Surgical experience on chronic constrictive pericarditis in African setting: review of 35 years’ experience in Cote d’Ivoire
Introduction
Chronic constrictive pericarditis (CCP) is the end stage of a chronic inflammatory process that produces a fibrous, thick, constrictive pericardium surrounding the heart with a limitation of diastolic ventricular filling (1). Basic pathophysiology of CCP is yet controversial (2); but it seems that the most important factor to be considered in its pathogenesis is the heart constriction and the limitation of the diastolic distensibility of the two ventricles in association with an inability to generate an adequate preload (2). The etiology in most of the cases is difficult to establish (1); it is even unknown in many patients. Nevertheless, in Africa, tuberculosis is the common cause (3,4) while in western countries (5,6) the main etiologies are: idiopathic, post-surgical, radiation injury. In case of CCP, clinical presentation is not specific making diagnosis not always easy: CCP is often confused with hepatic cirrhosis or furthermore restrictive cardiomyopathy and finally with endomyocardial fibrosis (1). In this context, cardiac catheterization still be an essential technique for accurate diagnosis. When the diagnostic is made, the most effective therapy is surgery. Classically, a pericardiectomy is indicated; surgical approach is not unique, the extent of pericardial resection changes according to teams and the surgical risk factors are variously appreciated as well (2). In literature, only a few publications and data on CCP treatment and results in Africa (7,8) are listed versus a large number of CCP articles from developed countries (9,10).Therefore, for filling the gap, the aim of this study is to report our experience, one of the largest surgical experience with CCP in Sub-Saharan Africa in terms of clinical and surgical outcomes and risk factors of early death after pericardiectomy.
Patients and methods
Between 1977 and 2012, 120 cases were recorded. There were 72 men and 48 women; average age was 28.8 years plus or minus 10.4 years standard deviation (SD) (extremes 8–51 years). Etiology was tuberculosis (119 cases) and bacterial infection (1 case). Diagnosis of tuberculosis was based on history, on close contact with tuberculosis, on pericardial calcifications at chest radiography and pathological findings. Average duration of the disease was 30 months (3 months–25 years). Clinical characteristics are in Table 1. Clinical feature of systemic venous congestion or a diastolic syndrome was observed in all the cases. Systemic venous congestion symptoms were as follows: dyspnea, hepatomegaly, raised jugular venous pressure or jugular venous distension, hepatojugular reflex, ascite, peripheral edema. Chest radiograph shown an average cardiothoracic ratio (CTR) of 0.55 (0.45–0.70) and pericardial calcifications (n=63, 52.5%) without pleural extravasations (n=99, 82.5%).

Full table
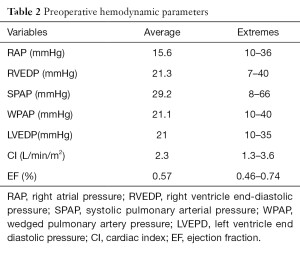
Electrocardiogram demonstrated a low voltage QRS complex (n=99, 82.5%), arrhythmias such as atrial fibrillation (n=55, 46%), T waves inverted (n=105, 87.5%), conduction disturbances: first degree Atrio-Ventricular block (3 cases) or right bundle branch block (6 cases). Echocardiography shown thick pericardial layers (100% of cases) with calcifications and was specific in diagnosing CP in 80 cases (66%). Cardiac catheterization confirmed a dip-and-plateau(square root sign), an equalization of end- diastolic pressures in right and/or left cardiac chambers ranged between 10 and 40 mmHg, a mean cardiac index (CI): 2.3 L/min/m2 (extremes: 1.3–3.6) (Table 2). The constriction was limited to the right cardiac cavities called right constriction (n=54, 45%) or to the right and left cardiac cavities called bilateral constriction (n=66, 55%).
Full table
Hemodynamic parameters and cineangiograms confirmed the diagnosis of pericardial constriction in all the patients. In five cases, there was an associated mitral or mitro-tricuspid regurgitation.
All the patients underwent pericardiectomy; 117 cases (97.5%) without cardiopulmonary bypass (CPB) and 3 cases (2.5%) with CPB. A total pericardiectomy was performed through a median sternotomy (97.5%, n=117) or a left antero-lateral thoracotomy (2.5%, n=3). Pericardial resection including the epicardium was performed in all patients; it was an “epicardo-pericardiectomy”. Pericardial resection started anteriorly from the left ventricle, then to the right ventricle, at last to the right and left atria. Pericardial resection was carried out from a phrenic nerve to the other; it was also carried superiorly on the ascending aorta and the pulmonary trunk, the diaphragmatic and inferior surfaces of the two ventricles. If there was no risk of hemorrhage, we used to liberate the intra-pericardial portions of the superior and inferior vena cava and the pulmonary veins. When calcifications were tightly adhesive to the myocardium, islands of pericardial calcareous layers were left in place in order to avoid heart laceration. Surgery revealed sub-acute CCP (n=12; 10%), fibrous CCP (n=36; 30%) and calcified CCP (n=72; 60%). Caseous material into the pericardial cavity had been also observed intra-operatively (n=12; 10%). After surgery, a definite diagnosis of tuberculous pericarditis had been done based on pericardium histological section in 48 patients. For statistical analysis: data were expressed as means; the Anova test or the Mann-Whitney test were used for the quantitative variables and the Fisher Exact test for the qualitative variables. A P value <0.05 was considered statistically significant.
Results
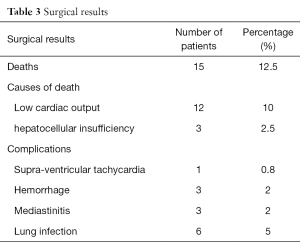
Hospital mortality was 12.5% (n=15). Causes of early deaths included low cardiac output (n=12) and hepato-cellular insufficiency due to a cirrhosis (n=3) (Table 3).
Full table
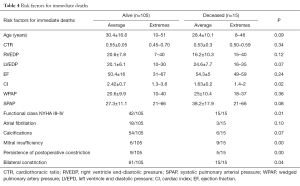
Using univariate statistical analysis (Table 4) significant risk factors for early mortality included functional class III or IV [New York Heart Association (NYHA)] (P=0.01), associated mitral insufficiency (P<0.05), low CI (P=0.02), and persistence of systemic venous congestion symptoms after surgery (P<0.05).
Full table
Early post-operative complications included transient supra-ventricular tachycardia (1 case), hemorrhage controlled by a reoperation (3 cases), mediastinitis (3 cases) and lung infection (6 cases).
Among the 105 patients who survived, 83 were followed-up from 1 month to 10 years (mean 4 years) and 22 were lost to follow-up. No late death was recorded. All the patients were at the functional class I or II (NYHA) with no systemic venous congestion clinical signs. Thirty patients underwent a cardiac catheterization late postoperatively; we observed a significant reduction even a normalization of the right and/or the left ventricular diastolic pressures (Table 5) and a disappearance of the dip-and-plateau after pericardiectomy.

Full table
Discussion
As limitation of this work, we should mention that it is a retrospective study.
The majority of acute pericarditis in tropical countries is attributable to tuberculosis (4) and complications include early or late pericardial constriction, which occurs between a few months and several years (2).
Because of the acquired immune deficiency syndrome (AIDS), an increase in prevalence of pericarditis, and specifically tuberculous pericarditis, has been observed in Africa (4,11). However, only two cases of the combination tuberculous CP and AIDS were seen in our study. In the opposite side, etiological pattern in developed world seems different: it was either idiophatic, secondary to mediastinal radiation for malignant diseases; post-cardiac surgery, or related to systemic diseases as mentioned in most of the recent series (5,6,12) with a significant impact on postoperative prognosis (9).
The commonest symptoms described in the literature (13,14) with a frequency of 90% to 98% are similar to the ones we found: hepatomegaly, raised jugular venous pressure. Ascites and peripheral edemas are less frequent (50% to 80%) (13).
Right ventricular failure coexisting with small or normal radiological heart size may suggest pericardial constriction in 50% of cases according to Galey (15). According to the same author cardiomegaly cannot exclude a pericardial constriction as we had observed in 60% of cases (n=72). In our study X-ray cardiomegaly was related either to caseous material surrounding the heart or secondary to associated valvular lesions.
Low voltage QRS complex and negative T waves in all electrographical derivations were highly suggestive of a possible pericardial constriction in our population.
Two-dimensional echocardiography was not 100% specific in our experience, and cardiac catheterization was performed in all cases. It provided to us definitive confirmation of the diagnosis of CCP. In their study, McCaughan et al. (14) demonstrated in all their cases of CCP an increase and an equalization of the right and left atrial and ventricular diastolic pressures with a characteristic dip-and-plateau (2).
Cardiac catheterization and angiocardiography enabled us to formally eliminate a right or a bilateral endomyocardial fibrosis, a restrictive myocardiopathy and to evaluate the severity of coexisting valvular lesions.
Neither Computed Tomography, nor Magnetic Resonance Imaging was used; these imaging techniques could have allowed us to appreciate the pericardial thickness and the importance of the constriction (1,2).
In our experience, hepatic cirrhosis has become an absolute contraindication for surgery; we had three deaths due to a hepatic cirrhosis with hepato-cellular insufficiency after a satisfactory pericardiectomy.
Several surgical approaches are suggested: left antero-lateral thoracotomy, bilateral anterior thoracotomy and median sternotomy (1,2,13,14).
Left antero-lateral thoracotomy allows a better exposure and liberation of the left ventricle in its anterior, lateral and inferior aspects; but limits access to the right atria, of the superior and inferior vena cava.
Bilateral anterior thoracotomy although allowing a good visualization of the two ventricles, but is less well tolerated than the left anterior thoracotomy or the median sternotomy.
Median Sternotomy is performed more rapidly; it provides an easy access to the right cavities and to the left ventricle; it allows extended pericardial decortication; it is furthermore indicated in cases of massive pericardial calcifications; it allows easy use of CPB if necessary. According to Mavitas (13), after a median sternotomy, the post-operative pain is decreased and the duration of hospitalization shorter.
The extension of the decortication remains controversial (16). A pericardial decortication is incomplete or partial when the two ventricles are not completely decorticated. It is total and complete when the two ventricles including lateral and diaphragmatic surfaces are decorticated (9). Radical decortications address all the surfaces of the heart including the atria, the vena cava, the pulmonary veins and the intrapericardial portions of the great vessels. The radical approach is not necessary, and may increase complications (14).
In severe cases with calcifications and/or dense myo-pericardial adhesions, we recommend a restriction of the decortication to the ventricles and careful liberation of the atria, vena cava and pulmonary veins. This approach is recommended if there is an absence of hemorrhagic risk, if a good cleavage planes for dissection exists, if the texture of the cardiac structures is not excessively thin and friable. In contrast in patients with an obvious increased hemorrhagic risk we leave calcified pericardial layers in place.
All the pericardial layers are addressed by the decortication. In calcified CCP, the epicardium was always very thick and highly adhesive to the myocardium, making it difficult to visualize a correct plane of dissection and increases bleeding risk during dissection between the myocardium and the epicardium. In these cases it is recommended to use the technique of “patchwork’’ sectioning of the visceral pericardium (17) or the one described by Faggian et al. (18). In our study, calcifications were always associated with a thick and very hemorrhagic epicarditis making the correct plane of dissection difficult to find.
The rate of hospital mortality of 12.5% in our study was moderately high. Ling et al. (9) report 6% of hospital deaths in 132 operated CP cases. Other authors have reported similar results (10,12,18-22); Merle et al. (3) in their short series of four patients mentioned one early death. The most frequent cause of early death was a low cardiac output (1,13), which corresponds to our observation.
Seifert et al. (19) demonstrated a correlation between the functional class and operative mortality; and identified a relationship between end-diastolic pressure of the right ventricle end-diastolic pressure (RVEDP) and risk of death after surgery. Contrary to Seifert et al. (19) and McCaughan et al. (14), increase of RVEDP was not always a significant risk factor; we did not find any significant difference between the patients who survived: RVEDP average 20.6±7.8 mmHg and those who died postoperatively: RVEDP average 16.2±10.3 mmHg.
According to McCaughan’s observation (14), opposite to ours, parameters such as the increase of right or left atrial pressure and increase of the pulmonary blood pressure had significantly influence on surgery risk. Also contrary to our study, calcifications or atrial fibrillation did not seem to be significant risk factors for death. Contrary to the same author, in our viewpoint, the CI could be a risk factor (P=0.02).
Concerning functional status, the immediate and late results after pericardiectomy were satisfactory (2,9,14,18), and similar to ours.
Conclusions
Based on our experience, CCP surgery can be performed safely with an acceptable hospital mortality and a significant improvement of patients’ functional status at long term after surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics committee of Bouake Teaching Hospital (No. 1/2015). All authors were contacted for this study to get oral consent to be part of it.
References
- Paul EA, Hassan N. The Pericardium. In: Sabiston DC. Surgery of the Chest. 5th edition. Philadelphia: Saunders, 1990:1230-12492.
- Kirklin J, Barrat-Boyes B. Pericardial Disease. In: Cardiac Surgery. 3rd Edition. Philadelphia: Elsevier (USA): 2003:1779-95.
- Merle H, Angate A, Delormas P, et al. La Chirurgie Thoracique en Cote d’Ivoire. Afrique Medicale 1961;1:25-33.
- Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005;112:3608-16. [Crossref] [PubMed]
- Little WC, Freeman GL. Pericardial Disease. Circulation 2006;113:1622-32. [Crossref] [PubMed]
- George TJ, Arnaoutakis GJ, Beaty CA, et al. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg 2012;94:445-51. [Crossref] [PubMed]
- Adebo OA, Adebonojo SA, Osinowo O, et al. Chronic constrictive pericarditis: hemodynamic changes following pericardiectomy. J Natl Med Assoc 1980;72:461-6. [PubMed]
- Tettey M, Sereboe L, Aniteye E, et al. Surgical Management of Constrictive Pericarditis. Ghana Med J 2007;41:190-3. [PubMed]
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999;100:1380-6. [Crossref] [PubMed]
- Ghavidel AA, Gholampour M, Kyavar M, et al. Constrictive pericarditis treated by surgery. Tex Heart Inst J 2012;39:199-205. [PubMed]
- Mutyaba AK, Balkaran S, Cloete R, et al. Constrictive pericarditis requiring pericardiectomy at Groote Schuur Hospital, Cape Town, South Africa: causes and perioperative outcomes in the HIV era (1990-2012). J Thorac Cardiovasc Surg 2014;148:3058-65.e1. [Crossref] [PubMed]
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004;43:1445-52. [Crossref] [PubMed]
- Mavitas B, Yamak B, Karircioglu F, et al. Ten Years Experience with pericardiectomy. Asian Cardiovascular and Thoracic Annals 1996;4:222-5. [Crossref]
- McCaughan BC, Schaff HV, Piehler JM, et al. Early and late results of pericardiectomy for constrictive pericarditis. J Thorac Cardiovasc Surg 1985;89:340-50. [PubMed]
- Galey JJ, Vanetti A. Long term follow-up of chronic constrictive pericarditis operated on. Ann Intern Med 1973;124:699-703.
- Arsan S, Mercan S, Sarigül A, et al. Long-term experience with pericardiectomy: analysis of 105 consecutive patients. Thorac Cardiovasc Surg 1994;42:340-4. [Crossref] [PubMed]
- Nataf P, Cacou BP, Dorent R, et al. A retrospective study of a series of 84 patients. Arch Mal Coeur Vaiss 1994;87:241-5. [PubMed]
- Faggian G, Mazzucco A, Tursi V, et al. Constrictive epicarditis after open heart surgery: the turtle cage operation. J Card Surg 1990;5:318-20. [Crossref] [PubMed]
- Seifert FC, Miller DC, Oesterle SN, et al. Surgical treatment of constrictive pericarditis: analysis of outcome and diagnostic error. Circulation 1985;72:II264-73. [PubMed]
- Cinar B, Enç Y, Göksel O, et al. Chronic constrictive tuberculous pericarditis: risk factors and outcome of pericardiectomy. Int J Tuberc Lung Dis 2006;10:701-6. [PubMed]
- Biçer M, Özdemir B, Kan İ, et al. Long-term outcomes of pericardiectomy for constrictive pericarditis. J Cardiothorac Surg 2015;10:177. [Crossref] [PubMed]
- Lin X, Xu RY, Liu JZ, et al. Effect of Right Heart Systolic Function on Outcomes in Patients with Constrictive Pericarditis Undergoing Pericardiectomy. Chin Med J (Engl) 2016;129:154-61. [Crossref] [PubMed]


