Antioxidant therapies for the management of atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice, representing a major public health problem. Accumulating evidence suggests oxidative stress may play an important role in the pathogenesis and perpetuation of AF (1-5). There are several redox signaling pathways that are possibly related to increased oxidative stress in the setting of AF, including mitochondrial DNA damage, increased activity of enzymes such as NADPH oxidase and xanthine oxidase, nitric oxide synthase uncoupling, activation of pro-arrhythmic transcription factors such as peroxi-some proliferator-activated receptor, c-fos and NF-κB. In the past few years experimental data and clinical evidence have tested the concept of antioxidant interventions to prevent AF. Besides statins, ACE-inhibitors (ACEIs) and/or angiotensin-receptor blockers (ARBs), and omega-3 polyunsaturated fatty acids, several other interventions with antioxidant properties, such as Vitamin C and E, thiazolidinediones, N-acetylcysteine, probucol, nitric oxide donors or precursors, NADPH oxidase inhibitors, Xanthine oxidase inhibitors have emerged as novel strategies in the prevention and treatment of AF (6-10). In this review, we aim to summarize recent evidence regarding antioxidant therapies in the prevention and treatment of atrial fibrillation.
Vitamin C and E
Recently, antioxidant vitamins C and E have been tested in the prevention of AF, especially postoperative AF (POAF) (11-14). These dietary vitamin supplementations have been proven to protect against the development and progression of AF in experimental models (15,16). Vitamin C is a potent water-soluble antioxidant that protects against oxidative stress derived by reactive oxygen and nitrogen species. In a canine model of AF, Carnes et al. (15) were the first to demonstrate that ascorbate attenuates the pacing-induced atrial remodeling and atrial peroxynitrite production. However, Shiroshita-Takeshita (16) and colleagues did not confirm the protective effects of Vitamin C and E against AF in their study. Additionaly, tachypacing-induced atrial effective refractory period shortening and AF promotion were not influenced by antioxidant vitamins, whereas simvastatin attenuated atrial remodeling and prevented AF. Recently, Lin et al. (17) investigated whether Vitamin C has direct electrophysiological effects on isolated rabbit pulmonary vein (PV) preparations. They demonstrated that ascorbic acid decreases PV spontaneous activity and attenuates the arrhythmogenic effects of hydrogen peroxide (H2O2). Given that PVs represent major sources of ectopic beats that trigger paroxysmal AF, the potential preventive effects of vitamin C on AF recurrence after PV isolation should be tested in future clinical trials.
The clinical evidence regarding Vitamin C and E on AF prophylaxis is mainly limited in the setting of POAF prevention. In a retrospective observational study, Carnes et al. (15) evaluated the effects of supplemental ascorbate on POAF prevention. A series of 43 consecutive patients scheduled for coronary artery bypass graft (CABG) surgery were given 2 g ascorbic acid the night before surgery, followed by 500 mg doses twice daily for the 5 days following CABG. Patients receiving ascorbate had a 16.3% incidence of POAF, in contrast to 34.9% in the control group (P=0.048). However, multivariate analysis after adjusting for other confounding factors demonstrated that β-blockers use exhibits the most protective effect (84% risk reduction, P=0.007) and ascorbate alone was not an independent protector for POAF. In particular, the two groups were not ideally matched regarding all risk factors for AF, and the incidence of diabetes, hypertension, and previous history of AF was higher in the control group compared to the treatment group. Finally, this study may not have enough power to evaluate POAF incidence.
Eslami et al. (18) examined the effects of ascorbic acid as an adjunct to β-blockers in a prospective, randomized trial. One hundred patients undergoing CABG surgery were randomized to the ascorbic acid or to the control group. All patients had been treated with β-blockers for at least for one week. Patients in the ascorbic acid group received 2 g of ascorbic acid on the night before the surgery and 1 g twice daily for 5 days following surgery. Patients in the control group did not receive ascorbic acid. Patients in both groups continued to receive β-blockers postoperatively. The incidence of POAF was 4% in the Vitamin C group and 26% in the control group (P=0.002). The authors concluded that ascorbic acid can be prescribed as an adjunctive therapy to β-blockers for the prophylaxis of POAF. Finally, Papoulidis et al. (19) evaluated the preventive effects of Vitamin C on POAF incidence in 170 patients undergoing isolated on-pump CABG. Importantly, all the patients were under β-blockers therapy preoperatively. The incidence of POAF was 44.7% in the Vitamin C group and 61.2% in the control group (P=0.041). Notably, patients with Vitmain C had a shorter hospital stay as well as conversion time from AF into sinus rhythm.
Very recently, in another randomized clinical trial (RCT) (20) which enrolled 152 patients scheduled for cardiac surgery, the combination of vitamin C (1 g/d) plus vitamin E (400 IU/d) reduced the risk of POAF in patients aged over 60 years indicating that the efficacy of the antioxidant interventions may be improved with aging. In a recent meta-analysis including five randomized controlled trials with 567 patients, Harling et al. (13) showed that the prophylactic use of vitamins C and E significantly reduced the incidence of POAF (OR: 0.43, 95% CI: 0.21 to 0.89) as well as the all-cause arrhythmia (OR: 0.54, 95% CI: 0.29 to 0.99) following cardiac surgery. However, the overall quality of enrolled studies was relatively poor. Undoubtedly, further well-designed studies with enough sample size are warranted in order to clarify this issue.
The clinical evidence relating to the potential role of antioxidant vitamins for secondary prevention of AF is sparse. Korantzopoulos et al. (21) prospectively studied 44 patients following successful electrical cardioversion of persistent AF. The patients randomized into Vitamin C group and control group. Within one week, AF recurred in 4.5% of patients in the vitamin C group and in 36.3% of patients in the control group (P=0.024). Moreover, inflammatory biomarkers decreased after cardioversion in patients receiving vitamin C. Another recently published study evaluated whether serum Vitamin E level was related to AF recurrence in patients undergoing electrical cardioversion (EC) (22). One hundred forty four consecutive patients who underwent successful EC were prospectively enrolled and followed for 3 months. It was indicated that low serum Vitamin E level is an independent predictor for the AF recurrence. Further studies are needed in order to examine the efficacy of antioxidant vitamin E in AF prevention.
Thiazolidinediones
Thiazolidinediones (TZDs) represent a class of insulin-sensiting agents with peroxisome proliferator-activated receptor-γ (PPAR-γ) activation effects, used to improve insulin resistance in patients with type 2 diabetes (23,24). Troglitazone, the first drug developed and used clinically, has been withdrawn from the market due to its liver toxicity. Pioglitazone and rosiglitazone are the only compounds that are available for clinical use now. Apart from their insulin-sensitizing effects, TZDs have several pleiotropic properties including anti-inflammatory and antioxidant (25,26). It has been demonstrated that PPAR-γ ligands inhibit the expression of inducible nitric oxide synthase (iNOS) and peroxynitrite production in mesangial cells and in cerebellar granule cells (27). Also, TZDs enhance endothelial nitric oxide (NO) bioavailability, reducing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent superoxide production, while they induce antioxidant enzymes such as Cu/Zn superoxide dismutase (Cu/Zn SOD) (28).
Recent experimental evidence indicates that TZDs, especially pioglitazone, prevent atrial electrical and structural remodeling through their anti-inflammatory and antioxidant properties. In a rabbit model of congestive heart failure, pioglitazone attenuated atrial structural remodeling and inhibited AF promotion, at the same degree as candesartan. Furthermore, the PPAR-γ activator suppressed transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α) and extracellular signal-regulated kinase (ERK) expression in atrial tissue. Therefore, the authors proposed that pioglitazone may inhibit AF by modulating inflammatory, oxidative stress, and hypertrophic signaling pathways involved in atrial remodeling (29). Very recently, Kume et al. showed that pioglitazone reduced inflammatory atrial fibrosis and vulnerability to AF in a pressure overload rat model of AF, possibly via the suppression of MCP-1-mediated inflammatory profibrotic processes (30). In an in vivo rat model, Xu et al. (31) reported pioglitazone inhibited age-related atrial structural remodeling and AF susceptibility via its antioxidant and anti-apoptotic effects. Gene and protein expression levels of antioxidant molecules such as Mn superoxide dismutase (MnSOD) and heat shock protein (HSP) 70 were significantly enhanced, whereas NADPH oxidase subunits p22phox and gp91phox were significantly reduced in aged rats treated with pioglitazone. Therefore, activation of antioxidant molecules and inhibition of NADPH-derived ROS production may be the mechanisms underlying the favorable effects of TZDs on aging-related atrial remodeling and AF promotion. However, experimental data on the effects rosiglitazone on atrial remodeling in the setting of diabetes is lacking. We have shown that rosiglitazone attenuates atrial structural remodeling reducing the interatrial activation time and the atrial interstitial fibrosis in alloxan-induced diabetic rabbits (31). Also, rosiglitazone treatment increased plasma antioxidant enzyme superoxide dismutase (SOD) activity and decreased oxidant stress and inflammatory markers including malondialdehyde (MDA), C-reactive protein (CRP), and TNF-α levels (32).
We have previously described two patients with diabetes who experienced a remarkable improvement in their paroxysmal AF episodes following treatment with rosiglitazone (33). However, two large RCTs, namely RECORD (34) and PROactive (35) which enrolled high-risk patients with type 2 diabetes failed to demonstrate a significant reduction of AF risk from TZDs compared with controls. The potential explanations were: firstly, AF was not a predefined endpoint and reported as an adverse event; secondly, there was a very low AF incidence in both treatment and control groups (1.5-2%). Also, another case-control study showed that pre-operative use of TZDs in diabetic patients undergoing cardiac surgery was associated with a non-statistically significant 20% reduction of POAF (36). In a prospective cohort study including 150 diabetic patients undergoing catheter ablation for AF, Gu et al. showed that previous pioglitazone use was independently associated with a lower recurrence of atrial tachyarrhythmias during a follow-up period of 23 months (37). Interestingly, in a recent observational study Chao et al. (38) investigated the possible association between TZDs use and development of new-onset AF in 12,605 patients with Type 2 diabetes. During a follow-up of 5 years, TZDs decreased the risk of new-onset AF by 31% after adjustment for age, underlying diseases and baseline medications. Although growing evidence suggests TZDs use prevents the development and recurrence of AF in diabetic patients, the cardiovascular safety considerations on rosiglitazone recently prompted the European Medicines Agency (EMA) to suspended this drug from the European market and patients taking rosiglitazone were advised to discuss alternative options with their physicians (39). Since November 18, 2011 the FDA does not allow rosiglitazone to be sold without a prescription from certified doctors. Patients are required to be informed of the risks associated with the use of rosiglitazone. Therefore, it is very hard for rosiglitazone to gain a therapeutic indication for AF in the future (40). Given the favorable cardiovascular effects of pioglitazone from a recent meta-analysis of 19 RCTs (including PROactive study) enrolling 16,390 patients (41), further large-scale randomized, controlled trials with long-term follow-up or a post hoc analysis from previous trials are still needed to evaluate the potential role of pioglitazone, as an upstream therapy for AF prevention in patients with diabetes (42,43).
N-acetylcysteine
N-acetylcysteine (NAC) is a precursor of L-cysteine and glutathione. As a source of sulfhydryl groups in cells, it may act as a scavenger of free radicals (44). In clinical practice, NAC is used as an antioxidant, mucolytic agent widely prescribed in chronic pulmonary disease. Carnes et al. (45) showed that atrial myocytes from AF patients incubated with NAC lead to a significant increase in the density of ICa,L. This observation suggests NAC may possibly attenuates atrial electrophysiological remodeling caused by rapid atrial activation. In a randomized study including 115 patients undergoing CABG or valve surgery, Ozaydin et al. (46) demonstrated that NAC markedly reduces the incidence of POAF lasting more than 5 minutes. A previous meta-analysis (47) evaluated potential beneficial effects of perioperative NAC on the prevention of complications after cardiothoracic surgery. In the sub-group analysis of six trials which reported POAF as study endpoints, the use of NAC significantly lowered the risk of developing POAF by 36%. In a more recent meta-analysis, Gu et al. (48) included 8 randomized trials incorporating 578 patients, and indicated that NAC significantly reduces the incidence of POAF by 38% (OR: 0.62, 95% CI: 0.41-0.93; P=0.021) compared with controls. It is worth mentioning that only one trial (46) included in this meta-analysis had specified POAF as a primary endpoint. The remaining seven studies reported POAF as a secondary endpoint. Therefore, future large-scale randomized studies with an adequate power are urgently in demand.
Probucol
Probucol is a lipid-lowering agent with potent anti-oxidant and anti-inflammatory effects. It has been used in clinical practice during the past two decades to decrease atherosclerosis and prevent restenosiss following stent implantation. However, the potential side effects including decreasing serum high-density lipoprotein cholesterol level and QT prolongation have limited the probucol’s worldwide clinical use (49,50). As a potent antioxidant, it may reduce the production of oxygen free radicals and act as a direct superoxide anion scavenger. In an isolated perfused rat model, probucol increased the expression of an important myocardial antioxidant enzyme, namely glutathione peroxidase, and prevented lipid peroxidation following ischemia reperfusion injury (51). Moreover, probucol inhibited NADPH oxidase activity in the aorta from cholesterol-fed rabbits (52). Our previous studies suggested that prophylactic treatment with probucol during the periprocedural period in patients undergoing coronary intervention protects against contrast-induced acute kidney injury (53,54). However, experimental studies regarding the possible benefits of probucol on atrial remodeling and AF prevention are lacking. Gong et al. showed that probucol attenuates atrial nerve sprouting and heterogeneous sympathetic hyperinnervation induced by rapid right atrial pacing, and markedly reduces the promotion and maintenance of AF. Also, probucol significantly reduces atrial oxidative stress and increases total antioxidant capacity (55). A further study from this group suggested that probucol attenuates structural remodeling and preventes atrial apoptosis, decreasing the left atrial MDA content in paced dogs (56). Very recently, we investigated the effects of probucol on atrial structural and electrical remodeling in alloxan-induced diabetic rabbits (57). After treatment for 8 weeks, the diabetic rabbits on probucol exhibited alleviation of oxidative stress displayed as decreased plasma MDA and increased plasma SOD levels compared with diabetic controls, while probucol significantly reduced left atrial interstitial fibrosis and AF inducibility (57). Therefore, it seems reasonable to speculate that the antioxidant effects of probucol may favorably affect atrial autonomic and structural remodeling.
Succinobucol (AGI-1067), a derivative of probucol, is a metabolically stable compound that has greater intracellular antioxidant efficacy in vitro than probucol without causing a significant prolongation of the QT interval (58). Surprisingly, the ARISE study (59) showed that use of succinobucol was associated with increased incidence of new-onset AF in patients with an acute coronary syndrome. Our previous meta-analysis suggested that increased CRP levels are associated with greater risk of immediate and short-term AF recurrence following electrical cardioversion (60,61). In this context, although succinobucol have potent antioxidant effects, its unfavourable influence on CRP levels may be a possible potential explanation for this undesirable effect (62,63). Undoubtedly, further studies are needed to elucidate the precise role of probucol and succinobucol in atrial remodeling and their clinical impact on AF.
Nitric oxide donors or precursors
Nitric oxide is an important endothelium-derived relaxing factor that plays a pivotal role in the maintenance of vascular tone. NO is synthesized from L-arginine through the effects of endothelial NO synthase (eNOS) with the critical cofactor tetrahydrobrobiopterin (BH4). BH4 depletion induces NOS uncoupling which shifts the enzymatic activity from NO production towards superoxide anion (O2•−) production (64). Endothelial dysfunction (ED) promotes oxidative stress and inflammation and also impairs NO dependent vaso-relaxation. Endothelial dysfunction with decreased NO production has been implicated on the development of atrial fibrillation (65,66).
It has been indicated that L-Arginine supplementation, as a NO precursor, increases plasma nitrite levels, decreases MDA release and attenuates ROS mediated myocardial injury (67). In a canine tachypacing model of heart failure, Nishijima et al. (68) found increased inducible NOS in the left atrium which was associated with BH4 depletion, NOS2 uncoupling, and increased superoxide anion production. These biochemical changes were associated with atrial electrophysiological changes with increased AF inducibility. BH4 supplementation reduced atrial oxidative stress and inducibility of atrial fibrillation. Thus, modulation of NOS activity may be an interesting therapeutic approach to prevent AF (69). At the clinical level, a pilot randomized placebo-controlled study examined the potential role of sodium nitroprusside (SNP), as a NO donor, in the prevention of POAF (70). Specifically, 100 consecutive patients undergoing CABG surgery were randomized to receive SNP (0.5 µg/Kg.min) or placebo (dextrose 5% in water) during the rewarming period. The occurrence of AF was significantly lower in the SNP group (P<0.005). Furthermore, the inflammatory biomarker CRP was higher postoperatively in the control group compared to the SNP group (P<0.05). However, a recent study didn’t find any association between the use of sodium nitroprusside during cardiothoracic surgery and POAF in a retrospective cohort of 1,025 patients undergoing bypass surgery (71). Therefore, anti-inflammatory and antioxidant effects of NO may have beneficial effects on the prevention of POAF. Further randomized controlled studies are urgently needed to clarify the role of NO and its donor or precursor on the AF prevention.
NADPH oxidases inhibitors
NADPH oxidases (NOXs) have been investigated as a key enzymatic source of ROS and seem to play an important role in the pathogenesis of hypertension, atherosclerosis, and heart failure (72-74). NOXs are multi-subunit transmembrane enzymes that utilize NADPH as an electron donor to reduce oxygen to superoxide anion and hydrogen peroxide. NOX2 and NOX4 are the most abundant NOX subtypes in cardiomyocytes.
Recent evidence indicates that NOX-derived ROS plays a pivotal role in the development and maintenance of AF. Dudley et al. (75) showed reduced NO and increased superoxide production production in the left atrial appendage (LAA), which was related to increased NADPH oxidase activity in an experimental model of atrial tachy-pacing. Of note, the NADPH oxidase inhibitor apocynin reduced the superoxide production by 91%. In addition, Kim et al. (76) investigated the sources of superoxide production from the right atrial appendage (RAA) of patients undergoing cardiac surgery. They indicated that the membrane-bound subunit gp91phox (NOX2) containing NADPH oxidase was the main source of atrial superoxide production in human atrial myocytes during sinus rhythm and AF. Also, NADPH-stimulated superoxide release from RAA homogenates was significantly increased in patients with AF. In a subsequent study, they measured atrial NADPH oxidase activity in RAA samples from 170 consecutive patients undergoing coronary artery bypass surgery. The multivariate analysis showed that atrial NADPH oxidase activity was the strongest independent risk factor for the development of POAF (77). Remarkably, recent clinical evidence (78) suggests that the behaviour of NADPH oxidase is related to the type of AF. Cangemi et al. demonstrated that NOX2 was upregulated in patients with paroxysmal/persistent AF compared with those with permanent AF and controls (78). Also, NOX4-derived hydrogen peroxide production is markedly increased in the LAA tissues of AF patients. Moreover, treatment of HL-1 atrial cells with angiotensin II, resulted in upregulation of NOX4 and H2O2 production (79). Bearing in mind the potent NADPH oxidase inhibitors such as NOX2 inhibitors and apocynin (80) may be served as potential candidate for the novel preventive agents on AF.
Xanthine oxidase inhibitors
Accumulating evidence suggests that xanthine oxidase (XO) is another important source of ROS and may relate to atrial remodeling and AF (81). In a pig model of rapid atrial pacing, increased XO activity in LAA and reduced superoxide production by 85% following administration of oxypurinol (a XO inhibitor) was demonstrated (75). However, no significant effect of oxypurinol on superoxide production in human RAA was demonstrated in another study (76). A potential explanation for this evident difference is that in the porcine model the increased XO activity was located in the LAA, whereas RAA specimens were examined in the human study. Of note, in a similar porcine model, it was demonstrated that after 1 week of rapid atrial pacing NO expression was decreased in the LAA but not in the RAA, indicating that the oxidative stress was enhanced only in the left atrium (82).
UA is a metabolic product of purine metabolism produced via the action of XO. Therefore, UA represents a marker of oxidative stress and inflammation (83,84). There is a positive association between UA levels and AF in different population. In a small observational study, we showed a stepwise increase of UA levels in patients with paroxysmal AF and permanent AF compared to controls (85). It was also demonstrated that high serum UA levels were independent predictor for AF presence in hypertensive patients (86) and AF recurrence following catheter ablation (87). In the ARIC study, a large prospective cohort study, elevated serum UA was associated with a greater risk of AF development during the follow-up period (88).
No clinical trial to date has examined the effect of allopurinol administration on AF. Only one observational study reported that patients receiving UA lowering agents had decreased AF prevalence (89). In a very recent experimental study using a dog model of atrial tachypacing, allopurinol suppressed AF promotion by preventing both electrical and structural remodeling, while it also reduced endothelial NOS protein expression without affecting the left ventricular ejection fraction or LA diameter (90). Finally, there are no data on the cardiovascular effects of the newly released non-purine XO inhibitor febuxostat.
Conclusions
A substantial body of evidence indicates that oxidative stress plays a critical role in the pathophysiology of atrial remodeling. However, the molecular pathways of this pathologic process are complex and depend on different underlying substrates and concomitant diseases. Antioxidant therapy seems to be a promising intervention strategy in the prevention of AF development and perpetuation, at least in the case of POAF (Figure 1). It should be acknowledged that antioxidant substances may be ineffective in many instances since they act at an advanced stage of the oxidative damage cascade. On the other hand, interventions that target early steps of ROS formation seem to be a more promising strategy.
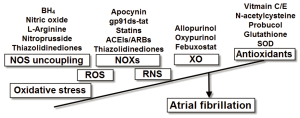 Figure 1. Molecular sources of oxidative stress and the potential antioxidant interventions for the management of atrial fibrillation. BH, tetrahydrobrobiopterin; SOD, superoxide dismutase; NOS, nitric oxide synthase; NOXs, nicotinamide adenine dinucleotide phosphate oxidases; XO, xanthine oxidase; ROS, reactive oxygen species; RNS, reactive nitrogen species
Figure 1. Molecular sources of oxidative stress and the potential antioxidant interventions for the management of atrial fibrillation. BH, tetrahydrobrobiopterin; SOD, superoxide dismutase; NOS, nitric oxide synthase; NOXs, nicotinamide adenine dinucleotide phosphate oxidases; XO, xanthine oxidase; ROS, reactive oxygen species; RNS, reactive nitrogen species Acknowledgements
This work was supported by grants (30900618, 81270245 to T.L.) from the National Natural Science Foundation of China.
Disclosure: The authors declare no conflict of interest.
References
- Korantzopoulos P, Kolettis TM, Galaris D, et al.The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation.Int J Cardiol 2007;115:135-43.
- Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol 2008;52:306-13.
- Huang CX, Liu Y, Xia WF, et al. Oxidative stress: a possible pathogenesis of atrial fibrillation. Med Hypotheses 2009;72:466-7.
- Negi S, Sovari AA, Dudley SC Jr. Atrial fibrillation: the emerging role of inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets 2010;10:262-8.
- Jeong EM, Liu M, Sturdy M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 2012;52:454-63.
- Savelieva I, Kakouros N, Kourliouros A, et al. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308-28.
- Savelieva I, Kakouros N, Kourliouros A, et al. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace 2011;13:610-25.
- Sovari AA, Dudley SC. Antioxidant therapy for atrial fibrillation: lost in translation? Heart 2012. [Epub ahead of print].
- Liu T, Li G. Antioxidant interventions as novel preventive strategies for postoperative atrial fibrillation. Int J Cardiol 2010;145:140-2.
- Sovari AA, Dudley SC Jr. Reactive oxygen species-targeted therapeutic interventions for atrial fibrillation. Front Physiol 2012;3:311.
- Rasoli S, Kakouros N, Harling L, et al. Antioxidant vitamins in the prevention of atrial fibrillation: what is the evidence? Cardiol Res Pract 2011;2011:164078.
- Rodrigo R, Vinay J, Castillo R, et al. Use of vitamins C and E as a prophylactic therapy to prevent postoperative atrial fibrillation. Int J Cardiol 2010;138:221-8.
- Harling L, Rasoli S, Vecht JA, et al. Do antioxidant vitamins have an anti-arrhythmic effect following cardiac surgery? A meta-analysis of randomised controlled trials. Heart 2011;97:1636-42.
- Rodrigo R. Prevention of postoperative atrial fibrillation: novel and safe strategy based on the modulation of the antioxidant system. Front Physiol 2012;3:93.
- Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res 2001;89:E32-8.
- Shiroshita-Takeshita A, Schram G, Lavoie J, et al. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004;110:2313-9.
- Lin YK, Lin FZ, Chen YC, et al. Oxidative stress on pulmonary vein and left atrium arrhythmogenesis. Circ J 2010;74:1547-56.
- Eslami M, Badkoubeh RS, Mousavi M, et al. Oral ascorbic acid in combination with beta-blockers is more effective than beta-blockers alone in the prevention of atrial fibrillation after coronary artery bypass grafting. Tex Heart Inst J 2007;34:268-74.
- Papoulidis P, Ananiadou O, Chalvatzoulis E, et al. The role of ascorbic acid in the prevention of atrial fibrillation after elective on-pump myocardial revascularization surgery: a single-center experience--a pilot study. Interact Cardiovasc Thorac Surg 2011;12:121-4.
- Rodrigo R, Gutiérrez R, Fernández R, et al. Ageing improves the antioxidant response against postoperative atrial fibrillation: a randomized controlled trial. Interact Cardiovasc Thorac Surg 2012;15:209-14.
- Korantzopoulos P, Kolettis TM, Kountouris E, et al. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol 2005;102:321-6.
- Ferro D, Franciosa P, Cangemi R, et al. Serum levels of vitamin E are associated with early recurrence of atrial fibrillation after electric cardioversion. Circ Arrhythm Electrophysiol 2012;5:327-33.
- Palee S, Chattipakorn S, Phrommintikul A, et al. PPARγ activator, rosiglitazone: Is it beneficial or harmful to the cardiovascular system? World J Cardiol 2011;3:144-52.
- Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab 2012;23:205-15.
- Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest 2004;27:982-91.
- Da Ros R, Assaloni R, Ceriello A. The preventive anti-oxidant action of thiazolidinediones: a new therapeutic prospect in diabetes and insulin resistance. Diabet Med 2004;21:1249-52.
- Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79-82.
- Hwang J, Kleinhenz DJ, Lassègue B, et al. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol 2005;288:C899-905.
- Shimano M, Tsuji Y, Inden Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm 2008;5:451-9.
- Kume O, Takahashi N, Wakisaka O, et al. Pioglitazone attenuates inflammatory atrial fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats. Heart Rhythm 2011;8:278-85.
- Xu D, Murakoshi N, Igarashi M, et al. PPAR-γ activator pioglitazone prevents age-related atrial fibrillation susceptibility by improving antioxidant capacity and reducing apoptosis in a rat model. J Cardiovasc Electrophysiol 2012;23:209-17.
- Liu T, Zhao H, Li J, et al. Rosiglitazone attenuates atrial structural remodeling and atrial fibrillation promotion in alloxan-induced diabetic rabbits. Eur Heart J 2010;31:abstr 705.
- Korantzopoulos P, Kokkoris S, Kountouris E, et al. Regression of paroxysmal atrial fibrillation associated with thiazolidinedione therapy. Int J Cardiol 2008;125:e51-3.
- Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125-35.
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89.
- Anglade MW, Kluger J, White CM, et al. Thiazolidinedione use and post-operative atrial fibrillation: a US nested case-control study. Curr Med Res Opin 2007;23:2849-55.
- Gu J, Liu X, Wang X, et al. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace 2011;13:1256-61.
- Chao TF, Leu HB, Huang CC, et al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol 2012;156:199-202.
- Abbas A, Blandon J, Rude J, et al. PPAR- γ agonist in treatment of diabetes: cardiovascular safety considerations. Cardiovasc Hematol Agents Med Chem 2012;10:124-34.
- Raschi E, Boriani G, De Ponti F. Targeting the arrhythmogenic substrate in atrial fibrillation: focus on structural remodeling. Curr Drug Targets 2011;12:263-86.
- Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180-8.
- Liu T, Korantzopoulos P, Li G, et al. The potential role of thiazolidinediones in atrial fibrillation. Int J Cardiol 2008;128:129-30.
- Liu T, Li G. Thiazolidinediones as novel upstream therapy for atrial fibrillation in diabetic patients: a review of current evidence. Int J Cardiol 2012;156:215-6.
- Zafarullah M, Li WQ, Sylvester J, et al. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 2003;60:6-20.
- Carnes CA, Janssen PM, Ruehr ML, et al. Atrial glutathione content, calcium current, and contractility. J Biol Chem 2007;282:28063-73.
- Ozaydin M, Peker O, Erdogan D, et al. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J 2008;29:625-31.
- Baker WL, Anglade MW, Baker EL, et al. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis. Eur J Cardiothorac Surg 2009;35:521-7.
- Gu WJ, Wu ZJ, Wang PF, et al. N-Acetylcysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: a meta-analysis of eight randomized controlled trials. BMC Cardiovasc Disord 2012;12:10.
- Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis 2009;207:16-23.
- Stocker R. Molecular mechanisms underlying the antiatherosclerotic and antidiabetic effects of probucol, succinobucol, and other probucol analogues. Curr Opin Lipidol 2009;20:227-35.
- Singla DK, Kaur K, Sharma AK, et al. Probucol promotes endogenous antioxidant reserve and confers protection against reperfusion injury. Can J Physiol Pharmacol 2007;85:439-43.
- Umeji K, Umemoto S, Itoh S, et al. Comparative effects of pitavastatin and probucol on oxidative stress, Cu/Zn superoxide dismutase, PPAR-gamma, and aortic stiffness in hypercholesterolemia. Am J Physiol Heart Circ Physiol 2006;291:H2522-32.
- Li G, Yin L, Liu T, et al. Role of probucol in preventing contrast-induced acute kidney injury after coronary interventional procedure. Am J Cardiol 2009;103:512-4.
- Yin L, Li G, Liu T, et al. Probucol for the prevention of cystatin C-based contrast-induced acute kidney injury following primary or urgent angioplasty: A randomized, controlled trial. Int J Cardiol 2012. [Epub ahead of print].
- Gong YT, Li WM, Li Y, et al. Probucol attenuates atrial autonomic remodeling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Chin Med J (Engl) 2009;122:74-82.
- Li Y, Sheng L, Li W, et al. Probucol attenuates atrial structural remodeling in prolonged pacing-induced atrial fibrillation in dogs. Biochem Biophys Res Commun 2009;381:198-203.
- Fu H, Liu T, Liu C, et al. Probucol preserves atrial structure and function by attenuates oxidative stress and increases stability of vulnerable atrial fibrillation in alloxan-induced diabetic rabbits. Cardiology 2012;123:48-9.
- Midwinter RG, Maghzal GJ, Dennis JM, et al. Succinobucol induces apoptosis in vascular smooth muscle cells. Free Radic Biol Med 2012;52:871-9.
- Tardif JC, McMurray JJ, Klug E, et al. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:1761-8.
- Liu T, Li G, Li L, et al. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol 2007;49:1642-8.
- Liu T, Li L, Korantzopoulos P, et al. Meta-analysis of association between C-reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am J Cardiol 2008;101:1749-52.
- Tardif JC, Grégoire J, L’Allier PL, et al. Effects of the antioxidant succinobucol (AGI-1067) on human atherosclerosis in a randomized clinical trial. Atherosclerosis 2008;197:480-6.
- Liu T, Li G. Probucol and succinobucol in atrial fibrillation: pros and cons. Int J Cardiol 2010;144:295-6.
- Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002;105:546-9.
- Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart 2009;95:102-6.
- Krishnamoorthy S, Lim SH, Lip GY. Assessment of endothelial (dys)function in atrial fibrillation. Ann Med 2009;41:576-90.
- Kiziltepe U, Tunçtan B, Eyileten ZB, et al. Efficiency of L-arginine enriched cardioplegia and non-cardioplegic reperfusion in ischemic hearts. Int J Cardiol 2004;97:93-100.
- Nishijima Y, Sridhar A, Bonilla I, et al. Tetrahydrobiopterin depletion and NOS2 uncoupling contribute to heart failure-induced alterations in atrial electrophysiology. Cardiovasc Res 2011;91:71-9.
- Bonilla IM, Sridhar A, Györke S, et al. Nitric oxide synthases and atrial fibrillation. Front Physiol 2012;3:105.
- Cavolli R, Kaya K, Aslan A, et al. Does sodium nitroprusside decrease the incidence of atrial fibrillation after myocardial revascularization?: a pilot study. Circulation 2008;118:476-81.
- Bolesta S, Aungst TD, Kong F. Effect of sodium nitroprusside on the occurrence of atrial fibrillation after cardiothoracic surgery. Ann Pharmacother 2012;46:785-92.
- Selemidis S, Sobey CG, Wingler K, et al. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 2008;120:254-91.
- Zhang M, Perino A, Ghigo A, et al. NADPH Oxidases in Heart Failure: Poachers or Gamekeepers? Antioxid Redox Signal 2012. [Epub ahead of print].
- Octavia Y, Brunner-La Rocca HP, Moens AL. NADPH oxidase-dependent oxidative stress in the failing heart: From pathogenic roles to therapeutic approach. Free Radic Biol Med 2012;52:291-7.
- Dudley SC Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 2005;112:1266-73.
- Kim YM, Guzik TJ, Zhang YH, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res 2005;97:629-36.
- Kim YM, Kattach H, Ratnatunga C, et al. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:68-74.
- Cangemi R, Celestini A, Calvieri C, et al. Different behaviour of NOX2 activation in patients with paroxysmal/persistent or permanent atrial fibrillation. Heart 2012;98:1063-6.
- Zhang J, Youn JY, Kim AY, et al. NOX4-Dependent Hydrogen Peroxide Overproduction in Human Atrial Fibrillation and HL-1 Atrial Cells: Relationship to Hypertension. Front Physiol 2012;3:140.
- Sovari AA, Morita N, Karagueuzian HS. Apocynin: a potent NADPH oxidase inhibitor for the management of atrial fibrillation. Redox Rep 2008;13:242-5.
- Korantzopoulos P, Letsas KP, Liu T. Xanthine oxidase and uric Acid in atrial fibrillation. Front Physiol 2012;3:150.
- Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation 2002;106:2854-8.
- Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis 2007;17:409-14.
- Glantzounis GK, Tsimoyiannis EC, Kappas AM, et al. Uric acid and oxidative stress. Curr Pharm Des 2005;11:4145-51.
- Letsas KP, Korantzopoulos P, Filippatos GS, et al. Uric acid elevation in atrial fibrillation. Hellenic J Cardiol 2010;51:209-13.
- Liu T, Zhang X, Korantzopoulos P, et al. Uric acid levels and atrial fibrillation in hypertensive patients. Intern Med 2011;50:799-803.
- Letsas KP, Siklódy CH, Korantzopoulos P, et al. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int J Cardiol 2011. [Epub ahead of print].
- Tamariz L, Agarwal S, Soliman EZ, et al. Association of serum uric acid with incident atrial fibrillation (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;108:1272-6.
- Kuwabara M, Niwa K, Niinuma H. Hyperuricemia is an independent risk factor of atrial fibrillation due to electrical remodeling through activation of uric acid transporter. J Am Coll Cardiol 2012;59:E663.
- Sakabe M, Fujiki A, Sakamoto T, et al. Xanthine Oxidase Inhibition Prevents Atrial Fibrillation in a Canine Model of Atrial Pacing-Induced left Ventricular Dysfunction. J Cardiovasc Electrophysiol 2012;23:1130-5.


