likely selected a sicker population, who were more likely to be at a higher risk for complications or surgical management. For example, our cohort had a high prevalence of moderate to severe valvular regurgitation. This was a known bias and was permitted because this cohort was collected through meticulous manual chart review. A future prospective multicenter trial will verify the utility of modern TEE imaging and the additive role of repeat TEE in diagnosis of IE.
Comparison with similar studies
The diagnosis of PVIE by TEE is more difficult, because prosthetic valves can be affected by different disease processes that mimic IE, including pannus formation, thrombus, prosthetic strands, loose suture material, mitral subvalvular tissue remnants, and micro-cavitation (24,25). Acoustic shadowing imposed by prosthetic valves can also render imaging difficult (26-28). The difficulties with diagnosing PVIE using echocardiographic imaging alone have led to increased adoption of a multimodality imaging approach to evaluation in appropriate cases utilizing 18-fluorodeoxyglucose positron emission tomography/computed tomography and cardiac computed tomography as adjuvant imaging modalities (29-32). Early in the disease course, signs of IE can be very subtle, and nearly a quarter of PVIE cases may be culture negative, partly due to the early administration of antibiotics, which further complicates the diagnosis of PVIE (33). Importantly, PVIE is characterized by a lower incidence of vegetations and a higher incidence of valvular and paravalvular complications, which are important echocardiographic considerations in suspected PVIE cases (34). Despite the important diagnostic utility of 2D-TEE in the detection of valvular and paravalvular complications of PVIE (11), a meta-analysis of 20 publications with a total of 496 patients with PVIE showed 2D-TEE missed the presence of vegetations and paravalvular complications in 18% and 14% of cases, respectively (35).
Explanations of findings
Due to the critical need for timely and accurate diagnosis and management of PVIE, there have been improvements in contemporary 2D and 3D TEE imaging techniques (35). In our study, we found that the sensitivity of TEE for PVIE dramatically improved from 2011 to 2019 from 70.8% to 93.7%, P=0.009. This may reflect improvements in both 2D and 3D imaging over this period. The modern TEE probes have improved imaging capabilities including higher frame rates and frequencies (36). We believe this improvement in spatial and temporal resolution helps capture and accurately assess fast moving structures, such as vegetations (37).
With the advent of modern probes and 3D imaging, the diagnostic performance of TEE in 2019 was comparable for both native valve and prosthetic valve IE (96.2% vs. 93.7%, respectively). Different reports have described the superior diagnostic value of 3D-TEE in PVIE cases, which stems from better visualization of subtle abnormalities and delineation of cardiac anatomy along with more detailed assessment of peri-annular infection (24,35). Through multiplanar imaging and volumetric reconstruction, 3D TEE can more accurately assess the size of irregular vegetations, help evaluate the risk for embolization, and detect perivalvular abscesses (38-42). 3D imaging is advantageous to 2D imaging in assessing these key factors to determine the need for potential valve surgery. The improved diagnostic performance in our study likely reflects improvements from both 2D and 3D TEE imaging.
Implications and actions
The current American Heart Association (AHA) and the European Society of Cardiology (ESC) guidelines strongly recommend repeating TEE in patients with an initial non-diagnostic TEE and persistent high suspicion of IE in three to five days (Class I indication, Level of evidence: C) (2,21,23,40,43,44). Our findings demonstrate that while a repeat TEE is still needed in certain clinical scenarios in the evaluation of IE, contemporary TEE imaging has further improved the diagnostic performance for IE. We found that with modern TEE imaging, 164/172 (95.3%) of patients with endocarditis were detected on the initial TEE. This improvement in sensitivity led to a decreased yield from additional TEEs in 2019, when compared to 2011, with 4.7% versus 12.8% of patients having evidence of IE being detected on a subsequent TEE (P=0.03).
Though we did not observe significant differences in the rate of complications, length of stay, 30-day and one year mortalities between the two groups, patients in 2019 had a higher rate of procedures, including valve surgery and device extraction, particularly those diagnosed early during the admission (within fifteen days from index admission). The time to diagnosis was also shorter in the 2019 group; however, this finding did not reach statistical significance. We attribute the increased rate of procedures in the latter group (70% in 2019 vs. 56% in 2011, P=0.04), when diagnosed within 15 days, to potentially earlier detection of endocarditis, leading to increased rates of definitive intervention. It should be noted this finding was not due to differences in vegetation size or embolic complications, which were comparable between 2011 and 2019.
Conclusions
Contemporary TEE imaging with modern TEE probes and 3D imaging was associated with improved diagnostic performance for IE on the initial examination, driven primarily by improved detection of PVIE. These findings were not explained by differences in baseline characteristics, including intravenous drug use, or the size of the vegetations.
Acknowledgments
We would like to acknowledge the Heart, Vascular and Thoracic Institute for assisting with data retrieval for this study.
Funding: BM was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number TL1TR002344. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the NIH.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-431/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-431/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-431/coif). BM received Education grant $5000 from Healthcare Delivery and Implementation Science Center (separate project) and was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number TL1TR002344. This grant contributed to drafting the manuscript, data analysis, manuscript submission, and manuscript revision. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Cleveland Clinic [19-792], and the need for informed consent was waived due to the retrospective analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alkhouli M, Alqahtani F, Alhajji M, et al. Clinical and Economic Burden of Hospitalizations for Infective Endocarditis in the United States. Mayo Clin Proc 2020;95:858-66. [Crossref] [PubMed]
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. Erratum in: Circulation 2015;132:e215 Erratum in: Circulation 2016;134:e113. Erratum in: Circulation 2018;138:e78-e79. [Crossref] [PubMed]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. [Crossref] [PubMed]
- Leone S, Ravasio V, Durante-Mangoni E, et al. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection 2012;40:527-35. [Crossref] [PubMed]
- Rajani R, Klein JL. Infective endocarditis: A contemporary update. Clin Med (Lond) 2020;20:31-5. [Crossref] [PubMed]
- Fernández-Hidalgo N, Almirante B, Tornos P, et al. Contemporary epidemiology and prognosis of health care-associated infective endocarditis. Clin Infect Dis 2008;47:1287-97. [Crossref] [PubMed]
- Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005;112:69-75. [Crossref] [PubMed]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200-9. [Crossref] [PubMed]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. [Crossref] [PubMed]
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69:325-44. [Crossref] [PubMed]
- Jassal DS, Aminbakhsh A, Fang T, et al. Diagnostic value of harmonic transthoracic echocardiography in native valve infective endocarditis: comparison with transesophageal echocardiography. Cardiovasc Ultrasound 2007;5:20. [Crossref] [PubMed]
- Pérez-Vázquez A, Fariñas MC, García-Palomo JD, et al. Evaluation of the Duke criteria in 93 episodes of prosthetic valve endocarditis: could sensitivity be improved? Arch Intern Med 2000;160:1185-91. [Crossref] [PubMed]
- Lang RM, Mor-Avi V, Dent JM, et al. Three-dimensional echocardiography: is it ready for everyday clinical use? JACC Cardiovasc Imaging 2009;2:114-7. [Crossref] [PubMed]
- Sifaoui I, Oliver L, Tacher V, et al. Diagnostic Performance of Transesophageal Echocardiography and Cardiac Computed Tomography in Infective Endocarditis. J Am Soc Echocardiogr 2020;33:1442-53. [Crossref] [PubMed]
- Estévez-Loureiro R, Franzen O, Winter R, et al. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. J Am Coll Cardiol 2013;62:2370-7. [Crossref] [PubMed]
- Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol 2008;52:446-9. [Crossref] [PubMed]
- Sugeng L, Shernan SK, Weinert L, et al. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr 2008;21:1347-54. [Crossref] [PubMed]
- Faletra FF, Berrebi A, Pedrazzini G, et al. 3D transesophageal echocardiography: A new imaging tool for assessment of mitral regurgitation and for guiding percutaneous edge-to-edge mitral valve repair. Prog Cardiovasc Dis 2017;60:305-21. [Crossref] [PubMed]
- Yong MS, Saxena P, Killu AM, et al. The Preoperative Evaluation of Infective Endocarditis via 3-Dimensional Transesophageal Echocardiography. Tex Heart Inst J 2015;42:372-6. [Crossref] [PubMed]
- González YO, Ung R, Blackshear JL, et al. Three-Dimensional Echocardiography for Diagnosis of Transcatheter Prosthetic Aortic Valve Endocarditis. CASE (Phila) 2017;1:155-8. [Crossref] [PubMed]
- Vieira ML, Grinberg M, Pomerantzeff PM, et al. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart 2004;90:1020-4. [Crossref] [PubMed]
- Eudailey K, Lewey J, Hahn RT, et al. Aggressive infective endocarditis and the importance of early repeat echocardiographic imaging. J Thorac Cardiovasc Surg 2014;147:e26-8. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Afonso L, Kottam A, Reddy V, et al. Echocardiography in Infective Endocarditis: State of the Art. Curr Cardiol Rep 2017;19:127. [Crossref] [PubMed]
- Lo Presti S, Elajami TK, Zmaili M, et al. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J Cardiol 2021;13:254-70. [Crossref] [PubMed]
- Evangelista A, Gonzalez-Alujas MT. Echocardiography in infective endocarditis. Heart 2004;90:614-7. [Crossref] [PubMed]
- Aguado JM, González-Vílchez F, Martín-Durán R, et al. Perivalvular abscesses associated with endocarditis. Clinical features and diagnostic accuracy of two-dimensional echocardiography. Chest 1993;104:88-93. [Crossref] [PubMed]
- San Román JA, Vilacosta I, Sarriá C, et al. Clinical course, microbiologic profile, and diagnosis of periannular complications in prosthetic valve endocarditis. Am J Cardiol 1999;83:1075-9. [Crossref] [PubMed]
- Wang TKM, Sánchez-Nadales A, Igbinomwanhia E, et al. Diagnosis of Infective Endocarditis by Subtype Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: A Contemporary Meta-Analysis. Circ Cardiovasc Imaging 2020;13:e010600. [Crossref] [PubMed]
- Wang TKM, Bin Saeedan M, Chan N, et al. Complementary Diagnostic and Prognostic Contributions of Cardiac Computed Tomography for Infective Endocarditis Surgery. Circ Cardiovasc Imaging 2020;13:e011126. [Crossref] [PubMed]
- Jain V, Wang TKM, Bansal A, et al. Diagnostic performance of cardiac computed tomography versus transesophageal echocardiography in infective endocarditis: A contemporary comparative meta-analysis. J Cardiovasc Comput Tomogr 2021;15:313-21. [Crossref] [PubMed]
- Haq IU, Haq I, Griffin B, et al. Imaging to evaluate suspected infective endocarditis. Cleve Clin J Med 2021;88:163-72. [Crossref] [PubMed]
- Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart 2003;89:258-62. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. Erratum in: Circulation 2014;129:e650. [Crossref] [PubMed]
- Habets J, Tanis W, Reitsma JB, et al. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur Radiol 2015;25:2125-33. [Crossref] [PubMed]
- Bai AD, Steinberg M, Showler A, et al. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J Am Soc Echocardiogr 2017;30:639-646.e8. [Crossref] [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46. [Crossref] [PubMed]
- Anwar AM, Nosir YF, Alasnag M, et al. Real time three-dimensional transesophageal echocardiography: a novel approach for the assessment of prosthetic heart valves. Echocardiography 2014;31:188-96. [Crossref] [PubMed]
- Singh P, Manda J, Hsiung MC, et al. Live/real time three-dimensional transesophageal echocardiographic evaluation of mitral and aortic valve prosthetic paravalvular regurgitation. Echocardiography 2009;26:980-7. [Crossref] [PubMed]
- Sordelli C, Fele N, Mocerino R, et al. Infective Endocarditis: Echocardiographic Imaging and New Imaging Modalities. J Cardiovasc Echogr 2019;29:149-55. [Crossref] [PubMed]
- Pérez-García CN, Olmos C, Islas F, et al. Morphological characterization of vegetation by real-time three-dimensional transesophageal echocardiography in infective endocarditis: Prognostic impact. Echocardiography 2019;36:742-51. [Crossref] [PubMed]
- Berdejo J, Shibayama K, Harada K, et al. Evaluation of vegetation size and its relationship with embolism in infective endocarditis: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2014;7:149-54. [Crossref] [PubMed]
- Sochowski RA, Chan KL. Implication of negative results on a monoplane transesophageal echocardiographic study in patients with suspected infective endocarditis. J Am Coll Cardiol 1993;21:216-21. [Crossref] [PubMed]
- Shafiyi A, Anavekar NS, Virk A, et al. Repeat transesophageal echocardiography in infective endocarditis: An analysis of contemporary utilization. Echocardiography 2020;37:891-9. [Crossref] [PubMed]


