Successful percutaneous closure of an extremely large secundum atrial septal defect during pregnancy
Case presentation
A 27-year-old Caucasian female at 20 weeks gestation presented with NYHA class II–III dyspnoea and lethargy. Prior to pregnancy, she reported progressive exercise intolerance over the previous year. During adolescence, she often struggled with exercise and physical activities. Her past medical history was only significant for infrequent episodic asthma.
Since the onset of pregnancy, the patient experienced worsening dyspnoea on exertion. Screening blood tests including full blood examination and iron studies were unremarkable. Ultrasound of her foetus demonstrated normal foetal development at 20 weeks gestation. A transthoracic echocardiogram (TTE) was requested following the detection of a systolic murmur. This demonstrated a large atrial septal defect (ASD) associated with haemodynamically significant, continuous left-to-right inter-atrial shunting (Qp:Qs shunt ratio: 2.2) and significant right ventricular dilatation. Estimated pulmonary artery systolic pressure was normal.
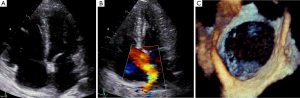
Transoesophageal echocardiogram (TOE) demonstrated an extremely large secundum type ASD (measuring 31, 38 and 30 mm at 0, 50 and 110 degrees respectively). Her right ventricle was moderate-to-severely dilated with low normal right ventricular ejection fraction (Figure 1).
The ladies exercise tolerance further worsened at 25 weeks gestation with her walking distance limited to 20 meters walking on flat ground. Her severe symptomatic limitation precluded attempting an exercise test and a cardiac magnetic resonance imaging study was not performed as the ASD and right ventricular size and function were felt to be adequately assessed on TTE and TOE. The patient’s case was then extensively discussed at a multi-disciplinary team meeting, involving obstetricians, cardiologists and cardiac surgeons. Conservative management of the ASD was strongly considered as the vast majority of ASDs are well tolerated during pregnancy. However, the decision was made to perform percutaneous closure in this case, given the size of the defect along with her progressive symptoms to NYHA class III. Open surgical management was also considered, however, the need for cardiopulmonary bypass, a sternotomy with the greater associated maternal and foetal risks mandated attempted closure using a percutaneous approach. The patient was also counselled regarding the risks of the procedure, including the requirement of fluoroscopy use (and the associated radiation exposure) for the procedure to be completed.
The procedure was performed under general anaesthesia, using fluoroscopic and TOE guidance at 26 weeks gestation. Femoral venous access was achieved with a 12 F sheath inserted into the right femoral vein. The defect was crossed with a guide wire using TOE guidance. A 40 mm sizing balloon was used which sized the defect at 38 mm. The defect was then closed percutaneous using a 40 mm Occlutech (Occlutech Figulla ASD, Jena, Germany) device with no residual shunting and sufficient rim attachment on TOE. Total procedure time was 1 hour and 13 minutes and total fluoroscopic screening time was 8.4 minutes. The total radiation dose area product was 445.51 dGray × cm2.
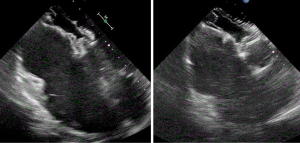
At 1 month post device deployment, a TTE demonstrated a well-seated ASD closure device without evidence of significant residual inter-atrial shunting (Figure 2). A significant reduction in the right ventricular size was displayed without interference to the aortic or mitral valves.
The patient experienced significant improvement in her dyspnoea and exercise tolerance following percutaneous ASD closure and delivered successfully at 38 weeks gestation via a well-tolerated normal vaginal delivery and a healthy infant birthweight.
Discussion
ASDs are one of the most common congenital acyanotic cardiac abnormalities with many patients asymptomatic until adulthood. Complications in adulthood include arrhythmias, pulmonary hypertension, embolic events and right heart failure, which may not present until beyond the fourth decade of life.
During pregnancy, a number of cardiovascular changes occur including an increase in total intravascular volume and an increase in cardiac output (by up to 30–50%). Detection of a murmur may prompt a TTE that may make the diagnosis of an ASD in previously asymptomatic patients. In those with large and haemodynamically significant defects, progressive dyspnoea and arrhythmias may develop during pregnancy (1).
Diagnosis of ASD during pregnancy requires a thorough clinical assessment to ensure the absence of heart failure symptoms, atrial arrhythmias, pulmonary hypertension or previous stroke. A small minority of patients diagnosed with an ASD during pregnancy require intervention (2). Indications for intervention include heart failure and pulmonary hypertension with severe hemodynamic compromise, NYHA class > II or recurrent stroke prior to pregnancy. However, in the absence of these, ASDs are considered a low-risk congenital heart lesion with minimal risk of maternal and fetal complications (3).
Maternal risks with an ASD include thromboembolic complications (5%) and arrhythmias (2%) (3). The presence of associated severe pulmonary hypertension carries a very high maternal (up to 50%), and fetal (up to 60%) mortality (3).
In circumstances where a diagnosis of an ASD is made during pregnancy, clinical assessment of symptomatic limitation can be challenging, as symptoms of normal pregnancy compared with those secondary to the haemodynamic effects of an ASD can be difficult to differentiate. Exercise testing (including with pulse oximetry), cardiac magnetic resonance imaging (to measure right ventricular volumes and ejection fraction), and in certain cases, right heart catheterisation may be useful in the prognostication and decision to perform ASD closure, particularly when the severity of symptoms appears out of proportion to the degree of left-to-right shunting and right heart volume overload. In the discussed case, the exercise deterioration was severe clinically and the information acquired on TTE and TOE provided the required data on right heart assessment.
Fortunately, a percutaneous closure was able to be performed in our case. In cases where an indication for ASD closure is present and percutaneous closure cannot be performed, referral and discussion of the case in a specialised multi-disciplinary unit with expertise in obstetric medicine, congenital heart disease and pulmonary hypertension should occur. Open surgical closure has been successfully performed during pregnancy in specific cases with reported successful maternal and fetal outcomes (4). Obstetric specific precautions if surgery is indicated include surgical timing (ideally during the 2nd trimeter), optimising fetal well-being (infusion of a high concentration of glucose at induction of cardiopulmonary bypass) and optimising maternal haemodynamics, oxygenation and haematocrit whilst on cardiopulmonary bypass (5).
Radiation dose exposure to the mother and the foetus is an important safety issue in undertaking percutaneous device closure in pregnancy. The radiation dose in our case was within an acceptable range for radiation exposure during percutaneous ASD closure (5). Timing of percutaneous ASD closure is also of relevance to minimise the risks of radiation exposure to the developing foetus. It would be preferable to delay ASD closure until the latter stage of the second trimester, at which time CNS development and organogenesis has largely occurred, minimising fetal risk (6,7).
This case demonstrates an extremely large ASD in a pregnant woman with an indication for defect closure. As demonstrated, with appropriate planning and patient selection, percutaneous closure can be successfully and safely performed, with minimal risks to both the mother and the foetus.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- INTECH. Pregnancy Issues in Women with Atrial Septal Defect. Available online: http://www.intechopen.com/books/atrial-septal-defect/pregnancy-issues-in-women-with-atrial-septal-defect
- Krishnamoorthy S, Butt M, Lip GY. Asymptomatic hypoxia in a young pregnant lady--unusual presentation of atrial septal defect. Int J Cardiol 2010;143:e34-6. [Crossref] [PubMed]
- Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol 2007;49:2303-11. [Crossref] [PubMed]
- Manivannan S, Dadlani G, Parsons M, et al. Surgical repair of atrial septal defect with severe pulmonary hypertension during pregnancy: a case report with literature review. Cardiol Young 2012;22:493-8. [Crossref] [PubMed]
- Arnoni RT, Arnoni AS, Bonini RC, et al. Risk factors associated with cardiac surgery during pregnancy. Ann Thorac Surg 2003;76:1605-8. [Crossref] [PubMed]
- Wagdi P, Ritter M. Patient radiation dose during percutaneous interventional closure of interatrial communications. J Cardiol 2009;53:368-73. [Crossref] [PubMed]
- Australian Clinical Guidelines for Radiological Emergencies - September 2012. Prenatal Radiation Exposure. Available online: http://www.health.gov.au/internet/publications/publishing.nsf/Content/ohp-radiological-toc~ohp-radiological-20-app-e~ohp-radiological-20.03-prenatal


