
Management of concomitant central venous disease
Introduction
Background
The presence of central venous disease (CVD) has great clinical importance for chronic kidney disease and end-stage renal disease (ESRD) population. CVD may limit the options for the creation of new vascular access (VA) and for those patients in whom VA is undertaken, may compromise the quality of the hemodialysis (HD) experience by causing symptomatic arm swelling and interrupting the ability to consistently received the prescribed HD. CVD, defined as more than 50% stenosis in the internal jugular, axillary, subclavian, or innominate veins (1). The most common mechanism is turbulent flow and mechanical trauma caused by current and prior central venous catheters resulting in intimal hyperplasia. The high blood flow volume from a concurrent arteriovenous fistula/graft (AVF/AVG) VA accelerates this process (2). Fifty percent of patients with CVD who did not undergo HD were asymptomatic (3), however, the clinical symptoms often unmasked with the initial creation of VA or during subsequent HD. The high venous pressures associated with a functioning VA may exceed the capacity of the collaterals resulting in venous hypertension. Clinically, symptomatic CVD manifests as ipsilateral upper extremity (UE) swelling, pain, decreased access flow, and inadequate dialysis (3-5). Rarely, CVD does lead to VA thromboses absent a lesion within the body or immediate outflow tract of the VA.
CVD is more prevalent among dialysis patients with rates reaching up to 50% (6,7) since the majority of end-stage renal disease patients initiate HD using a catheter (8). After 20 years from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI), these statistics have improved only slightly, leading to the latest change from “fistula first” to “fistula first, catheter last” (9).
Rationale and knowledge gap
With progression of CVD, the options for conventional UE VA may prematurely become exhausted, leading to catheter-dependence or the need for lower extremity vascular access (LEVA). LEVA has favorable primary and secondary patency rates (10,11), however it is associated with high infection rates of 18–41% (12,13). Additionally, contraindications for LEVA may exist for the patient with diabetes, peripheral artery disease or morbid obesity. In particular, the diabetic patient may be vulnerable to the development of neuroischemic foot wounds due to their attendant micro- and macrovascular disease, especially if VA ischemia develops. Sixteen percent of patients with LEVA reported lower extremity (LE) ischemia, often leading to major amputation (10-12,14). Conversely, a long-term tunneled dialysis catheter as the definitive was also found to be associated with higher mortality rates (15) compared to AVF/AVG.
Objective
Endovascular interventions including percutaneous transluminal angioplasty (PTA) and percutaneous transluminal stenting (PTS) are the mainstay therapies for CVD. We provide an overview of current endovascular interventions, utilization of hemodialysis reliable outflow (HeRO) grafts, and hybrid surgical approaches in the treatment of CVD.
Discussion
Endovascular interventions
According to the latest 2019 KDOQI VA guidelines (16), HD patients with symptomatic CVD are using primary PTA with PTS reserved for lesions that demonstrate intraprocedural recoil following PTA, i.e., provisional stenting (17,18). Rates of immediate lesion recoil are high due to the morphology of CVD including factors like long length and fibro intimal lesion morphology. These findings raise the question of whether primary stenting is the preferred approach in era of covered stent (CS) grafts. The immediate technical success of PTA is high ranging from 70% to 90%, however, recurrence is high, and multiple repeat interventions are generally required to maintain vessel patency (19). A few retrospective studies reported data on patency rates. Gür et al. (20) reported a primary patency rate of 42.4% with primary-assisted patency of 68.4% at 12 months. Another study reported higher a 12-month primary patency rate of 57% (21). A recent meta-analysis (22) that included eight comparative studies has compared these two modalities. This study showed that PTA was associated with better primary assisted patency rates compared to the PTS group at the 24-month follow-up only while primary patency rates were insignificant at 3, 6, 12 and 24 months. However, this simple comparison between the both techniques without accounting for previous treatments may be biased, since all included studies compared PTA as first-line therapy with PTS that was used either provisionally (i.e., following unsatisfactory PTA) or following re-stenosis/recurrence in a different setting.
Currently, two endovascular stent platforms are commercially available: bare metal stents (BMS) and CS (Table 1). These groups can be further classified as self-expanding and balloon expandable. The relative clinical benefits and the choice between these options may be challenging and depends on clinical factors such as target vein diameters and lengths, vessel tortuosity, proximity to the thoracic inlet and the need to preserve the internal juglar vein or prevent jailing of the contralateral innominate vessel. While CS appears to have better results according to the current literature, limited supporting data are available without firm recommendations from KDOQI (16,23). Table 2 lists a few studies that reported data on the safety and effectiveness of CS. Furthermore, a study by Chen et al. (27) reported that the use of CS (Viabahn®, W. L. Gore & Associates Inc., Flagstaff, AZ, USA) was an independent predictor for better primary patency rates. The CS favorable outcomes can be attributed to the inherent mechanism of prohibiting in-stent intimal hyperplasia formation via the covered design, thus, reducing the need for additional patency maintaining interventions vis-à-vis the BMS. While intimal hyperplasia may grow within the interstices of the BMS, a CS prevents this pattern. However, intimal hyperplasia may still occur only from the peripheral stent edges- resulting in a “candy wrapper” pattern.
Table 1
| Balloon expandable |
| iCAST (Atrium Medical Corporation, Hudson, NH, USA) |
| Gore Viabahn VBX (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) |
| Self-expanding |
| Gore Viabahn (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) |
| Covera Vascular (BD Interventional, Franklin Lakes, NJ, USA) |
| Fluency Plus Endovascular (BD Interventional, Franklin Lakes, NJ, USA) |
Table 2
| Study | Number of patients | Technical success | Primary patency | Primary assisted patency | Secondary patency | Stent used |
|---|---|---|---|---|---|---|
| Jones et al. 2011 (24) | 30 | 100% | 97%, 81%, 67%, and 45% at 3, 6, 12, and 24 months | 100%, 100%, 80%, and 75% at 3, 6, 12, and 24 months | – | Viabahna |
| Anaya-Ayala et al. 2011 (25) | 25 | 100% | 29% at 12 months | 85% at 12 months | 94% at 12 months | Viabahna [24]; Fluencyb [1] |
| Boutrous et al. 2019 (26) | 29 | 100% | 92.9%, 91.7% and 80% at 6, 12, and 24 months | – | 96.4%, 95.8%, and 93.3% at 6, 12, and 24 months | Viabahna |
a, W. L. Gore & Associates Inc., CA, USA; b, Bard Peripheral Vascular, Tempe, AZ, USA.
The type of CS chosen depends on factors such as the length and location of the lesion, the nominal vessel diameter, and the vessel tortuosity. Boutrous et al. (26) showed that a longer stent is associated with decreased patency rates (62.5 vs. 50 centimeters; P=0.002). In another study, larger vessel diameters (i.e., >12 mm) had higher primary patency rates as stents with larger diameters were used (27). Based on our previous experiences, for isolated innominate lesions, we would use either Bard Fluency Plus (BD Interventional, Franklin Lakes, NJ, USA) or Atrium iCAST (Hudson, NH, USA), both of which provide a benefit of accuracy for device deployment. For subclavian and axillary lesions to accommodate the dynamic forces that exist in the thoracic inlet region of the venous circulation, we favor the BD Interventional Covera (Franklin Lakes, NJ, USA), Gore Viabahn (Flagstaff, AZ, USA) or Atrium iCAST (Hudson, NH, USA).
In the thoracic inlet, extrinsic compression is an underappreciated cause of central stenosis and we employ thoracic outlet syndrome (TOS) decompression surgery when the pathology is confirmed to be contributory. In our practice, we perform a surgical decompression using a transaxillary or supraclavicular approach for all confirmed cases. The patients first receive a venogram followed by intravascular ultrasound then undergo an angioplasty to relieve their symptoms (i.e., swelling), subsequently on a different setting, the decompression is performed. Lastly, the patients undergo a non-provisional stent deployment in another different setting. A study by Lim et al. (28) included 18 TOS patients who had undergone a decompression procedure for their subclavian vein stenosis for their existing ipsilateral UE HD access. The results were satisfactory procedures with 1-year primary, primary-assisted and secondary patency rates of 42%, 69%, and 93%, respectively, with zero 30-day mortality rate. Though the main goal of the procedure was to maintain HD access, the absence of a control group did not help in addressing the indications for such procedure to maintain an existing HD access.
Use of the HeRO graft
The HeRO graft (Merit Medical Systems, South Jordan, UT, USA) was approved by Food and Drug Administration in 2008 (29). This composite system consisting of a prosthetic graft combined with an intraluminal catheter can be used to endoluminally traverse the central venous stenosis/occlusion, thus, offering new VA for HD patients who would otherwise not be a candidate for UE traditional surgical VA options (16) (Figure 1). Promising results were published by a multi-center study (30) that included 164 patients who were treated with HeRO graft. The primary and secondary patency rates were (6 months: 60%; 12 months: 48.8%; 24 months: 42.9%) and (6 months: 90.8%; 12 months: 90.8%; 24 months: 86.7%), respectively. Over two-thirds of the patients required an additional intervention, and only 4.3% had access-related infections. Less favorable outcomes were reported by another study (31) that included 25 patients where primary patency rates were 47%, 37%, and 26%, and secondary patency rates were 80%, 70%, and 64% at 3, 6, and 12 months, respectively.
Nassar et al. (32) compared the patency rates of HeRO graft with conventional AVG for 143 patients who were randomized into two groups. The patency rates were comparable between the two groups, (primary patency: HeRO, 34.8%; AVG, 30.6%) and (secondary patency: HeRO, 67.6%; AVG, 58.4%). Another study by Proksch et al. (33) of 75 HD patients compared PTS (n=44) with HeRO graft (n=31). The 1- and 2-year access circuit primary access was similar between the two groups. Also, no significant differences were found in all-cause 1- and 2-year mortality rates between the two groups, (3.7% vs. 4.8%) and (11.8% vs. 23.5%), respectively, P>0.5 in both.
A disadvantage of the HeRO graft is that it takes a few weeks to mature before it can be used. During this time, the patient is dependent on the central venous catheter (CVC), increasing their VA complications risk. Therefore, a group from Augusta University (29) decided to modify the HeRO graft with the use of an early-cannulation ACUSEAL vascular graft (W. L. Gore & Associates, Newark, Del) theoretically, obviating the need for a CVC The group published their 6-month outcomes with primary and secondary rates of 70% and 90%, respectively.
HeRO graft complications have been reported in a number of studies, where one of these complications is steal syndrome. Despite a few studies reporting it in lower rates (1.4–2.6%) (30,34,35), a study by Wallace et al. (36) found that 22% of the included patients suffered from this complication. The authors attributed it mainly to the patient underlying comorbidities such as diabetes, smoking, and peripheral artery disease as well as the large diameter of arterial inflow component (6 mm polytetrafluoroethylene graft). Additionally, two separate case reports described complications of graft migration into the right ventricle of the heart (37) and graft separation during a percutaneous thrombectomy procedure (38). Fortunately, both patients were managed successfully.
Hybrid surgical approach
Although often overlooked, the anterior chest wall AVG can be a viable option before considering LEVA, given its similar patency to UE grafts. Patients with known CVD are usually not suitable for chest wall AVGs. However, in our previous study (39), we successfully performed a hybrid procedure through creating a chest wall AVG with concomitant central venous stenting in a patient central venous stenosis, without infraclavicular venous options.
In this hybrid case, the patient was suffering from a severe bilateral CVD (bilateral axillo/subclavian and innominate vein stenosis/occlusion) with multiple failed UE AVGs. Additionally, the femoral VA was not an option given the patient’s severe peripheral artery disease, bilateral below-knee amputations, morbid obesity, and severe obstructive pulmonary disease. As a hybrid, same setting procedure a chest wall loop AVG was constructed with stenting of the patient’s subclavian vein using a 10 mm × 40 mm self-expanding CS (Fluency Plus; Bard Peripheral Vascular, Tempe, AZ, USA) (Figure 2). The patient tolerated the procedure well and along with a maintenance procedure, the graft was found to be patent and functional at 1-year follow-up (39). Despite the lack of studies investigating long-term patency rates of these hybrid chest wall AVG, we think that this case represents a new treatment option for patients with a concomitant CVD and could reduce the catheter dependence and the need for femoral access.
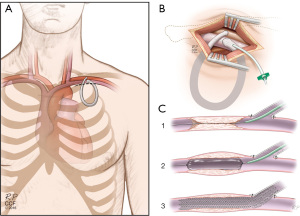
Additionally, this hybrid approach to permanent VA does raise the question of whether some currently available treatment options for CVD—like the aforementioned HeRO graft (Merit Medical Systems, South Jordan, UT, USA)—may be replaced by this technique. No comparative studies are available to answer this question. In addition, broad surgical application, acceptance, and experience with open chest wall AVGs would be necessary for such a claim to be valid.
Another case report by Ptohis et al. (40) described a hybrid approach where PTA was performed to the left brachiocephalic trunk followed by the insertion of a standard CVC as VA. The procedure went uneventful and the patient was doing well at the 6-month follow-up. Nevertheless, the reliance on CVC is problematic given its previously mentioned short- and long-term complications. Furthermore, it does not align with the ambitious Fistula first breakthrough initiative goal of reducing tunneled CVC use to less than 10% for HD patients (41).
Surgical approach to CV occlusion
The surgical approach can be considered in symptomatic patients either in whom endovascular management has failed or as a primary intervention. Different surgical techniques were described in the literature. Babadjanov et al. (42) reported a successful axillary to innominate venous bypass via a median sternotomy incision. Zubair et al. (43) created a chest wall graft using a bovine carotid artery conduit from left axillary artery to the right atrium via a small thoracotomy incision. Axillary-femoral bypass using a long prosthetic graft is another surgical bypass option. On the other hand, a central vein patch can be another option, Gradman et al. (44) repaired occluded five subclavian veins with an autogenous and polytetrafluoroethylene (PTFE) patches, avoiding potential high infection and thrombosis rates of surgical bypasses. Despite no large studies were found in literature evaluating the outcomes of these open techniques, the favorable results as reported in aforementioned studies signify the reliability of open repair as an alternative to traditional endovascular interventions.
Conclusions
Central Venous Occlusive disease is common amongst the ESRD patient population and is associated with significantly worse immediate and long term clinical outcomes for the dialyzed patient. This disease pattern reduces the available options for current and future VA creation and impairs the reliability of existing VA. Efforts to reduce the prevalence of CVD should be directed at catheter avoidance strategies. From the most reductive perspective, the frequency of this occurrence represents an abject failure of our medical system to identify and manage patients prior to the need for renal replacement therapy.
The current first-line treatment option for CVD relies on endovascular options such as PTA with or without PTS. Emerging data suggests that CS platforms demonstrate mid and late term patency benefits by mitigating the pattern of in-stent restenosis. Higher quality evidence is necessary to definitively answer this comparative technology question. That said, in our opinion, the current 2019 KDOQI guidelines do not accurately reflect the current strength of evidence that appears to support the preferential treatment of CSs for the lesion morphology associated with CVD.
Other treatment options that can be considered include the HeRO graft as well as hybrid surgical and open surgical approaches, however, further long-term investigations are needed to elucidate and stratify the comparative outcomes for these therapeutic modalities.
The appropriate therapy should be selected based upon a patient centered interdisciplinary discussion utilizing the locally available expertise in the area of VA creation and maintenance.
Acknowledgments
Funding: This research was funded by Robert Buckley Endowed Chair research fellowship funding.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population”. The article has undergone external peer review
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-570/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-570/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. LK served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Modabber M, Kundu S. Central venous disease in hemodialysis patients: an update. Cardiovasc Intervent Radiol 2013;36:898-903. [Crossref] [PubMed]
- Trerotola SO, Kuhn-Fulton J, Johnson MS, et al. Tunneled infusion catheters: increased incidence of symptomatic venous thrombosis after subclavian versus internal jugular venous access. Radiology 2000;217:89-93. [Crossref] [PubMed]
- Schwab SJ, Quarles LD, Middleton JP, et al. Hemodialysis-associated subclavian vein stenosis. Kidney Int 1988;33:1156-9. [Crossref] [PubMed]
- Nakhoul F, Hashmonai M, Angel A, et al. Extreme swelling of a limb with A-V shunt for hemodialysis resulting from subclavian vein thrombosis due to previous catheterization. Clin Nephrol 1998;49:134-6. [PubMed]
- Tatapudi VS, Spinowitz N, Goldfarb DS. Symptomatic central venous stenosis in a hemodialysis patient leading to loss of arteriovenous access: a case report and literature review. Nephron Extra 2014;4:50-4. [Crossref] [PubMed]
- Agarwal AK. Central vein stenosis. Am J Kidney Dis 2013;61:1001-15. [Crossref] [PubMed]
- Osman OO, El-Magzoub AR, Elamin S. Prevalence and Risk Factors of Central Venous Stenosis among Prevalent Hemodialysis Patients, a Single Center Experience. Arab J Nephrol Transplant 2014;7:45-7. [PubMed]
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2019;73:A7-8. [Crossref] [PubMed]
- Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2016;67:Svii-S305.
- Parekh VB, Niyyar VD, Vachharajani TJ. Lower Extremity Permanent Dialysis Vascular Access. Clin J Am Soc Nephrol 2016;11:1693-702. [Crossref] [PubMed]
- Tashjian DB, Lipkowitz GS, Madden RL, et al. Safety and efficacy of femoral-based hemodialysis access grafts. J Vasc Surg 2002;35:691-3. [Crossref] [PubMed]
- Taylor SM, Weatherford DA, Langan EM 3rd, et al. Outcomes in the management of vascular prosthetic graft infections confined to the groin: a reappraisal. Ann Vasc Surg 1996;10:117-22. [Crossref] [PubMed]
- Santoro D, Benedetto F, Mondello P, et al. Vascular access for hemodialysis: current perspectives. Int J Nephrol Renovasc Dis 2014;7:281-94. [Crossref] [PubMed]
- Geenen IL, Nyilas L, Stephen MS, et al. Prosthetic lower extremity hemodialysis access grafts have satisfactory patency despite a high incidence of infection. J Vasc Surg 2010;52:1546-50. [Crossref] [PubMed]
- Wasse H. Catheter-related mortality among ESRD patients. Semin Dial 2008;21:547-9. [Crossref] [PubMed]
- Lok CE, Huber TS, Lee T, et al. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]
- Bakken AM, Protack CD, Saad WE, et al. Long-term outcomes of primary angioplasty and primary stenting of central venous stenosis in hemodialysis patients. J Vasc Surg 2007;45:776-83. [Crossref] [PubMed]
- The American College of Radiology. ACR-SIR practice parameter for endovascular management of the thrombosed or dysfunctional dialysis access. Published 2022. Accessed October 20, 2022. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Dysfunc-DialysisMgmt.pdf
- Agarwal AK, Patel BM, Haddad NJ. Central vein stenosis: a nephrologist's perspective. Semin Dial 2007;20:53-62. [Crossref] [PubMed]
- Gür S, Oğuzkurt L, Gedikoğlu M. Central venous occlusion in hemodialysis access: Comparison between percutaneous transluminal angioplasty alone and nitinol or stainless-steel stent placement. Diagn Interv Imaging 2019;100:485-92. [Crossref] [PubMed]
- Banshodani M, Kawanishi H, Shintaku S, et al. Percutaneous transluminal angioplasty for central venous disease in dialysis patients: influence on cardiac function. J Vasc Access 2014;15:492-7.
- Wu TY, Wu CK, Chen YY, et al. Comparison of Percutaneous Transluminal Angioplasty with Stenting for Treatment of Central Venous Stenosis or Occlusion in Hemodialysis Patients: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 2020;43:525-40. [Crossref] [PubMed]
- Kundu S. Central venous obstruction management. Semin Intervent Radiol 2009;26:115-21. [Crossref] [PubMed]
- Jones RG, Willis AP, Jones C, McCafferty IJ, Riley PL. Long-term results of stent-graft placement to treat central venous stenosis and occlusion in hemodialysis patients with arteriovenous fistulas. J Vasc Interv Radiol 2011;22:1240-5. [Crossref] [PubMed]
- Anaya-Ayala JE, Smolock CJ, Colvard BD, et al. Efficacy of covered stent placement for central venous occlusive disease in hemodialysis patients. J Vasc Surg 2011;54:754-9. [Crossref] [PubMed]
- Boutrous ML, Alvarez AC, Okoye OT, et al. Stent-Graft Length Is Associated with Decreased Patency in Treatment of Central Venous Stenosis in Hemodialysis Patients. Ann Vasc Surg 2019;59:225-30. [Crossref] [PubMed]
- Chen B, Lin R, Dai H, et al. One-year outcomes and predictive factors for primary patency after stent placement for treatment of central venous occlusive disease in hemodialysis patients. Ther Adv Chronic Dis 2022;13:20406223211063039. [Crossref] [PubMed]
- Lim S, Alarhayem AQ, Rowse JW, et al. Thoracic outlet decompression for subclavian venous stenosis after ipsilateral hemodialysis access creation. J Vasc Surg Venous Lymphat Disord 2021;9:1473-8. [Crossref] [PubMed]
- Perry JW, Hardy D, Agarwal S, et al. Safety and efficacy of a modified HeRO dialysis device in achieving early graft cannulation: A single-institution experience. J Vasc Surg Cases Innov Tech 2017;3:175-9. [Crossref] [PubMed]
- Gage SM, Katzman HE, Ross JR, et al. Multi-center experience of 164 consecutive Hemodialysis Reliable Outflow [HeRO] graft implants for hemodialysis treatment. Eur J Vasc Endovasc Surg 2012;44:93-9. [Crossref] [PubMed]
- Gebhard TA, Bryant JA, Adam Grezaffi J, et al. Percutaneous interventions on the hemodialysis reliable outflow vascular access device. J Vasc Interv Radiol 2013;24:543-9. [Crossref] [PubMed]
- Nassar GM, Glickman MH, McLafferty RB, et al. A comparison between the HeRO graft and conventional arteriovenous grafts in hemodialysis patients. Semin Dial 2014;27:310-8. [Crossref] [PubMed]
- Proksch DM, Rodriguez LE, Rathore A, et al. A comparison of stenting versus hemodialysis reliable outflow graft for hemodialysis patients with recurrent central venous obstructions. J Vasc Surg Venous Lymphat Disord 2021;9:1136-44. [Crossref] [PubMed]
- Katzman HE, McLafferty RB, Ross JR, et al. Initial experience and outcome of a new hemodialysis access device for catheter-dependent patients. J Vasc Surg 2009;50:600-7, 607.e1.
- Fusselman M. Results of a customer-based, post-market surveillance survey of the HeRO access device. Nephrol News Issues 2010;24:30-3. [PubMed]
- Wallace JR, Chaer RA, Dillavou ED. Report on the Hemodialysis Reliable Outflow (HeRO) experience in dialysis patients with central venous occlusions. J Vasc Surg 2013;58:742-7. [Crossref] [PubMed]
- Anis M, Harris D, Elwing J. Not Quite a Hero (Hemodialysis Reliable Outflow Graft): A Rare Cause of Dyspnea and Positional Syncope in End Stage Renal Disease (ESRD). Chest 2016;150:908A. [Crossref]
- Patel N, Hussain J, Gemmete JJ, et al. Percutaneous retrieval of a fractured HeRO graft venous outflow component with endobronchial forceps. J Vasc Access 2019;20:339-41.
- Rowse JW, Kirksey L. A Hybrid Chest Wall Arteriovenous Graft in Central Venous Stenosis. J Assoc Vasc Access 2017;22:199-204.
- Ptohis N, Theodoridis PG, Raftopoulos I. Hybrid angioplasty-catheter placement procedure performed in a hemodialysis patient with central venous obstruction disease. Presentation of a case. J Vasc Access 2022;23:162-5.
- Lynch JR, Wasse H, Armistead NC, et al. Achieving the goal of the Fistula First breakthrough initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis 2011;57:78-89. [Crossref] [PubMed]
- Babadjanov J, Bernstein R, Kirksey L. Surgical reconstruction of central venous obstruction in salvaging upper extremity dialysis accesses. J Vasc Access 2017;18:e39-41.
- Zubair MM, Bennett ME, Peden EK. Left axillary to right atrium anterior chest wall graft using bovine carotid artery conduit. J Vasc Access 2018;19:187-90.
- Gradman WS, Bressman P, Sernaque JD. Subclavian vein repair in patients with an ipsilateral arteriovenous fistula. Ann Vasc Surg 1994;8:549-56. [Crossref] [PubMed]



