
Carotid massive intraplaque hemorrhage, lipid-rich necrotic core, and heavy circumferential calcification were associated with new ipsilateral ischemic cerebral lesions after carotid artery stenting: high-resolution magnetic resonance vessel wall imaging study
Highlight box
Key findings
• Our study found that the massive intraplaque hemorrhage, lipid-rich necrotic core, and heavy circumferential calcium were linked with new ipsilateral ischemic lesions (NIILs) in the brain following carotid artery stenting (CAS).
What is known and what is new?
• CAS is one of the main modalities for the treatment of carotid stenosis. The increased frequency of new infarcted injuries during the perioperative period is the primary issue for CAS. By using high-resolution magnetic resonance vessel wall imaging (HR-VWI), our work further validated the relationship between plaque composition and the emergence of NIILs in the brain after CAS.
What is the implication, and what should change now?
• Preoperative quantitative measurement for carotid plaques using HR-VWI may be useful for predicting NIILs after CAS. We need more prospective research to confirm our results.
Introduction
One of the primary reasons for death and disability globally is ischemic stroke (1). Carotid atherosclerosis is one of the key contributing factors to an ischemic stroke. For individuals with carotid stenosis who are at high surgical risk, carotid artery stenting (CAS) is a well-established therapy option that has comparable long-term effectiveness to carotid endarterectomy (CEA). Nevertheless, new ipsilateral ischemic lesions (NIILs) in the brain following CAS revealed by diffusion-weighted imaging-magnetic resonance imaging (DWI-MRI) are frequent, varying from 18% to 57%, despite the widespread use of embolic protection devices (EPDs) (2). Despite the fact that most individuals with NIILs on DWI are silent, they are much more likely than those without NIILs to have a symptomatic stroke (2). Moreover, ischemic brain lesions have already been connected to a higher chance of death, Alzheimer, and cognitive decline (3). Plaque components may affect the likelihood of developing new ischemic lesions following CAS (4). High-resolution magnetic resonance vessel wall imaging (HR-VWI) has been widely employed to assess the carotid plaque burden and components (5). It has enabled the visualization of vulnerable plaque features like intraplaque hemorrhage (IPH), and lipid-rich necrotic core (LRNC) etc. in vivo (6). It is also an excellent method for assessing calcification, the usefulness has been demonstrated with accuracies of 98% and specificity of 99% (7). In terms of plaque morphology, the best imaging technique for seeing the fibrous cap is MRI (8). More plaque stability is associated with thick, unbroken fibrous caps. Conversely, a fragile or rupturing fibrous cap connected to ulcerations is an important determinant of ischemic stroke. Unenhanced MRI are substantially less capable of displaying ulceration. One research found that 37.5% of carotid plaques ulcers diagnosed using contrast-enhanced magnetic resonance angiography (MRA) were overlooked on unenhanced time-of-flight (TOF) MRA, demonstrating the need for contrast injection for identifying ulceration by MRI (9).
In clinical settings, it's critical to quickly spot individuals who are in an elevated danger of cerebral embolism following CAS. Consequently, we examined the correlation between plaque burden, plaque components, individual characteristics, and the NIILs on DWI following CAS utilizing HR-VWI based on quantitative software.
We aimed to investigate the imaging characteristics associated with NIILs on DWI following CAS. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-543/rc).
Methods
Study patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University approved the study protocol with a waiver of informed consent due to the retrospective nature of the study (No. 2022135X). This was a case-control study. From January 2016 to June 2022, the patients who satisfied the following inclusion criteria and received preoperative HR-VWI and brain MRI in Beijing Anzhen Hospital within 7 days prior to surgery and within 3 days postoperatively were chosen, and their clinical and MRI imaging information was collected. Inclusion criteria were the following: (I) patients had >70% asymptomatic carotid stenosis or >50% symptomatic carotid stenosis for carotid atherosclerosis; (II) patients were treated with CAS for the first time; (III) patients had never undergone prior intervention (CEA, CAS, or both) of the ipsilateral carotid artery; (IV) comprehensive clinical information and satisfactory image quality (IQ). Exclusion criteria included the following: (I) patients had <50% carotid stenosis for carotid atherosclerosis; (II) patients were diagnosed with carotid stenosis for non-atherosclerosis, such as vasculitis; (III) patients were treated with CEA; (IV) patients had a history of previous ipsilateral CEA, stent, or both; (V) insufficient IQ; (VI) patients without complete digital subtraction angiography (DSA) imaging information; (VII) patients who were diagnosed with carotid subtotal occlusion.
Patient groupings
NIILs are hyperintense lesions on postoperative brain DWI that wasn’t visible on preoperative brain DWI. Patients were split into NIILs positive group and the NIILs negative group. Based on the existence or lack of stroke symptoms following CAS, the NIILs positive group was split into two subgroups: the NIILs symptomatic group versus the NIILs asymptomatic group.
Imaging protocol
On a 3.0T MRI scanner (Ingenia CX, Philips Healthcare, Best, the Netherlands), carotid HR-VWI was done using a 32-channel skull coil and an 8-channel cervical coil (TSImaging Healthcare, Beijing, China). The index carotid artery plaque, which was identified as the artery exhibiting moderate-to-severe stenosis, was the focus of the MRI scanning. The scanning process involved three-dimensional (3D) TOF MRA, two-dimensional T1-weighted imaging (2D T1WI), two-dimensional T2-weighted imaging (2D T2WI), and 3D simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) sequences (parameters are detailed in Table 1). 3D TOF MRA was used for the evaluation of stenosis degree. 2D T1WI, 2D T2WI and 3D SNAP were used to evaluate the burden and components of carotid plaque. A gadolinium-based contrast agent was given into the antecubital vein as just a burst at 3 mL/s with a dosage of 0.1 mmol/kg of body mass, followed immediately by a 20 mL saline wash, after the collection of the pre-contrast T1WI sequence. The contrast injection was followed by a post-contrast T1WI scan. The duration of the trial was around 30 minutes. Using a standard head coil, a brain MRI was done on a 3.0T MRI scanner (Ingenia CX, Philips Healthcare, Best, the Netherlands). The presence or absence of NIILs was assessed on DWI (spin echo-echo planar imaging; repetition time/echo time =2,429/80 milliseconds; slice thickness =5 mm; spacing =1 mm; B value =1,000 s/mm2; field of view =230 mm).
Table 1
| Name | 3D TOF | 2D T1WI | 2D T2WI | 3D SNAP |
|---|---|---|---|---|
| TE, ms | 4 | 10 | 50 | 6.8 |
| TR, ms | 20 | 800 | 4,800 | 12 |
| FOV, mm2 | 160×160 | 160×160 | 160×160 | 160×160 |
| Resolution, mm2 | 0.6×0.6 | 0.6×0.6 | 0.6×0.6 | 0.6×0.6 |
| Slice thickness, mm | 2 | 2 | 2 | 2 |
| No. of slices | 40 | 20 | 20 | 40 |
HR-VWI, high-resolution magnetic resonance vessel wall imaging; 3D, three-dimensional; TOF, time of flight; 2D, two-dimensional; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; SNAP, simultaneous non-contrast angiography and intraplaque hemorrhage; TE, echo time; TR, repetition time; FOV, field of view.
Imaging analysis
With the use of software (Vessel Explorer 2, TSimaging Healthcare, Beijing, China), two skilled radiologists with more than 2 years of cerebrovascular imaging expertise independently evaluated the HR-VWI images of the carotid arteries. The reviews were based on an agreement between the two reviewers, and in the event of a dispute, a third neuroradiologist with at least 5 years of experience reviewed the images again and helped with the negotiation of a resolution. Both of whom were blinded to the patient’s clinical features. To assess interobserver reproducibility, the HR-VWI image of 40 randomly selected subjects was reevaluated by 2 reviewers (YM Sun and HY Xu) 6 months after the initial review. The IQ was graded on a scale of 1 to 4 (1= poor, 2= marginal, 3= good, and 4= excellent). Only the images having an IQ score of 2 or above were included in the study (10).
Quantitative analysis of plaque characteristics
The lumen and wall borders were manually drawn segment by segment on the HR-VWI cross-sectional slices. The lumen area (LA), wall area (WA), maximum wall thickness (Max WT), and normalized wall index (NWI = WA/WA + LA ×100%) were automatically measured and calculated. The distance between the lumen and wall boundaries was automatically used to calculate wall thickness. Based on the established criteria, the presence or lack of plaque components like calcification, LRNC, and IPH were assessed (11). The plaque components on the HR-VWI cross-sectional slices were manually drawn segment by segment. Additionally, automatically calculated plaque component volumes and maximum area percentages.
Calcification assessment
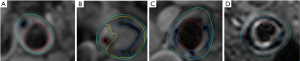
A prior study employed a 5-point grading scale to assess the extent of circumferential calcium. The following values are in order of increasing artery circumference: 0, none; 1, 90° or less; 2, 90° to 180°; 3, 180° to 270°; and 4, >270° (12). We computed the total circumferential scores of calcium and the Maximum circumference score of calcium in a single slice if the calcium was dispersed over multiple slices (Figure 1). According to the location distribution of calcium, carotid calcium was classified into four types: bottom, interior, surface, and non-calcification (12). Bottom calcium was described as calcium inside the plaques and far from the lumen, whereas surface calcium was described as calcium in touch with the lumen. Interior calcium was between the two. Similarly, a 4-point scale was used to grade the distribution of calcium in each location: 0, none; 1, bottom calcification; 2, interior calcification; 3, surface calcification. If there are multiple calcifications in a single slice, we chose the Maximum score for single calcification as the location score of this slice. We computed the total of the scores if the calcification was scattered throughout multiple slices (Figure 2).

CAS implantation
The lesion’s shape, arterial anatomy, plaque features, implant features, and operating experience were all taken into consideration by the physicians when choosing stents. The procedure was carried out in accordance with the previously disclosed protocol (13). For three days before CAS, aspirin (100 mg), clopidogrel (75 mg), or ticlopidine (250 mg) were consistently provided. The operations were carried out on anesthetic patients. Filterwire EZ (Boston Scientific, Natick, MA, USA) and SpiderFx (ev3, Plymouth, MN, USA) EPDs were used in all the patients. Based on the internal carotid artery (ICA) diameter shown on the DSA imaging, an appropriate balloon was chosen. The extent of stenosis determined whether a balloon was used for pre-dilatation or post-dilatation. In the stenotic lesion, open cell (Precise; Cordis, Hialeah, FL; or RX Acculink; Abbott, Chicago, IL, USA) or closed cell (Wallstent; Boston Scientific, Marlborough, MA, USA) stents were inserted. All patients were administered any kind of dual or triple antiplatelet agents for at least 3 months after the procedure and thereafter aspirin for the lifetime.
Statistical analysis
The median and interquartile range (IQR) were utilized to present continuous variables. The Mann-Whitney U test was utilized to contrast the differences between the NIILs positive group and the NIILs negative group, and the Kruskal-Wallis test and Dunn-Bonferroni post hoc analysis were used to contrast the variations between the three independent groups (NIILs positive subgroups and NIILs negative group). In categorical variables, percentages were used to express them. Comparisons between the NIILs positive group and NIILs negative group were made using the Chi-squared test. Univariate logistic regression analysis was used for determining the imaging characteristics connected to NIILs after CAS. The receiver operating characteristic (ROC) curves were analyzed; the area under the curve (AUC) was calculated for the imaging characteristics associated with NIILs after CAS. Two-sided P <0.05 indicated statistical significance in all analyses. The intraclass correlation coefficient (ICC) was utilized to assess the reproducibility of inter-observer results for HR-VWI. ICC values below 0.4 indicate poor reproducibility, between 0.6 and 0.75 indicate good reproducibility and over 0.75 indicate excellent reproducibility. Commercial SPSS 25.0 software was used for all statistical analyses.
Results
Patient baseline characteristics
From January 2016 to June 2022, 109 subjects were involved in the final study (Figure 3). Among 109 patients, 38 patients (34.9%) developed NIILs after CAS. Six patients (5.5%) developed symptomatic stroke with NIILs. Table 2 displays the baseline characteristics for all patients.
Table 2
| Variables | All patients (n=109) | NIILs+ (n=38) | NIILs− (n=71) | P value |
|---|---|---|---|---|
| Age (years), median [IQR] | 69 [65–75] | 71 [66–76] | 68 [62–73] | 0.06 |
| Sex (male), n (%) | 85 (78.0) | 31 (81.6) | 54 (76.1) | 0.51 |
| Hypertension, n (%) | 82 (75.2) | 31 (81.6) | 51 (71.8) | 0.26 |
| Hyperlipidemia, n (%) | 84 (77.1) | 32 (84.2) | 52 (73.2) | 0.19 |
| Diabetes, n (%) | 36 (33.0) | 15 (39.5) | 21 (29.6) | 0.30 |
| Coronary artery disease, n (%) | 32 (29.4) | 14 (36.8) | 18 (25.4) | 0.21 |
| Symptom stenosis, n (%) | 0.27 | |||
| Yes | 72 (66.1) | 25 (65.8) | 47 (66.2) | |
| No | 37 (33.9) | 13 (34.2) | 24 (33.8) | |
| Smoking, n (%) | 69 (63.3) | 25 (65.8) | 44 (62.0) | 0.69 |
| The location of the lesion, n (%) | 0.18 | |||
| Left | 45 (41.3) | 19 (50.0) | 26 (36.6) | |
| Right | 64 (58.7) | 19 (50.0) | 45 (63.4) | |
| Stent type, n (%) | 0.41 | |||
| Open cell type | 43 (39.4) | 13 (34.2) | 30 (42.3) | |
| Closed cell type | 66 (60.6) | 25 (65.8) | 41 (57.7) | |
| Stent length (cm), n (%) | 0.70 | |||
| L1 (<4.0 cm) | 41 (37.6) | 7 (18.4) | 31 (43.7) | |
| L2 (=4.0 cm) | 59 (54.1) | 26 (68.4) | 35 (49.3) | |
| L3 (>4.0 cm) | 9 (8.3) | 5 (13.2) | 5 (7.0) | |
| Pre-dilatation balloon frequency, n (%) | 0.27 | |||
| n=1 | 17 (15.6) | 8 (21.1) | 9 (12.7) | |
| n=2 | 85 (78.0) | 29 (76.3) | 56 (78.9) | |
| n=3 | 7 (6.4) | 1 (2.6) | 6 (8.5) | |
| Pre-dilatation balloon diameter, n (%) | 0.58 | |||
| D1 (<5 mm) | 14 (12.8) | 6 (15.8) | 8 (11.3) | |
| D2 (=5 mm) | 48 (44.0) | 18 (47.4) | 30 (42.3) | |
| D3 (>5 mm) | 47 (43.1) | 14 (36.8) | 33 (46.5) | |
| Post-dilatation balloon, n (%) | 57 (52.3) | 19 (50.0) | 38 (53.5) | 0.63 |
NIILs, new ipsilateral ischemic lesions; CAS, carotid artery stenting; IQR, interquartile range.
HR-VWI imaging characteristics
All the imaging characteristics of 109 individuals and comparisons between NIILs positive and negative groups are shown in Table 3. The Maximum area percentage of IPH and LRNC, the volume of IPH and LRNC, the Maximum circumference score of calcium in a single slice, and the Max WT in the NIILs positive group were larger compared to the NIILs negative group (all P<0.05). The findings of the univariate logistic analysis revealed that the Maximum area percentage of IPH and LRNC, the volume of IPH and LRNC, the Maximum circumference score of calcium in a single slice, and the Max WT were significantly associated with NIILs after CAS (Table 4). The ROC showed AUC of 0.70 [95% confidence interval (CI): 0.60–0.80] for the Max WT, 0.60 (95% CI: 0.50–0.72) for the maximum circumference score of calcium in a single slice, 0.83 (95% CI: 0.75–0.91) for the Maximum area percentage of LRNC, 0.80 (95% CI: 0.72–0.88) for the volume of LRNC, 0.92 (95% CI: 0.86–0.97) for the maximum area percentage of IPH, and 0.97 (95% CI: 0.92–1.00) for the volume of IPH (Figure 4). The results of the NIILs positive subgroups analysis revealed that there was no noticeable difference in the maximum area percentage of IPH and LRNC, the volume of IPH and LRNC, and the Max WT between the NIILs symptomatic group and NIILs asymptomatic group. However, compared with the NIILs asymptomatic group, the NIILs symptomatic group has a larger circumference of calcification (Table 5).
Table 3
| Variables | All patients (n=109) | NIILs+ (n=38) | NIILs− (n=71) | P value |
|---|---|---|---|---|
| Plaque burden | ||||
| Max WT (mm) | 6.1 (4.8–7.5) | 6.7 (5.9–8.7) | 5.7 (4.5–6.8) | 0.001 |
| NWI (%) | 82.1 (74.1–91.7) | 81.2 (74.2–90.5) | 81.7 (73.1–92.9) | 0.78 |
| Preoperative stenosis rate (%) | 69.5 (61.4–76.2) | 69.5 (66.2–77.0) | 68.2 (61.6–76.4) | 0.59 |
| Maximum area percentage of plaque components | ||||
| Calcification (%) | 11.4 (5.9–19.1) | 10.8 (5.3–18.5) | 13.0 (6.4–19.3) | 0.66 |
| LRNC (%) | 34.9 (10.0–54.7) | 54.6 (42.3–65.7) | 20.2 (5.7–36.7) | <0.001 |
| IPH (%) | 10.1 (3.6–27.2) | 30.1 (22.8–37.9) | 4.1 (2.9–10.4) | <0.001 |
| The volume of plaque components | ||||
| Calcification (mm3) | 47.0 (16.6–89.5) | 39.9 (13.0–77.9) | 51.4 (17.3–97.3) | 0.93 |
| LRNC (mm3) | 142.6 (25.9–355.7) | 318.0 (212.3–373.3) | 99.6 (44.1–208.2) | <0.001 |
| IPH (mm3) | 85.4 (12.9–142.6) | 251.7 (108.5–315.5) | 68.5 (33.7–105.4) | <0.001 |
| Scores of calcification | ||||
| Total circumferential score | 7.0 (3.0–11.0) | 7.0 (3.0–11.5) | 7.0 (3.0–11.0) | 0.71 |
| Max circumference score in a single slice | 2.0 (1.0–2.0) | 2.0 (1.0–2.3) | 1.0 (1.0–2.0) | 0.03 |
| Total location scores | 7.0 (4.0–11.0) | 8.0 (4.0–11.0) | 6.0 (3.0–11.0) | 0.40 |
Data are presented as the median (IQR). NIILs, new ipsilateral ischemic lesions; CAS, carotid artery stenting; Max, maximum; WT, wall thickness; NWI, normalized wall index; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage; IQR, interquartile range.
Table 4
| Variables | Univariable regression analysis | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Max WT | 1.53 | 1.20–1.96 | 0.001 |
| Maximum area percentage of LRNC | 1.05 | 1.03–1.07 | <0.001 |
| Volume of LRNC | 1.004 | 1.002–1.005 | <0.001 |
| Maximum area percentage of IPH | 1.17 | 1.11–1.24 | <0.001 |
| Volume of IPH | 1.06 | 1.03–1.08 | <0.001 |
| Maximum circumference score of calcification in a single slice | 1.66 | 1.04–2.63 | 0.03 |
NIILs, new ipsilateral ischemic lesions; CAS, carotid artery stenting; Max, maximum; WT, wall thickness; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage; OR, odds ratio; CI, confidence interval.
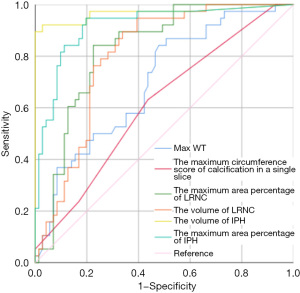
Table 5
| Variables | NIILs+ (n=38) | NIILs− (n=71) | P value | ||||
|---|---|---|---|---|---|---|---|
| Symptomatic group (n=6) | Asymptomatic group (n=32) | K-W test | Post-hoc | ||||
| SS vs. AS | SS vs. NS | AS. vs. NS | |||||
| Plaque burden | |||||||
| Max WT (mm) | 5.8 (4.8–6.6) | 6.9 (6.0–8.9) | 5.7 (4.5–6.8) | 0.001 | 0.20 | >0.99 | <0.001 |
| NWI (%) | 79.5 (72.9–89.6) | 81.8 (74.1–90.6) | 81.7 (73.1–92.9) | 0.61 | – | – | – |
| Preoperative stenosis rate (%) | 68.7 (65.4–77.3) | 69.1 (63.4–77.8) | 68.2 (61.6–76.4) | 0.58 | – | – | – |
| Maximum area percentage of plaque components | |||||||
| Calcification (%) | 14.5 (12.8–18.6) | 8.9 (4.5–18.7) | 13.0 (6.4–19.3) | 0.44 | – | – | – |
| LRNC (%) | 50.5 (19.0–80.2) | 54.6 (43.2–65.6) | 20.2 (5.7–36.7) | <0.001 | >0.99 | 0.06 | <0.001 |
| IPH (%) | 66.1 (40.7–67.8) | 28.4 (22.0–33.5) | 4.1 (2.9–10.4) | <0.001 | >0.99 | <0.001 | <0.001 |
| The volume of plaque components | |||||||
| Calcification (mm3) | 38.4 (26.2–230.3) | 43.1 (11.8–75.6) | 51.4 (17.3–97.3) | 0.88 | – | – | – |
| LRNC (mm3) | 272.9 (267.6–329.3) | 315.3 (198.1–327.4) | 99.6 (44.1–208.2) | <0.001 | 0.86 | 0.41 | <0.001 |
| IPH (mm3) | 264.7 (217.3–304.1) | 248.2 (107.6–296.5) | 68.5 (33.7–105.4) | <0.001 | >0.99 | <0.001 | <0.001 |
| Scores of calcification | |||||||
| Total circumferential score | 10.5 (6.8–25.5) | 6.5 (3.0–9.8) | 7.0 (3.0–11.0) | 0.17 | – | – | – |
| Max circumference score in a single slice | 3.0 (2.8–4.0) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.001 | 0.006 | 0.001 | >0.99 |
| Total location scores | 8.0 (6.3–21.5) | 7.0 (4.0–10.8) | 6.0 (3.0–11.0) | 0.39 | – | – | – |
Data are presented as the median (IQR). NIILs, new ipsilateral ischemic lesions; CAS, carotid artery stenting; Max, maximum; WT, wall thickness; NWI, normalized wall index; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage; SS, symptomatic stroke with NIILs; AS, asymptomatic stroke with NIILs; NS, NIILs negative group; K-W test, Kruskal-Wallis test; IQR, interquartile range.
Reproducibility evaluation
The interobserver reproducibility was excellent for Max WT (ICC, 0.87; 95% CI: 0.70–0.92), NWI (ICC, 0.86; 95% CI: 0.70–0.91), maximum area percentage of calcification (ICC, 0.88; 95% CI: 0.72–0.91), maximum area percentage of LRNC (ICC, 0.89; 95% CI: 0.81–0.93), maximum area percentage of IPH (ICC, 0.84; 95% CI: 0.71–0.94), the volume of calcification (ICC, 0.81; 95% CI: 0.71–0.90), the volume of LRNC (ICC, 0.87; 95% CI: 0.71–0.94), the volume of IPH (ICC, 0.85; 95% CI: 0.73–0.92), the total circumferential score of calcification (ICC, 0.81; 95% CI: 0.69–0.88), maximum circumference score of calcification in a single slice (ICC, 0.88; 95% CI: 0.70–0.93), and total location score of calcification (ICC, 0.78; 95% CI: 0.68–0.86).
Discussion
In this study, we found that large amounts of IPH, LRNC and heavy circumferential calcification were associated with NIILs after CAS. The circumference of calcification seems a key factor influencing clinical symptoms in NIILs positive patients than plaque components. Quantitative evaluation of carotid plaque burden and components by HR-VWI provides additional imaging information for clinical prediction of NIILs before the CAS procedure.
In patients with high surgical risk, CAS has become a viable replacement for CEA. However, despite the widespread use of EPDs, the main issue with CAS is the high incidence of postoperative ischemic brain lesions (14). One potential explanation is that during CAS, embolic particles and atherosclerotic debris come loose from unstable plaques and cause cerebral embolization (15). Large quantities of IPH or LRNC, which are important indicators of plaque instability, suggest plaque progression and heightened instability. With an increase in IPH and LRNC volume, the number of fragments discharged from the plaques burst by interventions may considerably rise, leading to more NIILs on DWI after CAS (16). Distal filtration cerebral EPDs also have several flaws. One of them is selective protection. Given that the mean filtering pore diameter is 100 µm, embolic particulates smaller than 100 µm will pass through the filter’s pores and collect in distal arteries, causing a cerebral embolism. All presently available filters may result in microembolization (17). Filter obstruction or flow standstill is another flaw of filtration EPDs. When embolic particles are larger, they can be captured by EPDs. The ICA, close to the filter device, may experience a considerable reduction in antegrade flow as a result of this massive embolic burden, which is known as a “slow-flow” phenomenon. Previous research has shown that this “slow-flow” characteristic raises the risk of perioperative stroke, and the embolization of vulnerable plaque components may have a harmful effect (18). Recent research found that despite the use of EPDs, CAS therapy for vulnerable plaques with the LRNC and IPH resulted in ipsilateral multiple cortical infarcts (17). This is consistent with our findings. Our study suggests that LRNC and IPH are associated with NIILs after CAS. Similarly, other studies have shown that the recent IPH (along with LRNC) was also strongly related to NIILs after CAS (16,19,20). Conversely, some researchers have concluded that there is no significant correlation between IPH and NIILs after CAS. Because the frequency of NIILs between the IPH positive and IPH negative groups did not appear to differ significantly, an earlier investigation discovered that IPH detected by magnetization prepared rapid gradient echo (MPRAGE) was not a major risk factor for cerebral embolism following CAS (21). In a subsequent investigation using high-resolution magnetic resonance imaging (HR-MRI), similar outcomes that demonstrated the security of protected CAS in individuals with IPH and ulceration were revealed (22). We speculate that these findings may be due to the fact that they did not quantify the volume of IPH. Accurately quantitative imaging analysis by HR-VWI could more accurately evaluate the relationship between plaque characteristics and clinical symptoms. Controversy still exists regarding the correlation between IPH and NIILs following CAS. To further understand the connection between plaque characteristics and clinical outcomes, multicenter prospective trials are needed.
Indeed, recent studies have shown that calcified carotid plaques are associated with new cerebral ischemic emboli following CAS (4,15). These studies, however, did not quantitatively assess calcified plaque. A recent study quantitatively assessed carotid calcification by computed tomography (CT) and found that calcification circumference, rather than calcification volume, was connected to new ischemic brain lesions following CAS (12), which agrees with the findings of our investigation. Our research discovered that whereas calcification circumference was substantially linked with NIILs following CAS, calcification volume, calcification location, and the Maximum area percentage of calcification were not. Compared with NIILs asymptomatic groups, the NIILs symptomatic group has a greater circumference of calcification. The expansion of the stent is due to the compression of the plaque and the stretching of the vessel wall (23). Because it is hard and difficult to compress, calcified plaque might obstruct stent expansion. As a result, when the carotid artery wall has significant circumferential calcification, the hard vessel wall exerts too much resistance, preventing the implanted stent from opening sufficiently (24). It takes much more energy to achieve sufficient stent inflation, which raises the possibility of overstretching the vascular wall. In this instance, vascular wall damage, plaque rupture, increased number of emboli released from the ruptured plaques, and in situ thrombus formation within the stent lead to reduced blood flow to the distal brain tissue, which may increase the risk of symptomatic stroke. It cannot be assumed that a big carotid circumference is filled with calcification based on a significant percentage area or volume owing to the eccentricity of the plaque morphology. Therefore, even though the volume of calcification is the same, arteries with varying degrees of circumferential calcification can sustain varying expansion strengths during the installation of stents (12). This explains why there was not a similar correlation between the volume of calcification, the Maximum area percentage of calcification, and the presence of NIILs following CAS. Similarly, due to the eccentric and scattered distribution of calcification, the Max circumference score of calcification in a single slice is a better indicator of the circumferential degree of calcification than the total circumferential score of calcification, which also explains why a similar relationship between the total circumferential score of calcification and NIILs was not observed after CAS.
The findings of the present investigation may have practical significance for the selection of a carotid artery stenosis treatment protocol. The quantitative preoperative assessment of carotid plaque using HR-VWI assists clinicians in selecting patients at high risk for NIILs following CAS. In patients with massive IPH, LRNC, and heavy circumferential calcification, if there is only a minor contraindication to surgery, CEA can be a better option than CAS. Alternatively, for patients at high risk for open surgery, reasonable medicinal medication and a more effective CAS treatment may be more secure for these high-risk NIILs patients. Future clinical research on individuals with severe IPH, LRNC, and substantial circumferential calcification would need to examine the link between treatment choice and clinical outcomes.
This study has a number of limitations. First, this was single-center research and there was a low number of cases in the NIILs symptomatic group (n=6). In previous studies, the incidence of symptomatic stroke with NIILs after CAS was 4.4% and 5.1%, respectively (16,19). In our study, the incidence of symptomatic stroke with NIILs after CAS was 5.5%, similar to the previous results. Future multicenter studies with larger sample sizes in symptomatic stroke after CAS are needed to demonstrate the results of this study. Second, since this is a retrospective study, clinicians may have a bias in stent selection. Typically, open cell stents are used if the plaque is hard with strong calcification, and closed cell stents are used if the plaque tends to disintegrate and bleed. In our study, this bias may have led to the fact that stent type was not significantly associated with NIILs after CAS. Prospective studies are required in the future to investigate the association between stent type and NIILs after CAS.
Conclusions
The massive IPH, LRNC, and heavy circumferential calcification were associated with NIILs after CAS. Preoperative quantitative assessment of carotid plaque using HR-VWI may be useful for predicting NIILs after CAS.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Beijing Municipality (grant No. 7222047 to Wei.Yu) and the International Medical Foundation of China (grant No. Z-2014-07-2101 to Wei.Yu).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-543/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-543/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-543/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of BeiJing AnZhen Hospital, Capital Medical University approved the study protocol with a waiver of informed consent due to the retrospective nature of the study (No. 2022135X).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vlisides PE, Moore LE. Stroke in Surgical Patients. Anesthesiology 2021;134:480-92. [Crossref] [PubMed]
- Feng Y, Bai X, Zhang X, et al. Risk factors for new ischemic cerebral lesions after carotid artery stenting: A systematic review and meta-analysis. Ann Vasc Surg 2021;77:296-305. [Crossref] [PubMed]
- Köklü E, Gencer ES. Plaque morphology effect on periprocedural asymptomatic cerebral embolism in carotid artery stenting using first-generation carotid stents: A diffusion-weighted magnetic resonance imaging study. Kardiol Pol 2022;80:307-14. [Crossref] [PubMed]
- Sabat J, Bock D, Hsu CH, et al. Risk factors associated with microembolization after carotid intervention. J Vasc Surg 2020;71:1572-8. [Crossref] [PubMed]
- Geiger MA, Flumignan RLG, Sobreira ML, et al. Carotid Plaque Composition and the Importance of Non-Invasive in Imaging Stroke Prevention. Front Cardiovasc Med 2022;9:885483. [Crossref] [PubMed]
- Tapis P, El-Koussy M, Hewer E, et al. Plaque vulnerability in patients with high- and moderate-grade carotid stenosis - comparison of plaque features on MRI with histopathological findings. Swiss Med Wkly 2020;150:w20174. [Crossref] [PubMed]
- Fabiano S, Mancino S, Stefanini M, et al. High-resolution multicontrast-weighted MR imaging from human carotid endarterectomy specimens to assess carotid plaque components. Eur Radiol 2008;18:2912-21. [Crossref] [PubMed]
- Bos D, van Dam-Nolen DHK, Gupta A, et al. Advances in Multimodality Carotid Plaque Imaging: AJR Expert Panel Narrative Review. AJR Am J Roentgenol 2021;217:16-26. [Crossref] [PubMed]
- Etesami M, Hoi Y, Steinman DA, et al. Comparison of carotid plaque ulcer detection using contrast-enhanced and time-of-flight MRA techniques. AJNR Am J Neuroradiol 2013;34:177-84. [Crossref] [PubMed]
- Che F, Mi D, Wang A, et al. Extracranial carotid plaque hemorrhage predicts ipsilateral stroke recurrence in patients with carotid atherosclerosis - a study based on high-resolution vessel wall imaging MRI. BMC Neurol 2022;22:237. [Crossref] [PubMed]
- Benson JC, Cheek H, Aubry MC, et al. Cervical Carotid Plaque MRI: Review of Atherosclerosis Imaging Features and their Histologic Underpinnings. Clin Neuroradiol 2021;31:295-306. [Crossref] [PubMed]
- Lv P, Ji A, Zhang R, et al. Circumferential degree of carotid calcification is associated with new ischemic brain lesions after carotid artery stenting. Quant Imaging Med Surg 2021;11:2669-76. [Crossref] [PubMed]
- Mazurek A, Malinowski K, Rosenfield K, et al. Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. J Clin Med 2022;11:4819. [Crossref] [PubMed]
- Rots ML, Meershoek AJA, Bonati LH, et al. Editor's Choice - Predictors of New Ischaemic Brain Lesions on Diffusion Weighted Imaging After Carotid Stenting and Endarterectomy: A Systematic Review. Eur J Vasc Endovasc Surg 2019;58:163-74. [Crossref] [PubMed]
- Xu X, Feng Y, Bai X, et al. Risk factors for silent new ischemic cerebral lesions following carotid artery stenting. Neuroradiology 2020;62:1177-84. [Crossref] [PubMed]
- Zhao G, Tang I, Tang H, et al. Predictors of Ipsilateral New Ischemic Lesions on Diffusion-Weighted Imaging after Carotid Artery Stenting in Asymptomatic Patients: A Retrospective Observational Study with Conventional Multicontrast MRI. Ann Vasc Surg 2021;74:95-104. [Crossref] [PubMed]
- Kim HJ, Rho MH. Massive Cerebral Microemboli after Protected Carotid Artery Angioplasty and Stenting Using a Distal Filter Embolic Protection Device for a Vulnerable Plaque with a Lipid Rich Necrotic Core and Intraplaque Hemorrhage: A Case Report. Taehan Yongsang Uihakhoe Chi 2020;81:739-45. [Crossref] [PubMed]
- Casserly IP, Abou-Chebl A, Fathi RB, et al. Slow-flow phenomenon during carotid artery intervention with embolic protection devices: predictors and clinical outcome. J Am Coll Cardiol 2005;46:1466-72. [Crossref] [PubMed]
- Zhao G, Tang X, Tang H, et al. Recent Intraplaque Hemorrhage Is Associated with a Higher Risk of Ipsilateral Cerebral Embolism During Carotid Artery Stenting. World Neurosurg 2020;137:e298-307. [Crossref] [PubMed]
- Ji A, Lv P, Dai Y, et al. Associations between carotid intraplaque hemorrhage and new ipsilateral ischemic lesions after carotid artery stenting: a quantitative study with conventional multi-contrast MRI. Int J Cardiovasc Imaging 2019;35:1047-54. [Crossref] [PubMed]
- Chung GH, Jeong JY, Kwak HS, et al. Associations between Cerebral Embolism and Carotid Intraplaque Hemorrhage during Protected Carotid Artery Stenting. AJNR Am J Neuroradiol 2016;37:686-91. [Crossref] [PubMed]
- Jeon SY, Lee JM. Protected carotid artery stenting in patients with severe stenosis. Medicine (Baltimore) 2022;101:e30106. [Crossref] [PubMed]
- Elsayed N, Yei KS, Naazie I, et al. The impact of carotid lesion calcification on outcomes of carotid artery stenting. J Vasc Surg 2022;75:921-9. [Crossref] [PubMed]
- Dong P, Ye G, Kaya M, et al. Simulation-Driven Machine Learning for Predicting Stent Expansion in Calcified Coronary Artery. Appl Sci (Basel) 2020;10:5820. [Crossref] [PubMed]


