Acute coronary syndrome in HIV patients: from pathophysiology to clinical practice
Introduction
Since 1998, when Keith Henry, in a letter to The Lancet (1), reported two cases of myocardial infarction in young men on protease inhibitors (PIs), multiple studies and databases (2,3), documented an increased risk of myocardial infarction and endothelial dysfunction in patients on highly active antiretroviral therapy (HAART) and PIs. Our understanding of the underlying histopathology and impact of this condition on cardiovascular health has advanced during the last decade.
Up-to-date treatment of HIV-infected patients is associated with improved long-term survival, but, at the same time, an increase in cardiovascular morbidity and mortality, including chronic stages of this disease. Moreover, the same drugs used in the HIV-treatment have been implicated in insulin resistance and in the atherosclerosis process due to adverse effects on dyslipidemia.
In a recent study (4) Guaraldi et al. show that specific and oftentimes multiple age-related non-infectious comorbidities were more common among HIV-infected patients than in the general population. In addition, HIV-specific cofactors (lower nadir CD4 cell count and more prolonged HAART exposure) were identified as risk factors.
These accumulating data demonstrate that cardiovascular morbidity and mortality play a crucial role in the prognosis of HIV patients on HAART therapy in developed countries.
Traditional cardiovascular risk factors in HIV patients
The risk of coronary heart disease in HIV patients is significantly influenced by traditional factors such as age, smoking, diabetes, and dyslipidemia. Triant et al. show that HIV patients admitted for acute myocardial infarction had significantly higher proportions of hypertension (21.2% vs. 15.9%), diabetes (11.5% vs. 6.6%), and dyslipidemia (23.3% vs. 17.6%) than a non-HIV cohort (P<0.0001 for each comparison) (5). HIV-infected men had also a higher prevalence of smoking (6). However, even after adjusting for traditional risk factors, rates of atherosclerosis were still higher in HIV infected subjects than in those who were not (5). Other studies of HIV-infected patients with acute coronary syndrome (ACS) found that this population was younger, more often male, and smokers compared with HIV-uninfected patients (7-12). In a recent article, Baccarat et al. share these findings reporting a mean age of first occurrence of ACS in HIV-infected patients of 50 years, with predominantly male-gender, and tobacco-smoking as the most prevalent coronary risk factor. The authors also report a much higher proportion of HIV-infected patients with ACS using illicit drugs compared with HIV-uninfected patients (23% vs. 6%, P<0.001) (13). In a recent meta-analysis performed by our group (D'Ascenzo et al.) investigating rates mid term outcomes of patients with HIV presenting with ACS in northern countries, we report an overall average incidence of traditional cardiovascular risk factors, except for diabetes, as can be expected in a young population (14).
HIV and cardiovascular risk: A linear association?
Although the underlying mechanisms are not fully understood, HIV infection has been shown to increase the risk of coronary events.
In the Kaiser Permanente database, comparing HIV-positive and -negative members, the hospitalization rate for coronary heart disease was significantly higher (6.5 vs. 3.8, P=0.003), as was the incidence of myocardial infarction (4.3 vs. 2.9, P=0.07). This data are supported by larger cohort study of almost 4000 HIV-infected patients and more than 1 million controls, describing that the risk of acute myocardial infarction was higher for HIV-positive patients than for HIV-negative patients even after adjusting for age, gender, race, hypertension, diabetes and dyslipidemia (5).
Several causative mechanisms have been supposed, including HIV-associated dyslipidemia, endothelial damage or dysfunction, inflammation and hypercoagulability.
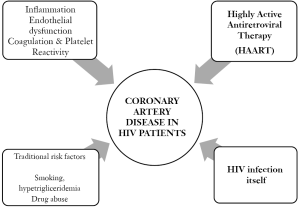
Pathogenesis of coronary artery disease (CAD) in HIV infection. (Figure 1)
Dyslipidemia and atherosclerosis
In the early stage of HIV infection, levels of total cholesterol and HDL-C are lower. The progressive lowering of CD4 cells lymphocyte counts have been associated with a reduced clearance of LDL-C particles, lower level of apolipoprotein B (15,16) and a decrease in high-density lipoprotein cholesterol (HDL-c). Also, the triglyceride levels may correlate to the degree of viremia (17). As supposed by Mujawar et al., these changes are triggered by deregulations of the intracellular lipid metabolism in HIV-infected macrophages due to the impairment of the ATP-binding cassette transporter A1 (ABCA1)-dependent cholesterol efflux (18).
Atherosclerosis in HIV patients appears to have a different pathogenetic features from atherosclerosis in the general population, with intermediate histologically features of lesions found in common CAD and transplant vasculopathy (19). A necroscopic study described diffuse and circumferential vessel involvement with unusual proliferation of smooth muscle cells mixed with abundant elastic fibres (20).
Furthermore, some postmortem examination studies had shown the presence of premature atherosclerosis in a high percentage of HIV-positive patients, including involvement in very young subjects, even before the introduction of protease inhibitors therapy (21,22).
Inflammation
Inflammation is associated with endothelial dysfunction in both treated and untreated HIV patients. Increased atherosclerosis with HIV infection can occur in the absence of antiretroviral therapy, detectable viremia, or overt immunodeficiency. Hsue et al. (23) compared carotid intima media thickness and levels of C-reactive protein (CRP) in HIV-positive and HIV-negative patients reporting greater values in all HIV patients groups, irrespective of level of viremia or antiretroviral therapy. Furthermore, CRP levels remained elevated in HIV infected subjects. This data suggests that persistent inflammation may account for early atherosclerosis in these patients.
Hypercoagulability
In addition to endothelial damage, HIV replication and immune activation may drive coagulation and fibrinolysis, in part, via up-regulation of tissue factor pathways. A positive correlation has also been noted among patients with untreated HIV infection and thrombocytopenia that usually worsens with advancing HIV disease (24,25). Beyond their role in acute setting of atherosclerotic events, chronic platelet activation is present in HIV infected patients, may promote atherogenesis, and increase the risk for thrombosis (26).
Antiretroviral therapy: treatment or poison?
In the decade many reports have investigated a possible association between myocardial infarction and HAART: several studies found a statistically significant association (27-30), while others did not (31,32). This heterogeneity arise from differences in study design (observational cohort studies vs. prospective randomized clinical trials), populations studied (differences in age, cardiovascular risk factor, previous exposition to ART treatment), and also from different outcome definition (33).
One of the most important was the Data Collection on Adverse Events of Anti-HIV Drugs (D.A.D) study (34) that prospectively followed more than 20,000 patients for a total of 94,469 person-years. The relative risk of myocardial infarction per year of protease inhibitor exposure was 1.16 (1.10-1-23; C.I. 95%) adjusting for hypertension, diabetes and Non-Nucleoside Reverse Transcriptase and remained significant even after adjusting for serum lipid level.
On the other hand, as recently reported, HIV patients with ACS had significantly lower viral loads (4769±3109, P<0.001) and numerically higher CD4 counts (298±184, P=0.11) (35,36) than patients with HIV/AIDS-related cardiomyopathy, thus suggesting that HIV infection probably plays a more limited role in the onset of coronaropathy.
The Strategies for Management of Antiretroviral Therapy (SMART) trial, one of the largest studies of antiretroviral treatment interruption, demonstrated that the rate of major cardiovascular events was higher if treatment was interrupted than with continuous treatment, with a hazard ratio of 1.57 (95% CI 1.0-2.46, P=0.05) (37). This association between treatment interruption and coronary events does not appear to be related to the level of viremia (38). These results suggest that suppression of HIV itself plays an important role in reducing pro-inflammatory cytokines. In fact elevated IL-6 level was significantly associated with the development of cardiovascular disease (OR 2.8, P=0.03). Moreover treatment-interruption may increase the risk of death as a consequence of further elevation of IL-6 and D-dimer levels (39).
Coronary artery disease
As we discussed above, several studies suggest that HIV patients are exposed to an increased risk of premature CAD linked predominantly to the hyperlipidaemia and insulin resistance that are associated with protease-inhibitor therapy.
Our group has recently conducted a meta-analysis (14) of 11 studies including 2442 HIV-patients presenting with ACS. The most common presentation was STEMI with a high prevalence of multivessel involvement. Both characteristic had an higher incidence than in contemporary ACS registries of non-HIV patients (40,41) and combined together could in part explain the higher rates of in-hospital events registered in HIV patients (42).
In contrast, other studies have reported a more favorable in-hospital outcome in absence of significant hemodynamic compromise (43).
PCI in HIV-infected patients has been associated with a high incidence of non-fatal reinfarction, restenosis and in-stent thrombosis (44). This worse outcome during follow-up appears related to both high prevalence of cardiovascular risk factors, impact of viral pathological process, and side effects of antiretroviral drugs. Moreover a high incidence of thrombo-embolic events and intraluminal demonstration of fresh thrombus has been reported, probably related to a prothrombotic state (36).
In a recent report of the Soweto Study Cohort (36) including 518 HIV patients admitted with a new diagnosis of cardiovascular disease between 2006 and 2008, Sliwa and colleagues report a relatively infrequent incidence of ACS (3%) in this population, despite a high number of patients already receiving HAART prior to admission. This finding is in agreement with a recent meta-analysis casting doubt on the potential role of publication bias and confounding in overestimation of HIV and PIs exposure risk (45).
Management of CVD in HIV
Modification of risk factors
The early detection and treatment of co-morbilities and modifiable risk factors through lifestyle changes such as smoking cessation, dietary changes, and exercise is likely to have a significant impact on cardiovascular risk in this population.
Because HIV infection by itself and HAART treatment likely increase the risk of plaque rupture and atherothrombosis (46,47), routine primary and secondary prevention should be considered in HIV infected subjects. However, as reported in some study (48), LDL goals are less frequently achieved in HIV-infected patients during follow-up.
Management of hyperlipidaemia
Specific guidelines for the evaluation and management of HAART related hyperlipidaemia have been developed by the Infectious Disease Society of America (IDSA) and Adult AIDS Clinical Trials Group (AACTG) (49). These recommendations are largely based on National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines, and advocate adjusting individual cholesterol level through estimation of Framingham predicted 10 years cardiovascular risk (50).
Currently there is no difference in hyperlipidemia goals treatment between HIV and not-HIV infected subjects. In the choice of specific lipid-lowering therapy it is critical to consider drug-drug interactions. In general, all PIs inhibiting CYP3A4, with the highest level of inhibition with ritonavir, followed by indinavir, nelfinavir, amprenavir, and saquinavir. Delavirdine, an NNRTI, is also an inhibitor of CYP3A4, whereas nevirapine and efavirenz result in induction of the enzyme. Therefore, the first choice agents for lowering LDL are pravastatin (not metabolized by CYP3A4), with fluvastatin (metabolized CYP2C9) as second choice. Rosuvastatin concentrations appear to be increased when used in combination with some NNRTIs (atazanavir, ritonavir, lopinavir), thus, in that setting, 10 mg should be considered the maximum safe dose (51,52). Similarly, atorvastatin should be used at lower doses in HIV patients. Finally during PIs therapy, simvastatin and lovastatin are not recommended because of the high-risk of rhabdomyolysis (53). Lack of data limits the precise estimation of benefits related to anti-inflammatory properties of statins.
Conclusions
Short-term benefits of HAART prevent cardiovascular disease in HIV patients, but the long-term benefit is incompletely understood and will require further data. The results of ongoing trials will provide important information on how to manage timing of HAART therapy, optimizing the risk-benefit ratio. The START trial (54) includes antiretroviral-naive HIV-positive people with CD4 counts greater than 500 cells/mm3. It is an international multi-center trial including about 90 sites in nearly 30 countries (including Australia). Participants are randomized to receive either early antiretroviral treatment or deferred treatment awaiting the first CD4 count <350 cells/mm3 or onset of clinical signs of advanced HIV disease. In each group, 2,000 patients will be recruited.
Others challenges and open issues include the best regimen in CAD patients, the role of anti-inflammatory drugs, and the long-term clinical outcomes of HIV patients in the modern era of HAART treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Henry K, Melroe H, Huebsch J, et al. Severe premature coronary artery disease with protease inhibitors. Lancet 1998;351:1328.[LinkOut]
- Friis-Møller N, Reiss P, El-Sadr W, et al. Exposure to PI and NNRTI and risk of myocardial infarction: results from the D:A:D Study. 13th Conference on Retroviruses and Opportunistic Infections; 2006; Denver, Colo.
- Klein D, Hurley L, Silverberg M, et al. Surveillance data for myocardial infarction hospitalizations among HIV+ and HIV- Northern Californians: 1994-2006. 14th Conference on Retroviruses and Opportunistic Infections; 2007; Los Angeles, Calif.
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120-6.[LinkOut]
- Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506-12.[LinkOut]
- Savès M, Chêne G, Ducimetière P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003;37:292-8.[LinkOut]
- David MH, Hornung R, Fichtenbaum CJ. Ischemic cardiovascular disease in persons with human immunodeficiency virus infection. Clin Infect Dis 2002;34:98-102.[LinkOut]
- Escaut L, Monsuez JJ, Chironi G, et al. Coronary artery disease in HIV infected patients. Intensive Care Med 2003;29:969-73.[LinkOut]
- Matetzky S, Domingo M, Kar S, et al. Acute myocardial infarction in human immunodeficiency virus-infected patients. Arch Intern Med 2003;163:457-60.[LinkOut]
- Ambrose JA, Gould RB, Kurian DC, et al. Frequency of and outcome of acute coronary syndromes in patients with human immunodeficiency virus infection. Am J Cardiol 2003;92:301-3.[LinkOut]
- Varriale P, Saravi G, Hernandez E, et al. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J 2004;147:55-9.[LinkOut]
- Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 2004;109:316-9.[LinkOut]
- Boccara F, Mary-Krause M, Teiger E, et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J 2011;32:41-50.[LinkOut]
- D'Ascenzo F, Cerrato E, Biondi-Zoccai G, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J 2011. [Epub ahead of print]
- Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003;289:2978-82.[LinkOut]
- Shahmanesh M, Das S, Stolinski M, et al. Antiretroviral treatment reduces very-low-density lipoprotein and intermediate-density lipoprotein apolipoprotein B fractional catabolic rate in human immunodeficiency virus-infected patients with mild dyslipidemia. J Clin Endocrinol Metab 2005;90:755-60.[LinkOut]
- Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:1045-52.[LinkOut]
- Mujawar Z, Rose H, Morrow MP, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006;4:e365.[LinkOut]
- Tabib A, Leroux C, Mornex JF, et al. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis 2000;11:41-6.[LinkOut]
- Mehta NJ, Khan IA. HIV-associated coronary artery disease. Angiology 2003;54:269-75.[LinkOut]
- Tabib A, Greenland T, Mercier I, et al. Coronary lesions in young HIVpositive subjects at necropsy. Lancet 1992;340:730.[LinkOut]
- Bharati S, Joshi VV, Connor EM, et al. Conduction system in children with acquired immunodeficiency syndrome. Chest 1989;96:406-13.[LinkOut]
- Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009;23:1059-67.[LinkOut]
- Blann AD, Seigneur M, Constans J, et al. Soluble P-selectin, thrombocytopenia and von Willebrand factor in HIV infected patients. Thromb Haemost 1997;77:1221-2.[LinkOut]
- Seigneur M, Constans J, Blann A, et al. Soluble adhesion molecules and endothelial cell damage in HIV infected patients. Thromb Haemost 1997;77:646-9.[LinkOut]
- Karmochkine M, Ankri A, Calvez V, et al. Plasma hypercoagulability is correlated to plasma HIV load. Thromb Haemost 1998;80:208-9.[LinkOut]
- D:A:D Study Group, Sabin CA, Worm SW, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371:1417-26.
- Durand M, Sheehy O, Baril JG, et al. Relation between use of nucleoside reverse transcriptase inhibitors (NRTI) and risk of myo-cardial infarction (MI): a nested case control study using Quebec's public health insurance database (QPHID). Presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention in Cape Town, South Africa, 2009.
- Lang S, Mary-Krause M, Cotte L, et al; the Clinical Epi Group of the French Hospital Database on HIV. Impact of specific NRTI and PI exposure on the risk of myocardial infarction: a case-control study nested within FHDH ANRS CO4. Presented at the 16th Conference on Retroviruses and Opportunistic Infections in Montreal, Canada, 2009.
- Strategies for Management of Anti-Retroviral Therapy/INSIGHT; DAD Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS 2008;22:F17-24.[LinkOut]
- Bedimo R, Westfall A, Drechsler H, et al. Abacavir use and risk of acute Presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention in Cape Town, South Africa, 2009.
- Brothers CH, Hernandez JE, Cutrell AG, et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKlinesponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr 2009;51:20-8.[LinkOut]
- Benson C, Ribaudo H, Zheng E, et al; the ACTG A5001/ALLRT Protocol Team. No Association of Abacavir Use with Risk of Myocardial Infarction or Severe Cardiovascular Disease Events: Results from ACTG A5001. Presented at the 16th Conference on Retroviruses and Opportunistic Infections in Montreal, Canada, 2009.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723-35.[LinkOut]
- Biondi-Zoccai G, D'Ascenzo F, Modena MG. Novel insights on HIV/AIDS and cardiac disease: shedding light on the HAART of Darkness. Eur Heart J 2011. [Epub ahead of print]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283-96.[LinkOut]
- Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008;13:177-87.[LinkOut]
- Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203.[LinkOut]
- Gale CP, Manda SO, Weston CF, et al. Evaluation of risk scores for risk stratification of acute coronary syndromes in the Myocardial Infarction National Audit Project (MINAP) database. Heart 2009;95:221-7.[LinkOut]
- Ang DS, Wei L, Kao MP, et al. A comparison between B-type natriuretic peptide, global registry of acute coronary events (GRACE) score and their combination in ACS risk stratification. Heart 2009;95:1836-42.[LinkOut]
- Jolly SS, Shenkman H, Brieger D, et al. Quantitative troponin and death, cardiogenic shock, cardiac arrest and new heart failure in patients with non- ST-segment elevation acute coronary syndromes (NSTE ACS): insights from the Global Registry of Acute Coronary Events. Heart 2011;97:197-202.[LinkOut]
- Matetzky S, Domingo M, Kar S, et al. Acute myocardial infarction in human immunodeficiency virus-infected patients. Arch Intern Med 2003;163:457-60.[LinkOut]
- Mestres CA, Chuquiure JE, Claramonte X, et al. Long-term results after cardiac surgery in patients infected with the human immunodeficiency virus type-1 (HIV-1). Eur J Cardiothorac Surg 2003;23:1007-16; discussion 1016.[LinkOut]
- Sliwa K, Carrington MJ, Becker A, et al. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2011. [Epub ahead of print]
- Hulten E, Mitchell J, Scally J, et al. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and metaanalysis of observational studies. Heart 2009;95:1826-35.[LinkOut]
- Boccara F, Mary-Krause M, Teiger E, et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J 2011;32:41-50.[LinkOut]
- Oliviero U, Bonadies G, Apuzzi V, et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis 2009;204:586-9.[LinkOut]
- Boccara F, Mary-Krause M, Teiger E, et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J 2011;32:41-50.[LinkOut]
- Dubé MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)- infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003;37:613-27.[LinkOut]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.[LinkOut]
- van der Lee M, Sankatsing R, Schippers E, et al. Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients. Antivir Ther 2007;12:1127-32.[LinkOut]
- Busti AJ, Bain AM, Hall RG 2nd, et al. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J Cardiovasc Pharmacol 2008;51:605-10.[LinkOut]
- Hare CB, Vu MP, Grunfeld C, et al. Simvastatin-nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis 2002;35:e111-2.[LinkOut]
- Strategic Timing of Antiretroviral Treatment (START). Available online: http://clinicaltrials.gov/ct2/show/NCT00867048