
Surgical creation of lower extremity fistula and grafts
Introduction
Background
The prevalence of patients with end-stage renal disease (ESRD) has steadily increased by an average of 2% per year (1). As of the end of 2019, there were 809,103 patients with ESRD, an increase of over 40% compared to 2009 (1). In the US and worldwide, intermittent hemodialysis (HD) is the most common renal replacement therapy for treating patients with ESRD. According to the US Renal Data System 2021 Annual Data Report (1), 62.6% of ESRD patients are on either in-center or home HD. In the US, intermittent HD is increasingly administered to patients above the age of 65. Reported outcomes suggest higher rates of arteriovenous fistula (AVF) nonmaturation and loss of patency for both AVF/AV graft (AVF/AVG) (2) as a consequence of an unfavorable age related co-morbidity profile (3).
Rationale and knowledge gap
The creation and maintenance of HD vascular access (VA) represents a significant challenge for the ESRD patient population. The failure to achieve consistently reliable and sustainable VA results in a significant reduction in quality of life for the individual patient and increased overall expenditures for our healthcare system. Over 80% of patients initiate HD using a catheter, despite its high all-cause mortality rate (1,4). For the incident patient over the first 12 months, catheter use decreases in favor of more optimal VA options such as AVF and to a lesser extent, AVG.
AVFs are associated with demonstrably lower risks of infection and mortality (5-7) relative to either catheters or AVGs. However, comparisons regarding patency are confounded by varying results across age and comorbidity profile subgroups. Recent publications suggest that AVGs have comparable secondary patency rates (8) and may also be a viable option as a revisional rescue procedure for the failing AVF. Contemporary advances in the medical management of the ESRD patient cohort has resulted in improved patient survival rates. However, increased life expectancy has simultaneously increased the need for advanced alternative solutions to meet the challenging needs of this clinical dilemma.
Lower limb vascular access (LLVA) may be a necessary consideration for patients in whom conventional upper extremity access sites have been exhausted. In some instances, the need to consider LLVA is hastened due to catheter induced central vein occlusion which renders the arms nonuseable. Historically, LLVA has been approached with trepidation because of the potential consequences of limb ischemia and infection (9). Prudent judgement is essential in selecting the appropriate patients for this level of access, and in combination with technical tips, may mitigate morbid complications.
Objective
This review highlights the available LLVA techniques in patients with exhausted upper extremity VA options along with an overview of the current literature on their short- and long-term outcomes.
LLVA surgical techniques
The current surgical approaches to LLVA can be divided into two main categories: (A) autologous AVF; (B) synthetic AVG. The autologous AVFs include both the femoral vein (FV) and great saphenous vein (GSV) transpositions, while the synthetic options include the upper- and mid-thigh synthetic AVGs (10) configurations.
FV transposition
This procedure creates a superficial femoral artery to FV AVF utilizing approximately 25–30 cm of non-reversed FV (9) (Figure 1). The careful selection of the appropriate body habitus is critical because of the need to superficialize the conduit. Avoidance of the high body mass index (BMI) patient and avoidance of those patients with anatomically short groin to knee length is advised to ensure sufficient vein length (10-15) for adequate future cannulation (9). Prior to construction of a FV transposition, we confirm and document that the ipsilateral lower extremity circulation is normal (palpable pulses or an ABI of greater than or equal to 0.1 with compressible arteries) to mitigate the risk of vascular access associated steal (VAAS).
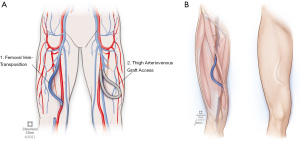
A history of neuropathic pain, foot ulceration or evidence of diabetic or ESRD small vessel vasculopathy are relative concerns and should be further elucidated. The FV should be at least 8 mm in diameter by ultrasound (9). The portion of FV harvested should begin just distal to the confluence of the profunda vein and end just proximal to the popliteal vein. To obtain sufficient length, the FV must be harvested to a point that lies distal to the belly of the sartorius (11). The vessel is transected distally at the popliteal vein, then tunneled laterally and subcutaneously towards the distal superficial femoral artery (SFA). It should be noted that an aperture made in the sartorius muscle belly proximally mitigates compression of the vein by the sartorius muscle (9) as the vessel moves from its in vivo anatomic bed to the superficialized conduit tunnel. Once tunneled, an end-to-side anastomosis is performed with the FV and distal SFA, ensuring an adequate thrill and palpable pulses (or Doppler signals similar to the preoperative exam) at the end of the procedure. Limiting the arteriotomy to 3–3.5 mm reduces the risk of VAAS. If the vein’s diameter is too large, vessel plication is performed to create a juxta-anastomotic taper. For patients in whom vein length is inadequate, supplementing the vein with a 4–7 mm prosthetic cuff can both mitigate steal and reduce any anastomotic tension. The FV harvest bed is drained with closed suction. Chronic anticoagulation is utilized for 3–4 months to reduce the risk of propagation of deep venous thrombosis.
Autogenous FV access in ESRD patients has good durability with higher primary and secondary patency rates at 12 months compared to the use of prosthetic ones. Orion et al. (12) showed that FV transposition has good long-term patency rates, where 5-year primary and secondary patency rates were 74% and 89%, respectively. Another study by Bourquelot et al. (13) reported 1- and 9-year primary patency rates of 91% and 45%, respectively, however, the study’s favorable patency rates are subject to a high degree of selection bias as the majority of their patients were young, non-diabetics with low BMI and normal blood vessels which is not typical for HD patients (Table 1).
Table 1
| Study | Number of patients | Patency rates | Ischemia complications | |
|---|---|---|---|---|
| Primary | Secondary | |||
| Orion et al., 2021 | 21 | 93%, 74%, and 74% at 1-, 3-, and 5-year follow-ups | 100%, 89%, and 89% at 1-, 3-, and 5-year follow-ups | 9.5%* |
| Farber et al., 2020 | 21 | 65.9% at 1-year follow-up | 94.7% at 1-year follow-up | 4.8% |
| Bourquelot et al., 2012 | 70 | 91% and 45% at 1- and 9-year follow-ups | – | 7% |
| Scollay et al., 2010 | 12 | – | – | 16% |
| Gradman et al., 2005 | 22 | – | 94% at 2-year follow-up | 0% |
| Hazinedaroğlu et al., 2004 | 15 | 86.7% at 1-year follow-up | – | 33% |
| Gradman et al., 2001 | 25 | 78% and 73% at 6- and 12-month follow-ups | 91% and 86% at 6-and 12-month follow-ups | 32% |
*, compartment syndrome only. FV, femoral vein.
Autogenous FV VA can be associated with a number of major and minor complications. The aforementioned VAAS complication (Table 1) was highlighted by Gradman et al. paper (14) which reported that 8 out of 25 patients (32%) underwent a secondary procedure to alleviate the ischemia symptoms. In their second experience/paper, they mentioned that the prophylactic measures of FV banding and tapering along with appropriate patient selection (i.e., ankle-brachial index of >0.85) significantly reduced number of patients with evidence of ischemia to 0% (13). Pike et al. (16) reported better results in their study that included 463 patients with overall VAAS rate of only 2.6% at the 6-month follow-up. Other major complications reported in literature include: bleeding, major edema and high-output heart failure (13,15).
Wound-related or minor complications such as infection, hematoma, delayed wound healing, lymphocele, and minor edema were reported by Orion et al. (12) (6/21), Farber et al. (17) (4/21), Hazinedaroğlu et al. (18) (5/15), Scollay et al. (19) (11/15), and Bourquelot et al. (13) (10/70). The wide variation in the reported complication rates might be due to the studies’ small sample size and different definitions of wound-related or minor complications. These complications may inherently increase hospital length of stay and hospital associated costs (9). However, complication rates seemed to decrease after the use of Jackson-Pratt (JP) drains at the wound site in regards to lymphocele development (9).
GSV transposition (GSV loop)
Historically GSV transpositions to the superficial femoral artery have not been frequently used and the data published include small patient samples (20-22). Common surgical technique for this AVF includes harvesting an ipsilateral GSV with a diameter of 3 mm or greater from the saphenofemoral junction down to the knee (with preservation of the vein at the saphenofemoral junction). After ligating all tributaries, the distal portion of the vein is ligated and divided just proximal to the knee and is tunneled superficially in a gentle curve laterally, towards the groin. The distal aspect of the vein is then anastomosed in an end-to-side fashion to either the common femoral artery or superficial femoral artery. Several publications report high complication rates and morbidity associated with saphenous vein harvest (20,22,23). Complications include possible hematoma development, ipsilateral lower extremity edema, increased risk of bleeding and pseudoaneurysm formation (22). The largest series describing GSV looped AV fistulas was a study that included thirty-one patients (23). Their single center study found the primary patency rate to be 75% at 1 year, but this dropped drastically to 45% at 2 years. These finding were similar to other subsequent studies done in the early 2000s (21).
Our groups’ use of the greater saphenous vein has been limited by our outstanding clinical outcomes with FV combined with our anecdotal observation that the GSV is unlikely to mature/dilate. For this reason, a significant number of patients will have cannulation issues given the expected 3–5 mm size of the average native GSV.
Femoral artery to FV synthetic AVG (upper thigh loop AVGs)
For patients in whom large thigh circumference, short thigh length, or venous sclerosis is present, thigh AVG may represent the next best option. Several synthetic materials that may be used for femoral artery to FV AVGs. Polytetrafluoroethylene (PTFE) and expanded PTFE (ePTFE) have successfully been used to establish adequate HD access since the early 1980s (Figure 1). A previous 14-year retrospective study found that PTFE AVGs had a median graft survival of 21 months and a 73 percent 1 year patency (24). This is similar across the literature regarding cumulative patency rates of upper thigh AVGs (25,26) with 1-year primary patency rates ranging from 53.9–71%, respectively, and 1- and 2-year secondary patency rates ranging from 62–90% and 54–90%, respectively (25,27-30).
Multi-layered PTFE grafts are an additional option that can be advantageous because of their ability to be cannulated within 24–48 hours of surgery (31). Theoretical concerns about using synthetic material in the thigh for access are largely related to perceived higher rates of infection compared to conventional arm access or autologous FV transposition, which may lead to significant morbidity (32). Based on the results of a systematic review of 15 studies (33), the infection rate in the thigh AVG (upper and mid) group was 18% compared to only 1.6% in the autologous FV transposition one. Alternative conduits such as FV homograft and bio synthetics have also been utilized as conduits in this position when autogenous vein is unavailable. Our institutional experience is limited in this regard and published series are small.
We undertake several steps to mitigate the infection potential: (I) a preoperative regimen of skin cleansing the night before surgery to reduce groin skin bioburden and (II) superficial femoral artery exposure performed 3–5 cm below the cutaneous groin crease. Importantly, the venous anastomosis is performed to the superficial FV. The prosthetic graft to FV anastomosis invariably develops intimal hyperplastic associated stenosis which is usually able to be treated. With a patent common FV and profunda femoris vein, FV stenosis is generally well tolerated and the patient remains asymptomatic without leg swelling.
It is axiomatic that optimization of aorto iliac and iliofemoral artery inflow is essential to optimize patency for VA. For those patients in whom infrainguinal occlusive disease is present, consideration should be given to open or endovascular options for prospective revascularization of the limb. Infrapopliteal occlusive disease represents the most concerning disease pattern and may be associated with prohibitive amputation risk.
The presence of a major amputation is not a contraindication to creation of leg access ipsilateral to the amputation. For those patients who underwent amputation for diabetic foot infection, the SFA is frequently patent and may be used as inflow. For those patients in whom multilevel arterial occlusive disease was the etiology of limb loss, SFA endarterectomy allows creation of the inflow for new thigh VA in the heretofore described proximal thigh (outside the groin region). As long as the profunda femoris artery is patent, the amputation is at limited risk for ischemic injury.
Conclusions
LLVA is a valuable access that may be considered for patients in whom standard upper extremity options have been exhausted. With careful patient selection, severe complications can be avoided and morbidity mitigated. For patients in whom successful leg access is created, the procedure significantly improves their quality of life and life expectancy compared to the alternative a tunneled dialysis catheter as their permanent and definitive access site. The decision of utilizing this approach should be based on the physician best clinical judgment and the alignment with End Stage Kidney Disease life-plan.
Acknowledgments
Funding: This research was funded by Robert Buckley Endowed Chair research fellowship funding.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population”. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-549/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (https://cdt.amegroups.com/article/view/10.21037/cdt-22-549/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. LK served as the unpaid Guest Editor of the special series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johansen KL, Chertow GM, Gilbertson DT, et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2022;79:A8-12. [Crossref] [PubMed]
- Siddiqui MA, Ashraff S, Carline T. Maturation of arteriovenous fistula: Analysis of key factors. Kidney Res Clin Pract 2017;36:318-28. [Crossref] [PubMed]
- Moist LM, Lok CE, Vachharajani TJ, et al. Optimal hemodialysis vascular access in the elderly patient. Semin Dial 2012;25:640-8. [Crossref] [PubMed]
- Vassalotti JA, Jennings WC, Beathard GA, et al. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial 2012;25:303-10. [Crossref] [PubMed]
- Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 2013;8:810-8. [Crossref] [PubMed]
- Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA Surg 2015;150:441-8. [Crossref] [PubMed]
- Ocak G, Halbesma N, le Cessie S, et al. Haemodialysis catheters increase mortality as compared to arteriovenous accesses especially in elderly patients. Nephrol Dial Transplant 2011;26:2611-7. [Crossref] [PubMed]
- Hajibandeh S, Burton H, Gleed P, et al. Impact of arteriovenous fistulas versus arteriovenous grafts on vascular access performance in haemodialysis patients: A systematic review and meta-analysis. Vascular 2022;30:1021-33. [Crossref] [PubMed]
- Rueda CA, Nehler MR, Kimball TA, et al. Arteriovenous fistula construction using femoral vein in the thigh and upper extremity: single-center experience. Ann Vasc Surg 2008;22:806-14. [Crossref] [PubMed]
- Parekh VB, Niyyar VD, Vachharajani TJ. Lower Extremity Permanent Dialysis Vascular Access. Clin J Am Soc Nephrol 2016;11:1693-702. [Crossref] [PubMed]
- Smith ST, Clagett GP. Femoral vein harvest for vascular reconstructions: pitfalls and tips for success. Semin Vasc Surg 2008;21:35-40. [Crossref] [PubMed]
- Orion KC, Kim TI, Rizzo AN 2nd, et al. Long-term outcomes of transposed femoral vein arteriovenous fistula for abandoned upper extremity dialysis access. J Vasc Surg 2021;74:225-9. [Crossref] [PubMed]
- Bourquelot P, Rawa M, Van Laere O, et al. Long-term results of femoral vein transposition for autogenous arteriovenous hemodialysis access. J Vasc Surg 2012;56:440-5. [Crossref] [PubMed]
- Gradman WS, Cohen W, Haji-Aghaii M. Arteriovenous fistula construction in the thigh with transposed superficial femoral vein: our initial experience. J Vasc Surg 2001;33:968-75. [Crossref] [PubMed]
- Gradman WS, Laub J, Cohen W. Femoral vein transposition for arteriovenous hemodialysis access: improved patient selection and intraoperative measures reduce postoperative ischemia. J Vasc Surg 2005;41:279-84. [Crossref] [PubMed]
- Pike SL, Farber A, Arinze N, et al. Patients with lower extremity dialysis access have poor primary patency and survival. J Vasc Surg 2019;70:1913-8. [Crossref] [PubMed]
- Farber A, Cheng TW, Nimmich A, et al. Femoral vein transposition is a durable hemodialysis access for patients who have exhausted upper extremity options. J Vasc Surg 2020;71:929-36. [Crossref] [PubMed]
- Hazinedaroğlu SM, Tüzüner A, Ayli D, et al. Femoral vein transposition versus femoral loop grafts for hemodialysis: a prospective evaluation. Transplant Proc 2004;36:65-7. [Crossref] [PubMed]
- Scollay JM, Skipworth RJ, Severn A, et al. Vascular access using the superficial femoral vein. J Vasc Access 2010;11:312-5.
- Lynggaard F, Nordling J, Iversen Hansen R. Clinical experience with the saphena loop arteriovenous fistula on the thigh. Int Urol Nephrol 1981;13:287-90. [Crossref] [PubMed]
- Pierre-Paul D, Williams S, Lee T, et al. Saphenous vein loop to femoral artery arteriovenous fistula: a practical alternative. Ann Vasc Surg 2004;18:223-7. [Crossref] [PubMed]
- Illig KA, Orloff M, Lyden SP, et al. Transposed saphenous vein arteriovenous fistula revisited: new technology for an old idea. Cardiovasc Surg 2002;10:212-5. [Crossref] [PubMed]
- Kinnaert P, Vereerstraeten P, Toussaint C, et al. Saphenous vein loop fistula in the thigh for maintenance hemodialysis. World J Surg 1979;3:95-8, 132-3. [Crossref] [PubMed]
- Korzets A, Ori Y, Baytner S, et al. The femoral artery-femoral vein polytetrafluoroethylene graft: a 14-year retrospective study. Nephrol Dial Transplant 1998;13:1215-20. [Crossref] [PubMed]
- Vogel KM, Martino MA, O'Brien SP, et al. Complications of lower extremity arteriovenous grafts in patients with end-stage renal disease. South Med J 2000;93:593-5. [Crossref] [PubMed]
- Cull JD, Cull DL, Taylor SM, et al. Prosthetic thigh arteriovenous access: outcome with SVS/AAVS reporting standards. J Vasc Surg 2004;39:381-6. [Crossref] [PubMed]
- Tashjian DB, Lipkowitz GS, Madden RL, et al. Safety and efficacy of femoral-based hemodialysis access grafts. J Vasc Surg 2002;35:691-3. [Crossref] [PubMed]
- Ong S, Barker-Finkel J, Allon M. Long-term outcomes of arteriovenous thigh grafts in hemodialysis patients: a comparison with tunneled dialysis catheters. Clin J Am Soc Nephrol 2013;8:804-9. [Crossref] [PubMed]
- Geenen IL, Nyilas L, Stephen MS, et al. Prosthetic lower extremity hemodialysis access grafts have satisfactory patency despite a high incidence of infection. J Vasc Surg 2010;52:1546-50. [Crossref] [PubMed]
- Han S, Song D, Yun S. Long Term Outcomes of Arteriovenous Grafts for Hemodialysis in Lower Extremities. Vasc Specialist Int 2016;32:180-5. [Crossref] [PubMed]
- Glickman MH, Stokes GK, Ross JR, et al. Multicenter evaluation of a polyurethaneurea vascular access graft as compared with the expanded polytetrafluoroethylene vascular access graft in hemodialysis applications. J Vasc Surg 2001;34:465-72; discussion 472-3. [Crossref] [PubMed]
- Rosenberg N, Henderson J, Lord GH, et al. An arterial prosthesis of heterologous vascular origin. JAMA 1964;187:741-3. [Crossref] [PubMed]
- Antoniou GA, Lazarides MK, Georgiadis GS, et al. Lower-extremity arteriovenous access for haemodialysis: a systematic review. Eur J Vasc Endovasc Surg 2009;38:365-72. [Crossref] [PubMed]


