
Distal stent graft-induced new entry (dSINE) after frozen elephant trunk: a scoping review
Highlight box
Key findings
• Proper device selection, along with accurate angio-CT scan analysis, are crucial elements to limit dSINE occurrence. A rigorous radiological follow-up is key to their early detection and to provide the best clinical outcome for the patient
What is known and what is new?
• dSINE are potentially lethal complications of endovascular or hybrid aortic repair;
• This review aims to collect the expertise across the scientific literature and to recognize the supposed risk factors for dSINE occurrence.
What is the implication, and what should change now?
• dSINEs are frequent complications after FET. Although not emergent, they require rigorous follow-up and proper treatment due to their asymptomatic nature and potential harm.
Introduction
During the last decades major complications after the frozen elephant trunk (FET) procedures progressively decreased thanks to improvements of the technique and better patients selection. Although good early results have been reported, during follow-up, many patients require reoperation to address the downstream thoracic aorta. This scenario can be caused by aortic diameter progression or endoleaks, even if the etiology is multifactorial without independent predictors for redo (1). Endovascular extension or secondary hybrid approach are often planned due to disease progression (2). The use of the FET in aortic dissections is often cause of distal stent graft-induced new entry (dSINE). “Stent Induced New Entry” is a clinical condition first described by Dong and colleagues in 2010 (3). It has been uniformly defined as “a new tear caused by the stent graft itself, excluding those created by natural disease progression or any iatrogenic injury from endovascular manipulation” (4). SINEs are potentially lethal complications, as the intimal tear induced by the stent-graft could evolve into a patent false lumen, with a gradual increase in pressure that can lead to aneurysmatic expansion and potential rupture (3,4). An important distinction must be performed between dSINEs and endoleaks, the latter being defined as leakage of blood into the aneurysmal sac outside the endograft but not leakage outside the vascular compartment (5).
Multiple risk factors for the formation of SINE have been described in recent literature, the most relevant being the oversizing of the stent-graft, the so-called “spring back force” (the mechanical tendency of the stent to retrieve its initial straight shape), and the natural fragility of the aortic wall (6). Although dSINE is not an emergent condition but remains a relatively frequent complication, occurring in 15% to 18% of the patients (7).
Although thoracic endovascular aortic repair (TEVAR) and FET have different indications in treating aortic pathologies, the supposed dSINE’s causes are basically the same after both interventions. More recently, few studies on dSINE after FET focused attention on the shear stress between the distal end of the stent graft and the dissection membrane, which seems to be high enough to contribute to dSINE formation (8). Moreover, the need of a further endovascular procedure to correct SINEs—though it has proven excellent clinical results—negates the benefit of a single-stage hybrid procedure.
In this article, the purpose was to collect and critically review the literature on dSINE after FET to create a model of prevention. We also aim to describe our center experience regarding this adverse event. We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-234/rc).
Methods
Literature search criteria
The initial search performed using PubMed databases from inception to January 2022 yielded 102 articles, selecting publications in English, with no time restrictions. Further selection was performed by filtering literature using ‘frozen elephant trunk’ OR ‘stented elephant trunk’ OR ‘antegrade stenting descending thoracic aorta’ OR ‘dSINE’, ‘distal stent-graft induced new entry’ as either keywords or MeSH terms. We found 7 case reports and 9 retrospective studies focusing on dSINE after total arch replacement (TAR) with FET technique. Case reports, editorial, expert opinion and comment types of publication were excluded, as well as review articles, because of the potential doubling of results. In the end, eight studies were included in the quantitative review. The primary aim of this scoping review was to define the dSINE incidence and reintervention rate. Then, a descriptive approach to detect and display supposed risk factors and predictors for the occurrence of this condition has been adopted. No protocol is available for this scoping review (Figure 1).
Data extraction and appraisal
All data were extracted from article text and tables. Two investigators independently reviewed each retrieved article (F Campanini, A Costantino). The results were reviewed by two senior investigators (G Murana, L Di Marco). All values are represented as numbers (percentages), mean ± standard deviation or median. Freedom from development of dSINE has been analyzed by using Kaplan-Meier method. This retrospective study was approved by local institutional review board (Gaetano Domenico Gargiulo and Davide Pacini) and did not require the patient informed consent (IRB 2021/Disp/AOUBo).
Graft selection and measurement method
The choice of the device in Bologna is based on a consistent experience with both Thoraflex and E-vita grafts. The former is preferred for its ease of deployment and the circle-shaped design of the stent, while the latter is used when a longer coverage in the descending thoracic aorta is needed.
The size selection of the graft is based on angio-computed tomography (CT), electrocardiogram (EKG)-gated evaluation. The distal landing zone diameter is performed by measuring the multiplanar reconstruction (MPR) CT images, starting from zone 2 according to Ishimaru aortic map, then, after having selected the proper stent length, then the true lumen perimeter/diameter at the estimated site of the distal end of the stent is measured. In acute setting we do not perform any oversizing, while in chronic aortic dissection we do not exceed a 20% oversizing.
Angio-CT evaluation according to the Bologna protocol
CT is uniformly considered to be the best modality for the detection and location of aortic pathological conditions (i.e., dSINE), providing high sensitivity and specificity. Our standard approach is based on the recommendations of the latest consensus document on acute aortic dissection (9), and it involves contrast-enhanced, EKG-gated (electrocardiography-gated), CT, which reduces motion artifacts within the entire length of thoracic aorta (8). It should always include delayed images acquisition, especially after stent graft repair, to detect endoleaks. In patients candidate to FET for aortic dissection (acute or chronic), double oblique reconstruction allow a more realistic measure of aortic diameters along the entire stent graft length. In this way, it is possible to make an exact prediction of the distal landing zone level, which is the most important zone to consider for avoiding stent graft oversizing. Angio-CT scans are performed postoperatively before hospital discharge. According to CT scan findings, planned CT scans are performed at 3 months, 6 to 12 months, and annually thereafter. The “degeneration” stent graft is generically used to identify aortic erosion or rupture at the extremity of the endoprosthesis. In this group of conditions, we recognize dSINE as a new communication between true and false lumen, induced by the stent graft itself, with or without type IB endoleak (Figure 2).

Results
Literature review
Table 1 summarizes retrospective single and multiple center experiences, reporting follow-up data with dSINE occurrence after FET. According to the literature review, 8 studies were identified and analyzed. A total number of 544 FET procedures have been performed, and dSINE occurred in 69 patients (12.7%). Mean time between surgery and the diagnosis of dSINE ranged (when reported) from 12.6 to 30.6 months.
Table 1
| Author | Source, year | Patients (n) | Pathology | Type of stent | dSINE, n (%) | Type of study | Time from FET to first detection of dSINE (months), mean ± SD | Follow-up (months), mean ± SD | Risk factors detail |
|---|---|---|---|---|---|---|---|---|---|
| Sun (9) | JTCVS, 2011 | 44 | 19 TAAD, 25 TCAD | – | 4 (9.1) | Single center retrospective | N/A | 38.0±17.0 | N/A |
| Huang (7) | JTCVS, 2013 | 20 | 8 TRAD, 12 TCAD | – | 12 (60.0) | Single center retrospective | 12.6±9.9 | 27.9±11.9 | Oversizing, taper ratio, ovality of the stent |
| Furutachi (10) | ICVTS, 2019 | 20 | 20 TAAD | Frozenix | 3 (15.0) | Single center retrospective | N/A | 11.0 (median) | Oversizing, aortic remodelling |
| Yamane (11) | AVD, 2020 | 15 | 15TCAD | Frozenix | 5 (33.3) | Single center retrospective | N/A | 41.6 | N/A |
| Czerny (8) | ATS, 2020 | 100 | 3 TRAD, 3 TCAD | – | 6 (6.0) | Multicentric retrospective | 16.3±22.7 | 44.7±38.7 | Oversizing, ovality of the stent |
| Kreibich (12) | ATS, 2020 | 126 | – | Thoraflex/E-vita | 16 (12.7) | Multicentric retrospective | 13.0 (median) | 27.0 (median) | Oversizing, remodelling, mechanical dynamics of the stent |
| Nomura (13) | AVD, 2021 | 70 | 44 TAAD, 26 TCAD | Frozenix | 9 (12.9) | Single center retrospective | 17.7±11.7 | 24.0 (median) | N/A |
| Osswald (14) | EJCS, 2021 | 149 | 105 TAAD, 44 TCAD | Thoraflex | 14 (9.4) | Single center retrospective | N/A | 30.4 (mean) | N/A |
| Summary | – | 544 | – | – | 69 (12.7) | – | N/A | N/A | – |
ATS, Ann Thorac Surg; AVD, Ann Vasc Dis; EJCS, Eur J Cardiothorac Surg; ICVTS, Interact Cardiovasc Thorac Surg; JTCVS, J Thorac Cardiovasc Surg; dSINE, distal stent graft-induced new entry; FET, frozen elephant trunk; N/A, not applicable; SD, standard deviation; TAAD, type A acute aortic dissection; TRAD, type A residual aortic dissection; TCAD, type A chronic aortic dissection.
Across the studies examined in this review, TAR with stented elephant trunk was performed to treat acute and chronic aortic dissection (type A or B according to Stanford classification).
During the follow-up period (ranged between 11 and 46 months), most patients that developed dSINE have received endovascular treatment, whereas a couple of them needed open surgery. Only few patients with the diagnosis of dSINE had not been treated because of the small size, so they have been treated conservatively by follow-up angio-CT scans, with the choice for a conservative approach.
Results in Bologna
From January 2007 to December 2021, 225 FET procedures have been performed in our center for the treatment of acute and chronic aortic syndromes. Details are shown in Table 2. Both Thoraflex (114, 50.7%) and E-vita Open (111, 49.3%) grafts were used.
Table 2
| Variables | All patients (n=225) | dSINE patients (n=54) | No dSINE patients (n=171) | P value |
|---|---|---|---|---|
| Acute type A, n (%) | 47 (20.9) | 4 (7.4) | 43 (25.1) | 0.03 |
| Residual type A, n (%) | 117 (52.0) | 30 (55.6) | 87 (50.9) | 0.80 |
| Chronic type A, n (%) | 20 (8.9) | 6 (11.1) | 14 (8.2) | 0.08 |
| Acute type B, n (%) | 16 (7.1) | 4 (7.4) | 12 (7.0) | 0.64 |
| Chronic type B, n (%) | 25 (11.1) | 10 (18.5) | 15 (8.8) | 0.37 |
| Thoraflex, n (%) | 114 (50.7) | 28 (51.9) | 86 (50.3) | 0.65 |
| E-vita Open, n (%) | 111 (49.3) | 26 (48.1) | 85 (49.7) | 0.64 |
| Distal anastomosis, n (%) | ||||
| Zone 2* | 114 (50.7) | 30 (55.6) | 84 (49.1) | 0.95 |
| Zone 3* | 111 (49.3) | 24 (44.4) | 87 (50.9) | 0.81 |
| Stent >10 cm | 117 (52.0) | 29 (24.8) | 88 (75.2) | 0.536 |
| Stent diameter (cm), mean ± SD | 29.8 | 29.9±4.0 | 29.8±3.7 | 0.531 |
*, according to Ishimaru aortic map. Zone 2 is defined as the aortic sector between the origins of the left carotid and left subclavian artery. Zone 3 is the aortic sector immediately after the left subclavian artery. dSINE, distal stent graft-induced new entry; SD, standard deviation.
Most patients were treated for type A residual dissection (117, 52.0%). We reported an occurrence of 54 cases of dSINE (24.0%). Among them, 28 patients (51.9%) have been implanted the Thoraflex device, while 26 (48.1%) the E-vita one. In all cases the occurrence of dSINE was occasionally found during follow-up.
The mean time between the surgical procedure and the diagnosis of dSINE was 27.2±33.6 months, median 19.5 months. A reintervention with TEVAR was necessary in 53 (98.1%). Whereas only 1 (1.9%) patient has not been treated.
Freedom from dSINE was strongly different in patients undergoing E-vita Open implantation compared to Thoraflex hybrid where it was 57.7% vs. 60.7% at 1 year and 30.8% vs. 7.1% at 3 years, respectively (Log-rank =0.05) (Figure 3).
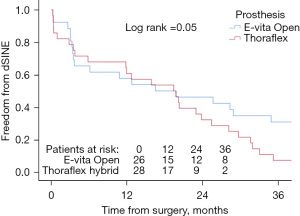
Our experience supports the findings of other authors, with higher freedom from dSINEs after the implantation of Thoraflex hybrid prosthesis compared to E-vita graft (10,11,13,15-17).
Description of supposed risk factors
Despite the lack of data related to such a specific topic, which made it impossible to perform a systematic statistical analysis of the supposed risk factors for the development of dSINEs after FET, a collection of the observed predictors is presented in a descriptive manner.
Across all the papers that were included in this review, it is noticeable how aortic remodeling, distal stent graft oversizing and the spring-back force of the devices are key elements that contribute to the development of this adverse event. The changes in the structure and the mechanical properties if the aortic walls are involved in the development of new entries, as the dissecting membrane becomes thicker and stiffer over time, therefore being more susceptible to injuries. Specifically, dSINE itself is patency of false lumen which remains perfused, thereby negatively influencing the aortic remodeling (14). Furthermore, several authors have stressed the importance of proper stent graft size selection, as oversizing is considered one of the determining factors for the occurrence of this adverse event, due to the shear force applied to the dissecting membrane (1,3,4,7,8,10,11,17).
Discussion
The primary findings of this review article shows that dSINEs are a frequent complication in patients treated either with hybrid devices or endoprostheses, which occurs between 15 to 18 percent of patients that have been treated with FET. This complication yields a high mortality rate, and it is related to multiple factors which contribute to the development of this adverse event. Improper oversizing, mechanical spring-back forces of the stent graft, and the aortic remodeling itself have been identified as risk factors.
Literature shows a relative abundance of material concerning SINE in TEVAR, but only few articles about this condition after a hybrid TAR.
Extensive search and analysis of the state of the art led to the individuation of the predictors of dSINE after FET, namely distal stent graft oversizing, springback forces, stent-graft length, and ovality of the stent. Distal stent oversizing in acute aortic dissection is considered as the size difference between the calculated pre-dissection aortic diameter and the size of the stent-graft itself. In a chronic setting, it is assumed as the difference between the calculated true lumen diameter and the stent graft diameter (12,13,18).
A proper selection of the stent-graft size is key to the long-term success of the surgical procedure. An oversizing ratio between 10–20% at the distal end is considered effective in the prevention of endoleaks or distal migration. The taper ratio of the thoracic aorta is not considered a predictor of dSINE in hybrid aortic arch repair (7). The spring-back force is the tendency of a stent-graft to retrieve its straight position once implanted. This causes stress on the false lumen wall, and it is responsible for an increased risk of dSINE development (11,13,19). Japanese studies have widely analyzed this aspect, with the implantation of Frozenix hybrid prosthesis. Stent graft length is another potentially relevant parameter, with a greater tendency for shorter grafts to develop SINE, as the landing zone is not in a straight portion of the aorta (10). Conversely, a longer thoracic descending aorta coverage, though more prone to develop spinal cord injury, could provide lower parietal shear stress at the distal end of the stent-graft (15). The ovality of stent graft is considered as the ratio between the long and short axis of the true lumen at the distal end of the stent-graft. A more oval profile of the TL at the distal end of the graft was associated with a higher tendency to develop dSINE, probably related to stronger stress against the aortic dissection membrane (8).
Aortic membrane changes over time
To understand the mechanism that leads to the development of dSINE, it’s also important to consider the physiological evolution of the dissection membrane over time. The morphology of the dissected aorta is susceptible to changes, as the intimal flap is highly mobile and relatively thin in the acute setting, while it becomes much stiffer, thicker, and less mobile over time. This structural evolution seems to be crucial in understanding the different behavior of the dissection membrane. In the acute setting, the dissection flap may be led to its anatomic position by the expansion of the stent-graft. Conversely, in chronic dissection, the thickened intimal flap cannot reapproximate the outer aortic wall, being susceptible to lesions by the radial force exerted by the stent-graft itself (Figures 4,5) (20).
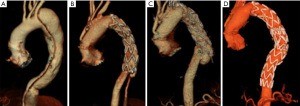
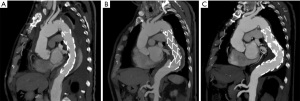
The aortic structure in patients with connective tissue disorders (e.g., Marfan syndrome) is an element that must be considered. In fact, these conditions are an important risk factor for the development of aortic disease. The weakened, abnormal structure of the aorta is susceptible to injuries and lesions, particularly during the stent deployment. Hybrid TAR with FET technique proved to be a valid therapeutic strategy, with better clinical outcomes compared to endovascular treatment, as shown by Sun and colleagues. Despite the increased fragility of the aorta in these patients, the incidence of dSINE in the Marfan group was in line with the general population (9).
Devices mechanical characteristics
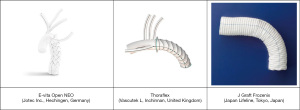
Finally, the mechanical characteristics of the device itself play an important role in tissue remodelling. The different available prostheses have physical and structural characteristics that can affect, with different patterns, the aortic wall. As shown by Kreibich et al. (12), a mechanical stress analysis was performed to showcase the differences between the radial forces of the stent-grafts of Thoraflex and E-vita prosthesis. Thoraflex stent-graft resulted to be much stiffer at the distal end than the E-vita one, which showed constant stiffness. A less rigid, more flexible design may be less prone to affect the dissection membrane. A relevant disclaimer should be done as the “ex vivo” prosthesis analysis may not be fully representative of its behavior once implanted in a patient (21). Recent studies performed by Berger et al. showed no significant differences in dSINE development between Thoraflex and E-vita Open (15), whereas in a study performed by Charchyan et al. (16) Thoraflex Hybrid device seems to have an advantage over the other prostheses, showing a lower incidence of dSINEs, which contradicts the findings of Kreibich et al. (12). Specifically, Charchyan et al. individuated two predictors for the occurrence of dSINE, connective tissue disorders and stent graft diameter at its distal end. As shown by this review, the “Z” shaped nitinol stent graft of the E-vita might be unfavourable compared to the standard, circle-shaped one of the Thoraflex device (16). Moreover, in a systematic review made by Jobouri et al., Thoraflex seems to perform better as promoter of maximum false lumen thrombosis as well as true lumen expansion, being associated with lower incidence of dSINEs (17).
As shown by the publications reporting data on Asian casuistry, the J Graft Frozenix (Japanese Lifeline, Tokyo, Japan) has been frequently used as the device of choice for hybrid TAR surgery. According to the evidence shown by Nomura et al., Frozenix seems to have a strong spring-back force, although no comparative mechanical stress analysis with the “competitor” devices has been performed yet (13) (Figure 6).
According to the literature reviewed, the occurrence of dSINE development was 13.9% out of 395 FET implantation. Many of these articles were missing data at follow-up and included different kinds of endograft implantation. In Bologna we observed a higher incidence of this complication (23.6%). This can be explained by the fact that, in contrast with other articles considered, all our patients underwent a complete regular follow-up, which included angio-CT scan.
Limitations
The primary limitation of the study was the lack of data. The little amount of material found made it impossible to perform a statistical risk factors analysis. In fact, according to the small number of papers available and the heterogeneity of data displayed, which were impossible to match, we have opted for a descriptive approach to summarize the major findings across the literature on the supposed risk factors and predictors for the development of this adverse event.
Conclusions
Although not emergent, dSINE can be potentially lethal, with mortality rates that can range up to 25% (7). Several mechanisms can favor this condition, such as stent graft length and size, the site of the distal anastomosis in the arch and the anatomic features of the descending thoracic aorta. Due to dSINE’s asymptomatic nature and potential harm, a rigorous follow-up including angio-CT should be planned.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mohamad Bashir, Edward P. Chen and A. Mohammed Idhrees) for the series “Frozen Elephant Trunk” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-234/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-234/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-234/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kreibich M, Berger T, Rylski B, et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg 2020;159:392-399.e1. [Crossref] [PubMed]
- Meisenbacher K, Osswald A, Bischoff MS, et al. TEVAR Following FET: Current Outcomes of Rendezvous Procedures in Clinical Practice. Thorac Cardiovasc Surg 2022;70:314-22. [Crossref] [PubMed]
- Dong Z, Fu W, Wang Y, et al. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg 2010;52:1450-7. [Crossref] [PubMed]
- Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [Crossref] [PubMed]
- Mauro MA, Murphy KP, Thomson KR, et al. Index. In: Expert Radiology. Image-Guided Interventions (Third Edition). Boston: Elsevier; 2020:929-64.
- Takagaki M, Midorikawa H, Yamaguchi H, et al. Rapidly Progressed Distal Arch Aneurysm with Distal Open Stent Graft-Induced New Entry Caused by “Spring-Back” Force. Ann Vasc Dis 2020;13:343-6. [Crossref] [PubMed]
- Huang CY, Weng SH, Weng CF, et al. Factors predictive of distal stent graft-induced new entry after hybrid arch elephant trunk repair with stainless steel-based device in aortic dissection. J Thorac Cardiovasc Surg 2013;146:623-30. [Crossref] [PubMed]
- Czerny M, Eggebrecht H, Rousseau H, et al. Distal Stent Graft-Induced New Entry After TEVAR or FET: Insights Into a New Disease From EuREC. Ann Thorac Surg 2020;110:1494-500. [Crossref] [PubMed]
- Sun L, Li M, Zhu J, et al. Surgery for patients with Marfan syndrome with type A dissection involving the aortic arch using total arch replacement combined with stented elephant trunk implantation: the acute versus the chronic. J Thorac Cardiovasc Surg 2011;142:e85-91. [Crossref] [PubMed]
- Furutachi A, Takamatsu M, Nogami E, et al. Early and mid-term outcomes of total arch replacement with the frozen elephant trunk technique for type A acute aortic dissection. Interact Cardiovasc Thorac Surg 2019;29:753-60. [Crossref] [PubMed]
- Yamane Y, Katayama K, Furukawa T, et al. Mid-Term Results of Frozen Elephant Trunk Technique for Chronic Aortic Dissection. Ann Vasc Dis 2020;13:137-43. [Crossref] [PubMed]
- Kreibich M, Bünte D, Berger T, et al. Distal Stent Graft-Induced New Entries After the Frozen Elephant Trunk Procedure. Ann Thorac Surg 2020;110:1271-9. [Crossref] [PubMed]
- Nomura Y, Tonoki S, Kawashima M, et al. Distal Stent Graft-Induced New Entry after Total Arch Replacement with Frozen Elephant Trunk for Aortic Dissection. Ann Vasc Dis 2021;14:362-7. [Crossref] [PubMed]
- Osswald A, Schucht R, Schlosser T, et al. Changes of stent-graft orientation after frozen elephant trunk treatment in aortic dissection. Eur J Cardiothorac Surg 2021;61:142-9. [Crossref] [PubMed]
- Berger T, Weiss G, Voetsch A, et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur J Cardiothorac Surg 2019;56:572-8. [Crossref] [PubMed]
- Charchyan ER, Breshenkov DG, Belov YV. Hybrid aortic repair in patients with type III aortic dissection and concomitant proximal aortic lesion. Khirurgiia (Mosk) 2020;28-37. [Crossref] [PubMed]
- Jubouri M, Kayali F, Saha P, et al. Incidence of Distal Stent Graft Induced New Entry vs. Aortic Remodeling Associated wth Frozen Elephant Trunk. Front Cardiovasc Med 2022;9:875078. [Crossref] [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Canaud L, Gandet T, Sfeir J, et al. Risk factors for distal stent graft-induced new entry tear after endovascular repair of thoracic aortic dissection. J Vasc Surg 2019;69:1610-4. [Crossref] [PubMed]
- Mehanna M, Elhamami M, Abolkasem A, et al. Aortic remodelling and false lumen changes after the frozen elephant trunk technique using the thoraflex hybrid stented graft for aortic dissection. Egypt Heart J 2021;73:74. [Crossref] [PubMed]
- Yamauchi T, Masai T, Takano H, et al. Equations for Estimating the Predissected Diameter of the Descending Aorta From Computed Tomographic Images at the Onset of Aortic Dissection. J Am Heart Assoc 2018;7:e009196. [Crossref] [PubMed]



