
Impact on early outcome after endovascular repair of type B dissection without proximal landing zone using Castor single-branched stent graft—a retrospective cohort study
Highlight box
Key findings
• Proximal landing zone (PLZ) from left common carotid artery (LCCA) to left subclavian artery (LSA) was significantly associated with the occurrence of bird-beak configuration in thoracic endovascular aortic repair (TEVAR) with Castor. Bird-beak was a significant risk factor for aortic-related adverse events.
What is known and what is new?
• TEVAR with LSA revascularization using Castor single-branched stent-graft showed safe early outcomes.
• It is more susceptible to aortic adverse events when the PLZ LCCA-LSA is less than 10 mm in length. This should be carefully considered, taking into account the risks and benefits.
What is the implication, and what should change now?
• Applying the bird-beak configuration found in this study to the clinic may improve the clinical evaluation of the disease and reduce the occurrence of complications. The model constructed in this study needs to be verified by multi-center data.
Introduction
Acute type B aortic dissections (TBADs) belong to the group of acute aortic syndrome, which comprises a number of life-threatening medical conditions. Without timely and appropriate management, TBADs have a high mortality rate (1-4). The primary initial management strategies for TBADs include medical management, thoracic endovascular aortic repair (TEVAR), and open surgical repair (5-7).
The advantages of TEVAR include short operation time, quick postoperative recovery, and lower complication rates. With continued advancement of endovascular therapy, TEVAR is increasingly replacing surgical treatment as the preferred treatment option for appropriate anatomical morphologies (5). A prerequisite of TEVAR is the need for a healthy proximal landing zone 3 (PLZ 3) (>2.0 cm), which is particularly challenging if the dissection involves the left subclavian artery (LSA) (5,8-10). Several different procedures, including hybrid operations, branched arch grafts, fenestrated arch grafts, in situ fenestration, chimneys, snorkels, periscopes, and sandwiches, can be utilized. Single branch arch grafts with LSA revascularization are a safe option, with simple operations and smooth LSA flow (11-13). However, the bird-beak configuration (describing a gap between the unopposed endograft and the vessel) is more commonly observed after TEVAR with branching grafts. This configuration has the potential to cause significant hemodynamic disruptions and inadequate sealing, elevating the risk of migration, endoleakage, or collapse of the stent graft (14,15). The causes and prognosis of the bird-beak configuration have been widely studied in relation to the aortic arch, but its occurrence in branching stents has been rarely reported.
The aim of this study was to assess the impact of aortic arch morphology on early outcome following endovascular repair using Castor single-branched stent graft for patients with TBADs involving the LSA. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-379/rc).
Methods
Study population
This is a retrospective cohort study. Between January 2019 and December 2021, a total of 113 consecutive patients with TBADs but without an adequate PLZ were retrospectively reviewed at Beijing Anzhen Hospital, Capital Medical University. Of these patients, 41 (33 males and 8 females, with an average age of 63.1±9.2 years) underwent TEVAR with the Castor single branch stent graft (MicroPort Medical, Shanghai, China) to revascularize the LSA (Figure 1). The inclusion criteria were as follows: (I) age between 18 and 80 years; (II) TBADs with acute presentation (1); (III) involvement of the LSA with a PLZ 3 that could cover the origin of the LSA; (IV) a distance of over 15 mm between the left common carotid artery (LCCA) and the entry tear of the distal aortic arch; and (V) TEVAR with the Castor single branch stent graft. The exclusion criteria were as follows: (I) aortic connective tissue disease, such as Marfan/Loeys-Dietz syndrome; (II) a history of TEVAR; (III) the shortest distance between the distal end of the LCCA and the proximal end of the LSA is less than 5 mm; and (IV) a lack of an appropriate access route for the stent graft, such as diameters of the external iliac artery or common femoral artery <7 mm, or severe stenosis and calcification of the LSA or the left brachial artery (LBA).
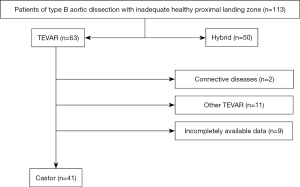
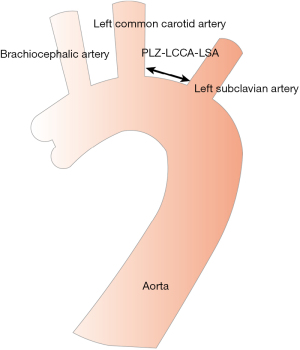
The PLZ in the aortic arch was classified from 0 to 12 according to the Society for Vascular Surgery/Society of Thoracic Surgeons (SVS/STS) classification (5,6). In cases where the PLZ was located in zone 2, however, the distance between the LCCA and the LSA (PLZ LCCA-LSA) was considered important (Figure 2). Thus, we divided the patients into two groups based on this distance: group A with a distance of ≤10 mm and group B with a distance of >10 mm.
TEVAR procedure and follow-up
The TEVAR procedure was performed by interventional diagnosis and treatment department of Beijing Anzhen Hospital. After local sterilization and anesthesia, 11-F and 6-F sheaths (Terumo Corporation, Tokyo, Japan) were inserted separately through the femoral arteries, and 8-F sheaths (Terumo Corporation) were inserted through the LBA by percutaneous puncture. Intravenous heparin (6,000 U) was administered. Initially, ascending aorta angiography was performed to obtain measurements of the thoracic aorta. The stent graft diameter was generally oversized to the non-dissected aortic maximum dimension in the PLZ by 5–15% for patients. The stent was released only after confirming its location via angiography. Postoperative angiography showed no obvious Ia endoleak or stent displacement.
Prior to the procedure and during the follow-up period (at 1 and 6 months), each patient underwent clinical assessment, as well as computed tomography angiography (CTA) or digital subtraction angiography (DSA). The PLZ diameter, PLZ LCCA-LSA length, aortic arch type, and working position angle were evaluated. Morphological risk factors were analyzed in relation to the bird-beak configuration and aortic-related adverse events.
Definition of outcome and complication
Technical success was defined as the completion of the procedure with patent target vessels as confirmed and absence of significant type Ia and/or III endoleak by the final angiography. The primary endpoints of this study were aortic adverse events at the 6-month follow-up. Secondary endpoints were defined as all adverse events (including mortality, re-intervention, new-onset stroke, paraplegia, renal complications, aortic-related adverse events).
Aortic-related adverse events included aneurysm formation or growth, aortic rupture, aortic branch vessel complications, stent migration, retrograde dissection, new distal dissection, Ia endoleak, and mortality.
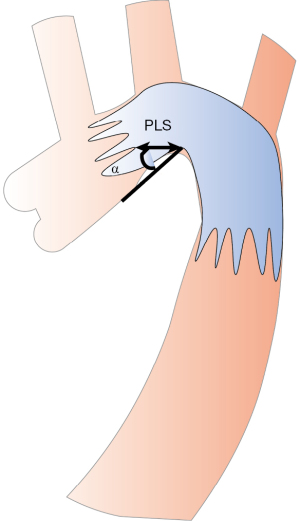
The bird-beak configuration, identified radiologically after TEVAR, refers to the wedge-shaped gap between the undersurface of the endograft and the lesser curvature of the arch (16). This configuration is typically characterized by two specific features: the angle between the undersurface of the endograft and the aortic wall (α angle) and the length of the longitudinal segment of the endograft that protruding longitudinal segment (PLS). These features have been previously defined and investigated in studies (14,15) (Figure 3).
The definitions of endoleak were as follows: type Ia—endoleak from the proximal stent-graft attachment site; type Ib—endoleak from the distal stent-graft attachment site; and type II—blood retrograde via branch arteries arising from the sealed segment (17).
According to the vertical distance from the opening of the brachiocephalic trunk to the top of the aortic arch and the multiple of the diameter of the dominant common carotid artery, we systematically classified the aortic arch into three types. Less than 1 was Myla type I aortic arch, 1–2 was Myla type II aortic arch, and greater than 2 was Myla type III aortic arch (18).
Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM Corporation, Armonk, NY, USA). All sample sizes were taken into account with clinical data. Continuous variables were presented as mean ± standard deviation (SD) if normally distributed or as medians with interquartile range (IQR) if the assumption of normality was not met. Mean differences were assessed using independent group t-tests or Mann-Whitney U tests. Categorical variables were presented as frequencies with percentages. The Chi-squared or Fisher’s exact tests were used for categorical variables, depending on the sample size. P value tests were two-sided. A P value <0.05 was considered statistically significant. Univariate and multivariate analyses were used to assess the relationship between morphological risk factors and outcomes. The relationship between the risk of bird-beak configuration and PLZ was assessed with logistic regression analysis. Meanwhile, the relationship between the risk of aortic-related adverse events and bird-beak configuration was assessed with logistic regression analysis. Follow-up data were analyzed by Kaplan-Meier life table analysis and the Gehan-Breslow-Wilcoxon test. Kaplan-Meier curves were generated using GraphPad Prism version 9.2.0 for MAC (USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Beijing Anzhen Hospital (No. 2023208X) with a waiver for informed consent due to the retrospective nature of the study.
Results
Patient baseline characteristics and morphology measurements
Table 1 shows baseline characteristics and details of aortic anatomy of the 41 patients included in this analysis. The mean age of the patients was 63.1±9.2 years, and 80.5% of them were male. The mean body mass index (BMI) was 26.5±3.3 kg/m2. There were no significant differences in comorbidities between the two groups. For detailed demographic data, please refer to Table 1.
Table 1
| Variable | Total (n=41) | Group A (n=18; 43.9%) | Group B (n=23; 56.1%) | P value |
|---|---|---|---|---|
| Age (year), mean ± SD | 63.1±9.2 | 61.7±9.0 | 74.2±9.4 | 0.403 |
| Male, n (%) | 33 (80.5) | 14 (77.8) | 19 (82.6) | 0.713 |
| BMI (kg/m2), mean ± SD | 26.5±3.3 | 26.7±3.6 | 26.4±3.0 | 0.825 |
| Smoking, n (%) | 30 (73.2) | 14 (77.8) | 16 (69.6) | 0.726 |
| Hypertension, n (%) | 38 (92.7) | 16 (88.9) | 22 (95.7) | 0.573 |
| Diabetes mellitus, n (%) | 8 (19.5) | 5 (27.8) | 3 (13.0) | 0.429 |
| COPD, n (%) | 1 (2.4) | 0 | 1 (4.3) | >0.99 |
| Cardiac disease, n (%) | 23 (56.1) | 9 (50.0) | 14 (60.9) | 0.486 |
| History of stroke, n (%) | 4 (9.8) | 2 (11.1) | 2 (8.7) | >0.99 |
| History of neoplasm, n (%) | 7 (17.1) | 4 (22.2) | 3 (13.0) | 0.679 |
| Previous renal insufficiency, n (%) | 4 (9.8) | 2 (11.1) | 2 (8.7) | >0.99 |
| PLZ diameter (mm), mean ± SD | 31.5±3.2 | 30.7±2.7 | 32.1±3.4 | 0.155 |
| PLZ LCCA-LSA (mm), median (IQR) | 10.0 (7.5–13.5) | 7.0 (6.0–8.0) | 13.0 (10.0–15.0) | <0.001 |
| Aortic arch type, n (%) | 0.666 | |||
| I | 16 (39.0) | 6 (33.3) | 10 (43.5) | |
| II | 12 (29.3) | 5 (27.8) | 7 (30.4) | |
| III | 13 (31.7) | 7 (38.9) | 6 (26.1) | |
| Dominant vertebral artery, n (%) | 0.871 | |||
| Right dominant | 12 (29.3) | 5 (27.8) | 7 (30.4) | |
| Equally dominant | 8 (19.5) | 3 (16.7) | 5 (21.7) | |
| Left dominant | 21 (51.2) | 10 (55.6) | 11 (47.8) | |
| Symptom, n (%) | 32 (78.0) | 14 (77.8) | 18 (78.3) | >0.99 |
Group A: PLZ LCCA-LSA ≤10 mm; group B: PLZ LCCA-LSA >10 mm. SD, standard deviation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PLZ, proximal landing zone; LCCA, left common carotid artery; LSA, left subclavian artery; IQR, interquartile range.
Among the preoperative CTA data, there were 18 patients with a PLZ LCCA-LSA less than 10 mm and 23 patients with a PLZ LCCA-LSA greater than 10 mm. The median PLZ LCCA-LSA length of the patients was 10 mm (IQR 7.5–13.5 mm), and the mean PLZ diameter was 31.5±3.2 mm. Of the patients, 16 (39.0%) had a type I aortic arch, 12 (29.3%) had a type II aortic arch, and 13 (31.7%) had a type III aortic arch. The dominant vertebral arteries were right (12, 29.3%), left (21, 51.2%), and equal (8, 19.5%). Additionally, in 78.0% of these cases (32/41), TBADs were combined with chest pain.
There were no statistically significant differences in baseline characteristics between groups A and B (all P>0.05). However, the PLZ LCCA-LSA length for the entire cohort was 10.0 mm (IQR 7.5–13.5 mm), while it was 7.0 mm (IQR 6.0–8.0 mm) in group A and 13.0 mm (IQR 10.0–15.0 mm) in group B. There was a significant difference in PLZ LCCA-LSA length with P<0.001.
TEVAR details and follow-up outcomes
The perioperative results and aortic-related adverse events occurring at any time during the follow-up period are summarized in Table 2. All patients received general anesthesia, and 97.6% of procedures were carried out successfully. Only one patient failed to complete the TEVAR because the patient would not cooperate. The median surgical time was 97 min (IQR 80–132 min). During the follow-up period, no perioperative mortality, new-onset stroke, rupture, or renal complications were observed. However, one case of paraplegia was observed 6 hours after the operation, and a cerebrospinal fluid (CSF) drain was immediately inserted. Unfortunately, sensory and motor disorders were not significantly relieved after 7 days. There was no statistically significant difference in these outcomes between group A and group B.
Table 2
| Variable | Total (n=41) | Group A (n=18; 43.9%) | Group B (n=23; 56.1%) | P value |
|---|---|---|---|---|
| TEVAR success, n (%) | 40 (97.6) | 17 (94.4) | 23 (100.0) | 0.439 |
| TEVAR time (min), median [IQR] | 97 [80–132] | 101 [71–152] | 97 [86–120] | 0.703 |
| Perioperative mortality, n (%) | 0 | 0 | 0 | NA |
| Re-intervention, n (%) | 6 (14.6) | 2 (11.1) | 4 (17.4) | 0.679 |
| New-onset stroke, n (%) | 0 | 0 | 0 | NA |
| Paraplegia, n (%) | 1 (2.4) | 0 | 1 (4.3) | >0.99 |
| Renal complications, n (%) | 0 | 0 | 0 | NA |
| Aortic-related adverse events, n (%) | 11 (26.8) | 8 (44.4) | 3 (13.0) | 0.036 |
| Rupture, n (%) | 0 | 0 | 0 | |
| Bird-beak phenomenon, n (%) | 12 (29.3) | 10 (55.6) | 2 (8.7) | 0.002 |
| Retrograde type A aortic dissection, n (%) | 1 (2.4) | 0 | 1 (4.3) | >0.99 |
| Ia endoleak, n (%) | 4 (9.8) | 3 (16.7) | 1 (4.3) | 0.303 |
| Stent migration, n (%) | 6 (14.6) | 5 (27.8) | 1 (4.3) | 0.070 |
Group A: PLZ LCCA-LSA ≤10 mm; group B: PLZ LCCA-LSA >10 mm. TEVAR, thoracic endovascular aortic repair; IQR, interquartile range; NA, not applicable; PLZ, proximal landing zone; LCCA, left common carotid artery; LSA, left subclavian artery.
All patients completed the follow-up data collection, and the median follow-up time was 6 months (IQR 3–10 months). Thirty-day outcomes: aortic-related adverse events and the bird-beak phenomenon were observed with an incidence of 2 (4.9%) and 6 (14.6%), respectively. Aortic-related adverse events included 1 (2.4%) retrograde type A aortic dissection, 1 (2.4%) Ia endoleaks. There were no significant differences in aortic-related adverse events, bird-beak phenomenon and re-intervention between groups A and B. Six-month outcomes: aortic-related adverse events and the bird-beak phenomenon were observed with an incidence of 11 (26.8%) and 12 (29.3%), respectively. Aortic-related adverse events included 1 (2.4%) retrograde type A aortic dissection, 4 (9.8%) Ia endoleaks, and 6 (14.6%) stent migrations. There were significant differences in aortic-related adverse events (8/18, 44.4% vs. 3/23, 13.0%, P=0.036) and the bird-beak phenomenon (10/18, 55.6% vs. 2/23, 8.7%, P=0.002) between groups A and B. The detailed follow-up outcomes are presented in Table 2.
Freedom from aortic-related adverse event
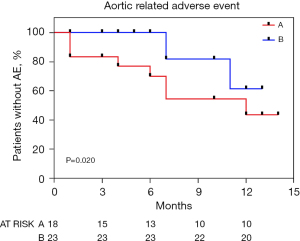
The Kaplan-Meier curve in Figure 4 indicates the rate of aortic-related adverse events for groups A and B. The aortic-related adverse event rate was significantly higher in Group A, with event-free rates of 83.3%, 83.3%, and 72.2% at 1, 3, and 6 months, respectively (P=0.020) (Figure 4).
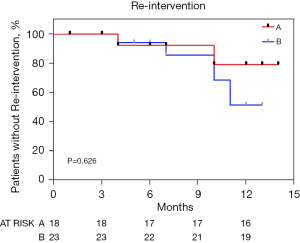
Freedom from re-intervention
The Kaplan-Meier curve in Figure 5 indicates the rate of re-intervention for each group. There were no significant differences in re-intervention rates between groups A and B at event-free rates of 100.0%, 100.0%, and 94.4% in group A and 100.0%, 100.0%, and 95.7% in group B at 1, 3, and 6 months, respectively (P=0.626) (Figure 5).
Risk factors for bird-beak configuration on the DSA or postoperative CTA
During TEVAR, a bird-beak configuration occurred in twelve patients after stent graft release. Univariable and multivariable logistic regression analyses revealed that PLZ 2 length [odds ratio (OR) 0.79, 95% CI: 0.64–0.97; P=0.026] was significantly associated with the occurrence of bird-beak configuration on the first postoperative DSA or CTA. The results are presented in Table 3.
Table 3
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| PLZ diameter (mm) | 1.08 (0.87–1.34) | 0.469 | |||
| PLZ length (mm) | 0.87 (0.73–1.03) | 0.025 | 0.79 (0.64–0.97) | 0.026 | |
| Aortic arch type | 1.95 (0.83–4.54) | 0.120 | |||
| Main body stent graft oversizing rate | 1.01 (0.86–1.15) | 0.988 | |||
| Working position angle (LAO) | 1.04 (0.96–1.12) | 0.320 | |||
OR, odds ratio; CI, confidence interval; PLZ, proximal landing zone; LAO, left anterior oblique position.
Risk factors for aortic-related adverse events
We conducted an analysis of aortic-related adverse events with bird-beak as a risk factor. Univariable and multivariable logistic regression analyses revealed that bird-beak (OR 17.19, 95% CI: 2.24–131.81; P=0.006) was a significant risk factor for aortic-related adverse events. The results are presented in Table 4.
Table 4
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| PLZ diameter (mm) | 1.03 (0.83–1.29) | 0.733 | |||
| PLZ length (mm) | 0.94 (0.81–1.1) | 0.488 | |||
| Aortic arch type | 2.46 (0.99–6.11) | 0.052 | |||
| Main body stent graft oversizing rate | 1.04 (0.90–1.20) | 0.573 | |||
| Bird-beak configuration | 17.33 (3.18–94.29) | 0.001 | 17.19 (2.24–131.81) | 0.006 | |
| Working position angle (LAO) | 1.02 (0.94–1.10) | 0.558 | |||
OR, odds ratio; CI, confidence interval; PLZ, proximal landing zone; LAO, left anterior oblique position.
Discussion
This retrospective cohort study assessed short-term outcomes of TEVAR surgery in TBADs involving the LSA and placed particular emphasis on the bird-beak configuration. The findings indicated that TEVAR reconstruction of the LSA was a safe and effective procedure, even in cases where the PLZ 3 was inadequate, and there were no serious complications. No post-operative deaths, new strokes, renal impairments, or dissection/ruptures were observed. Our data included an analysis of potential risk factors for bird-beak configuration, which we identified as a risk factor for aortic-related complications. The occurrence of bird-beak configuration was relatively common, which is consistent with previous literature reports (19,20). During the follow-up period, some patients experienced bird-beak configuration, Ia endoleak, and stent migration. Significant differences in bird-beak configuration and aortic-related adverse events were observed between groups A and B, defined as distance between the left carotid artery and the LSA ≤10 mm or >10 mm, respectively. Specifically, when PLZ LCCA-LSA was less than 10 mm, bird-beak configuration and aortic-related adverse events were more likely to occur.
The management of the LSA in cases where there is an inadequate PLZ remains a topic of debate (21-24). In order to improve the effectiveness of treating aortic dissection, the SVS and the STS introduced a new classification system in 2020 and 2022. This system provides a standardized nomenclature for describing and reporting aortic dissection (5,6). With the expansion of indications for TEVAR from the descending thoracic aorta to include arch pathologies, the well-known complication of bird-beak configuration has emerged as a new concern. Undoubtedly, previous studies (16,19,25,26) have demonstrated a correlation with the anatomic complexity of the arch, which combines tortuous, angulated, and curved landing zones along with specific hemodynamic conditions. The results of our analysis indicate that a bird-beak phenomenon was observed in 29.3% of cases of TEVAR with LSA revascularization. We also found a significant correlation between the PLZ LCCA-LSA and the occurrence of bird-beak configuration. Specifically, as the length of PLZ LCCA-LSA increases, the risk of bird-beak occurrence decreases. However, due to the limited number of common stent front-end specifications (5, 10, 15, and 20 mm) that cannot completely match the shape of the aortic arch, the risk of bird-beak configuration still exists.
Although we were unable to conduct further subgroup analysis on the length, angle, and classification of the aortic arch in relation to bird-beak configuration, our findings suggest that TEVAR surgery requires careful preoperative planning and intraoperative caution to minimize the risk of complications.
The presence of bird-beak configuration following TEVAR for an aortic arch pathologic condition is significantly associated with an increased risk of endoleak formation (14,15). In TEVAR, bird-beak configuration is more commonly observed in cases of aortic dissection involving the aortic arch, thereby increasing the risk of endoleak formation. To prevent type Ia endoleak and bird-beak configuration in landing zone 2, appropriate stent grafts must be carefully selected. Therefore, bird-beak configuration was analyzed as a risk factor in the analysis of aortic-related adverse events. After adjusting for confounders, bird-beak configuration remained an independent risk factor for aortic-related adverse events in the multifactorial analysis. Our findings emphasize the clinical significance of bird-beak configuration following TEVAR with LSA revascularization and its association with adverse clinical events. Detection of bird-beak configuration can aid in predicting adverse clinical events after TEVAR.
In some cases, Ia endoleak and stent displacement may occur simultaneously, highlighting the importance of ensuring an adequate landing zone involving the aortic arch. In patients with LSA revascularization, a longer landing zone 2 may be required to avoid Ia endoleak and stent displacement due to the pulling effect of the branch stent on the landing area. The indications for TEVAR in patients with a PLZ 2 length of less than 10 mm should be carefully considered, weighing the risks and benefits.
There are several important considerations to keep in mind when interpreting the results of this single-center retrospective study. Firstly, our cohort only includes patients who underwent interventional therapy for aortic diseases, and therefore, patients who underwent surgery or hybrid surgery were not included. This may lead to selection bias in our results. Additionally, the limited number of cases may lead to biased results. The range of 95% CI is too wide, which may be related to the small sample size and few adverse events. This also led to a certain decrease in the certainty of our results.
Despite these limitations, our study contributes to the understanding of the impact of LSA revascularization on overall survival and the need for intervention. However, prospective research on the long-term prognosis and response to treatment is necessary to better select indications for interventional therapy.
Conclusions
This study demonstrated that TEVAR with LSA revascularization resulted in satisfactory short-term outcomes and safety in cases of TBADs with involvement of the LSA. However, it is important to note that a PLZ LCCA-LSA length of less than 10 mm may increase the risk of aortic adverse events and should be carefully considered when weighing the risks and benefits of the procedure.
Acknowledgments
We would like to thank the American Journal Experts (AJE) for their help in polishing the paper.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-379/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-379/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-379/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-379/coif). P.S. serves as the Editor-in-Chief of Cardiovascular Diagnosis and Therapy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Beijing Anzhen Hospital (No. 2023208X) with a waiver for informed consent due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J 2018;39:739-749d. [Crossref] [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Mussa FF, Horton JD, Moridzadeh R, et al. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016;316:754-63. [Crossref] [PubMed]
- MacGillivray TE, Gleason TG, Patel HJ, et al. The Society of Thoracic Surgeons/American Association for Thoracic Surgery clinical practice guidelines on the management of type B aortic dissection. J Thorac Cardiovasc Surg 2022;163:1231-49. [Crossref] [PubMed]
- Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71:723-47. [Crossref] [PubMed]
- Boufi M, Alexandru G, Tarzi M, et al. Systematic Review and Meta-Analysis of Ex-Situ and In-Situ Fenestrated Stent-Grafts for Endovascular Repair of Aortic Arch Pathologies. J Endovasc Ther 2023; Epub ahead of print. [Crossref] [PubMed]
- Dake MD. Endovascular stent-graft management of thoracic aortic diseases. Eur J Radiol 2001;39:42-9. [Crossref] [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Xie W, Xue Y, Li S, et al. Left subclavian artery revascularization in thoracic endovascular aortic repair: single center's clinical experiences from 171 patients. J Cardiothorac Surg 2021;16:207. [Crossref] [PubMed]
- Jing Z, Lu Q, Feng J, et al. Endovascular Repair of Aortic Dissection Involving the Left Subclavian Artery by Castor Stent Graft: A Multicentre Prospective Trial. Eur J Vasc Endovasc Surg 2020;60:854-61. [Crossref] [PubMed]
- Chang H, Jin D, Wang Y, et al. Chimney Technique and Single-Branched Stent Graft for the Left Subclavian Artery Preservation During Zone 2 Thoracic Endovascular Aortic Repair for Type B Acute Aortic Syndromes. J Endovasc Ther 2023;30:849-58. [Crossref] [PubMed]
- Fang C, Wang C, Liu K, et al. Early Outcomes of Left Subclavian Artery Revascularization Using Castor Single-Branched Stent-Graft in the Treatment of Type B Aortic Dissection or Intramural Hematoma. Ann Thorac Cardiovasc Surg 2021;27:251-9. [Crossref] [PubMed]
- Ueda T, Fleischmann D, Dake MD, et al. Incomplete endograft apposition to the aortic arch: bird-beak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. Radiology 2010;255:645-52. [Crossref] [PubMed]
- Marrocco-Trischitta MM, Spampinato B, Mazzeo G, et al. Impact of the Bird-Beak Configuration on Postoperative Outcome After Thoracic Endovascular Aortic Repair: A Meta-analysis. J Endovasc Ther 2019;26:771-8. [Crossref] [PubMed]
- Boufi M, Guivier-Curien C, Deplano V, et al. Risk Factor Analysis of Bird Beak Occurrence after Thoracic Endovascular Aortic Repair. Eur J Vasc Endovasc Surg 2015;50:37-43. [Crossref] [PubMed]
- Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 2010;52:1022-33, 1033.e15.
- Casserly IP, Sachar R, Yadav JS. Manual of Peripheral Vascular Intervention. Philadelphia: Lippincott Williams and Wilkins; 2005.
- Kudo T, Kuratani T, Shimamura K, et al. Type 1a endoleak following Zone 1 and Zone 2 thoracic endovascular aortic repair: effect of bird-beak configuration. Eur J Cardiothorac Surg 2017;52:718-24. [Crossref] [PubMed]
- Kudo T, Kuratani T, Shimamura K, et al. Determining the Optimal Proximal Landing Zone for TEVAR in the Aortic Arch: Comparing the Occurrence of the Bird-Beak Phenomenon in Zone 0 vs Zones 1 and 2. J Endovasc Ther 2020;27:368-76. [Crossref] [PubMed]
- Zhao Z, Qin J, Yin M, et al. In Situ Laser Stent Graft Fenestration of the Left Subclavian Artery during Thoracic Endovascular Repair of Type B Aortic Dissection with Limited Proximal Landing Zones: 5-Year Outcomes. J Vasc Interv Radiol 2020;31:1321-7. [Crossref] [PubMed]
- Melissano G, Civilini E, Bertoglio L, et al. Results of endografting of the aortic arch in different landing zones. Eur J Vasc Endovasc Surg 2007;33:561-6. [Crossref] [PubMed]
- Liu D, Luo H, Lin S, et al. Comparison of the efficacy and safety of thoracic endovascular aortic repair with open surgical repair and optimal medical therapy for acute type B aortic dissection: A systematic review and meta-analysis. Int J Surg 2020;83:53-61. [Crossref] [PubMed]
- Liu J, Yang F, Chen L, et al. Management and Outcomes of Non-A Non-B Aortic Dissection. Eur J Vasc Endovasc Surg 2022;64:497-506. [Crossref] [PubMed]
- Cao L, Ge Y, He Y, et al. Association between aortic arch angulation and bird-beak configuration after thoracic aortic stent graft repair of type B aortic dissection. Interact Cardiovasc Thorac Surg 2020;31:688-96. [Crossref] [PubMed]
- Torsello GF, Argyriou A, Stavroulakis K, et al. One-Year Results From the SURPASS Observational Registry of the CTAG Stent-Graft With the Active Control System. J Endovasc Ther 2020;27:421-7. [Crossref] [PubMed]


