Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: rationale and design of the CARAT study
Introduction
For more than a quarter century, lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins has formed a foundation for risk reduction strategies in patients with established coronary heart disease. However, residual risk of patients with acute coronary syndrome (ACS) remains high despite effective statin treatment (1), and some of this residual risk may be modifiable by additional lipoprotein interventions (2).
High-density lipoprotein (HDL) as a target and experience with new therapies
Population studies consistently demonstrate an inverse, curvilinear relationship between HDL-C levels and cardiovascular risk, regardless of atherogenic lipid levels (3,4), while patients suffering from HDL deficiency resulting from genetic defects in gene coding for proteins involved in HDL synthesis or maturation present an increased risk for cardiovascular disease (5). In addition, meta-analyses of trials of statins indicate that an inverse association of HDL-C concentration with cardiovascular risk persists among patients treated with statins (4,6,7), although there are some exceptions to these observations (8,9). Favorable effects of HDL have been attributed to its role in lipid transport and to beneficial effects on inflammatory, oxidative, apoptotic and thrombotic pathways implicated in atherosclerosis (10). Intervention studies in animal models have demonstrated that direct infusion of HDL-like particle or transgenic expression of its major proteins has a favorable impact on the extent and histologic phenotype of experimental atherosclerosis (11-13). Small studies in human have indicated favorable effects of HDL-like particle infusion on endothelial function and atherosclerosis (14-17).
Orally bioavailable drugs that increase HDL-C concentration, including niacin, fibrates, and cholesteryl ester transfer protein (CETP) inhibitors have not been found to provide clinical benefit when added to statins (18-23). At least to date, these findings suggest that raising HDL-C may not be sufficient to further reduce cardiovascular risk in statin-treated patients. In contrast, favorable effects of infusing delipidated forms of HDL on lipid transporting factors, endothelial function and atherosclerotic plaque volume (14-17) suggest that administering an engineered HDL particle, as opposed to indirectly altering its production or remodelling, might provide a more effective therapeutic approach. This would be consistent with recent reports that increasing the number of HDL particles, as opposed to HDL-C, may be more relevant to reducing cardiovascular risk (24).
Emergence of plaque imaging to evaluate anti-atherosclerotic drugs
Advances in arterial wall imaging have extended the ability to visualize the extent and progression of atherosclerosis, in addition to the dimensions of the arterial lumen. In particular, serial application of intraarterial ultrasonography has evolved to assess the impact of medical therapies on disease progression (16,25-36). Serial IVUS imaging within an epicardial coronary artery enables precise quantitation of the volumetric burden of atherosclerotic plaque within an anatomically matched coronary segment, defined by the location of fiduciary side branches, after treatment. Using this approach, studies have demonstrated the benefits of LDL-C lowering with statins (26-28) and antihypertensive agents (29) in CAD patients and use of pioglitazone in patients with type 2 diabetes and CAD (30). Subsequent analyses revealed that HDL-C raising contributes to the benefits of statins (37) and pioglitazone (38) and identified patients who were more likely to have atherosclerosis regression under treatment with the CETP inhibitor, torcetrapib (39). The clinical relevance of these findings is supported by observations that plaque burden at baseline and its progression on serial imaging both associate with cardiovascular events (40,41).
Experience with HDL infusions and imaging
Several studies have utilized IVUS to determine a potential benefit of engineered HDL mimetics of different compositions. In a study of 57 patients with an ACS, weekly intravenous infusions of saline or reconstituted HDL containing palmitoyl-oleyl phospatidylcholine and apolipoprotein A–I Milano dimer (ETC-216) at a concentration of 15 or 45 mg/kg for 5 weeks was evaluated. Coronary IVUS imaging was performed at baseline and 2 weeks following the final infusion. Administration of both doses of ETC-216 was associated with regression of coronary atherosclerosis compared to baseline. In fact, there was no greater regression at the higher dose, suggesting a potential for relative saturation of lipid mobilization (16). Subsequent analyses revealed the greatest degree of regression at sites containing the largest amount of atheroma at baseline and that regression was accompanied by reverse remodeling of the artery wall (42).
The Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) study aimed to determine the effect of infusing HDL particles that contained wild-type apoA–I (CSL-111) and soy bean lecithin in 183 patients who received weekly infusions of either saline or CSL-111 at a dose of 40 or 80 mg/kg. The higher dose was terminated due to unacceptably high rates of liver enzyme elevation. Significant reductions in plaque burden compared with baseline were observed in the CSL-111 treated patients, although a placebo-controlled comparison failed to meet statistical significance. The investigators also reported favorable effects of infusing CSL-111 on surrogate ultrasonic measures of plaque composition and quantitative coronary angiography (36).
An alternative approach to administration of HDL involves the infusion of autologous HDL after it has undergone selective delipidation. An early, proof-of-concept study in 28 patients with an ACS demonstrated a non-significant trend towards greater regression with seven autologous delipidated HDL infusions compared with placebo (43). The findings of these studies suggested that it was the infusion of lipid-deplete forms of HDL, as opposed to the specific protein, that appeared to exert a favorable effect on the artery wall. These programs continue to undergo clinical development.
CER-001
CER-001 is an engineered negatively-charged lipoprotein particle mimicking pre-beta HDL containing recombinant human apoA–I and the natural phospholipids, sphingomyelin (SM) and dipalmitoyl phosphatidylglycerol (DPPG) in a molar ratio of 32.3:1, and with a protein:phospholipid weight ratio of 1:2.7. The particles are synthesized with the same negative charge as observed with natural pre-beta HDL particles. In this respect, CER-001 is quite distinct from other reconstituted HDL particles undergoing clinical development. The presence of a negative charge added to the SM prevents fusion between particles and promotes more rapid and sustained cholesterol and lipids mobilization. Animal studies using both LDL receptor knockout mice and carotid flow cessation in apoE knockout mice demonstrated a reduction in arterial wall cholesterol content with CER-001 infusions (44,45). Phase 1 studies in healthy volunteers demonstrated that CER-001 promoted increases in cholesterol content of circulating HDL particles, consistent with lipid mobilization, at doses as low as 3 mg/kg (46).
A number of studies were subsequently performed to evaluate the impact of infusing CER-001 on atherosclerotic plaque in humans. A small open-label study of infusing CER-001 8 mg/kg in patients with familial hypoalphalipoproteinemia demonstrated an increase in cellular cholesterol efflux and cholesterol content of circulating HDL and reductions in both magnetic resonance imaging derived measures of aortic and carotid plaque burden and positron emission tomography detected fluorodeoxyglucose signalling as a measure of plaque inflammatory activity (47). Similarly, a small open-label study evaluating the effect of infusing CER-001 8 mg/kg in patients with homozygous familial hypercholesterolemia demonstrated similar reductions in aortic and carotid plaque burden on magnetic resonance imaging (48).
The Can HDL Infusions Significantly Quicken Atherosclerosis Regression (CHI-SQUARE) study evaluated the effect of infusing CER-001 at a dose of 3, 6 or 12 mg/kg or placebo weekly on six occasions in 507 patients with an ACS. The primary analysis of the study demonstrated that the primary endpoint, total atheroma volume, decreased by 3.13, 1.50 and 3.05 mm3 in the 3, 6, and −12 mg/kg groups of CER-001 respectively, compared with 2.85 mm3 with placebo, failing to meet statistical significance. However, a subsequent blinded analysis by a different core laboratory focusing on anatomically matched arterial segments in patients with suitable image quality demonstrated disease regression with the 3 mg/kg dose. In intention to treat analysis, total atheroma volume declined by −4.76 mm3, compared with −2.85 mm3 with placebo. There was a trend towards diminishing regression with increasing CER-001 dose. Per protocol analysis supported these findings, with significantly greater plaque regression (−6.28 vs. −3.63 mm3 with CER-001 3 mg/kg compared with placebo, P=0.03). A similar observation for plaque regression at the lowest CER-001 dose was found on examination of percent atheroma volume (PAV), which in previous trials has associated more closely with clinical outcomes (46). The mechanism underlying the diminishing effect with increasing CER-001 dose remains uncertain. Whether this reflects saturation of cholesterol efflux transporters or the effects of increasing administration of the lipid contents of CER-001 is unknown.
Further analysis demonstrated much greater regression with CER-001 3 mg/kg in those patients whose baseline PAV was greater than 30%, suggesting a more substantial effect on regression in patients with a greater burden of atherosclerotic plaque at baseline (49). In composite, these findings suggest that CER-001 may have an anti-atherosclerotic effect in humans, which appears to be more robust in patients with a greater baseline plaque burden and is observed at lowest tested dose. An adequately powered placebo-controlled trial is therefore required to test the hypothesis that serial, low-dose infusions of CER-001 promote coronary plaque regression.
Methods
Study design
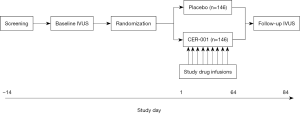
The CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial (CARAT) study (NCT02484378) is a double-blind, randomized, placebo-controlled trial performed in up to 40 sites in Australia, Hungary, Netherlands and the United States. The study (Figure 1) includes a screening period up to 14 days and up to 84 days from randomization to the end of study IVUS imaging. Institutional review board approval was obtained from each site prior to commencement. The study compares the effects of ten weekly intravenous infusions of CER-001 (3 mg/kg) with matching placebo infusions on coronary atheroma volume in patients with an ACS and higher plaque burden. Study patients are adults of at least 18 years of age, who undergo clinically indicated coronary angiography within 7 days of presentation with an ACS. The ACS can be either biomarker positive or negative, although the latter group will be limited to 25% of the study cohort. In addition, qualifying patients must have a PAV of at least 30% in the proximal 10-mm of the target artery at baseline, suitable image quality obtained with an approved IVUS catheter (Boston Scientific OptiCross; Volcano Revolution, Natick, MA, USA) to undergo plaque analysis, and be able to be randomized within 14 days of index event presentation. This minimum plaque burden requirement was selected on the basis of observations that CER-001 may produce greater disease regression in patients with the greatest plaque burden at baseline. The vessel chosen for imaging must not be a bypass graft, have undergone revascularization, have luminal stenosis greater than 50%, or be deemed the culprit vessel for the index or a previous myocardial infarction. Major exclusion criteria include HbA1c greater than 10%, fasting triglycerides >500 mg/dL, significant heart failure, liver or kidney disease and the presence of imaging within a vessel that is deemed not acceptable for the study by the core laboratory. Patients who meet all inclusion and no exclusion criteria are randomly assigned in 1:1 ratio to receive ten weekly infusions of CER-001 or placebo. Patients receive evidence-based background therapy, including anti-platelet drugs and maximally tolerated intensive statin treatment in accordance with national guidelines, as determined by treating physicians. IVUS imaging is repeated within the same coronary artery within 7–21 days following the final infusion.
Results
The primary endpoint of the study is the nominal change from baseline to follow up in PAV compared with placebo. This analysis is performed by the core laboratory using cross-sectional IVUS images spaced 0.5-mm apart in a matched arterial segment, defined by the presence of a proximal and distal side branch. In each cross-sectional image, the leading edges of the lumen and external elastic membrane will be traced by analysts, blinded to treatment status. Where calcification causes an acoustic shadow extending at least 90o, the external elastic membrane leading edge is not defined and thus that image is not included for analysis. Additional imaging endpoints include the nominal change in total atheroma volume throughout the vessel and within the 10-mm segment found to contain the largest burden of plaque at baseline. Cases with less than 15 matched, evaluable images at both time points will not be included in the primary analysis. Exploratory efficacy parameters include systemic lipoprotein and inflammatory markers (such as C-reactive protein) and surrogate measures of plaque composition. A sample size of 292 patients is chosen under the assumption of a 15% non-completion rate, yielding 248 patients with evaluable IVUS imaging at both time points. This sample size provides 86% power to demonstrate a difference in the change in PAV between the treatment groups of 1.0% with a standard deviation of 2.6%. In addition, adverse events and major adverse cardiovascular events will be collected.
CARAT is an academically led trial involving a partnership between the academic research organizations South Australian Health and Medical Research Institute (SAHMRI) and Cleveland Clinic Coordinating Center for Clinical Research (C5Research), contract research organizations (InterEuropa), and the pharmaceutical sponsor Cerenis SA. The study is led by an academic executive steering committee that will oversee all aspects of the trial. All imaging analysis will be performed by the core laboratory located within the Vascular Research Center at SAHMRI, with data management and statistical analysis provided by SAHMRI Clinical Research.
Conclusions
Despite the failure to date of oral, small molecule approaches that target HDL-C to provide cardiovascular benefit when added to statins, infusion of a simple, engineered HDL particle is an alternative with substantial supporting evidence from preclinical and early clinical studies. CER-001 has a number of properties that suggest that it should have a favorable impact on coronary atherosclerosis in humans. The CARAT study will draw on the information obtained from prior studies in humans to determine whether CER-001 promotes coronary plaque regression after ACS.
Acknowledgements
The study was sponsored by Cerenis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement:Institutional review board approval was obtained from each site prior to commencement (No. HREC/15/RAH/172) and written informed consent was obtained from all patients.
References
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005;46:1225-8. [Crossref] [PubMed]
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372:2387-97. [Crossref] [PubMed]
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8-15. [Crossref] [PubMed]
- Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301-10. [Crossref] [PubMed]
- Pisciotta L, Hamilton-Craig I, Tarugi P, et al. Familial HDL deficiency due to ABCA1 gene mutations with or without other genetic lipoprotein disorders. Atherosclerosis 2004;172:309-20. [Crossref] [PubMed]
- Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485-94. [Crossref] [PubMed]
- Reiner Z. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients: a clinical update. Nutr Metab Cardiovasc Dis 2013;23:799-807. [Crossref] [PubMed]
- Ridker PM, Genest J, Boekholdt SM, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet 2010;376:333-9. [Crossref] [PubMed]
- Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089-99. [Crossref] [PubMed]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res 2004;95:764-72. [Crossref] [PubMed]
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005;25:2416-21. [Crossref] [PubMed]
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990;85:1234-41. [Crossref] [PubMed]
- Badimon JJ, Badimon L, Galvez A, et al. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest 1989;60:455-61. [PubMed]
- Bisoendial RJ, Hovingh GK, Levels JH, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation 2003;107:2944-8. [Crossref] [PubMed]
- Spieker LE, Sudano I, Hürlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation 2002;105:1399-402. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Weibel GL, Alexander ET, Joshi MR, et al. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler Thromb Vasc Biol 2007;27:2022-9. [Crossref] [PubMed]
- AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [Crossref] [PubMed]
- Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014;349:g4379. [Crossref] [PubMed]
- Toth PP, Barylski M, Nikolic D, et al. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract Res Clin Endocrinol Metab 2014;28:353-68. [Crossref] [PubMed]
- HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203-12. [Crossref] [PubMed]
- ACCORD Study Group, Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563-74. [Crossref] [PubMed]
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849-61. [Crossref] [PubMed]
- Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 2013;128:1189-97. [Crossref] [PubMed]
- Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET Protein Inhibitor, RVX-208, on Progression of Coronary Atherosclerosis: Results of the Phase 2b, Randomized, Double-Blind, Multicenter, ASSURE Trial. Am J Cardiovasc Drugs 2016;16:55-65. [Crossref] [PubMed]
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078-87. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556-65. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071-80. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292:2217-25. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 2008;299:1547-60. [Crossref] [PubMed]
- Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304-16. [Crossref] [PubMed]
- Nissen SE, Tuzcu EM, Brewer HB, et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006;354:1253-63. [Crossref] [PubMed]
- Nicholls SJ, Bakris GL, Kastelein JJ, et al. Effect of aliskiren on progression of coronary disease in patients with prehypertension: the AQUARIUS randomized clinical trial. JAMA 2013;310:1135-44. [Crossref] [PubMed]
- Tardif JC, Grégoire J, L'Allier PL, et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 2004;110:3372-7. [Crossref] [PubMed]
- Tardif JC, Grégoire J, L'Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007;297:1675-82. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007;297:499-508. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Wolski K, et al. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol 2011;57:153-9. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Brennan DM, et al. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 2008;118:2506-14. [Crossref] [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [Crossref] [PubMed]
- Puri R, Nissen SE, Shao M, et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J 2013;34:3182-90. [Crossref] [PubMed]
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol 2006;47:992-7. [Crossref] [PubMed]
- Waksman R, Torguson R, Kent KM, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 2010;55:2727-35. [Crossref] [PubMed]
- Tardy C, Goffinet M, Boubekeur N, et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 2014;232:110-8. [Crossref] [PubMed]
- Tardy C, Goffinet M, Boubekeur N, et al. HDL and CER-001 Inverse-Dose Dependent Inhibition of Atherosclerotic Plaque Formation in apoE-/- Mice: Evidence of ABCA1 Down-Regulation. PLoS One 2015;10:e0137584. [Crossref] [PubMed]
- Tardif JC, Ballantyne CM, Barter P, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 2014;35:3277-86. [Crossref] [PubMed]
- Kootte RS, Smits LP, van der Valk FM, et al. Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res 2015;56:703-12. [Crossref] [PubMed]
- Hovingh GK, Smits LP, Stefanutti C, et al. The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am Heart J 2015;169:736-742.e1. [Crossref] [PubMed]
- Kataoka Y, Andrews J, Duong M, et al. Greater regression of coronary atherosclerosis with the pre-beta high-density lipoprotein mimetic CER-001 in patients with more extensive plaque burden. Circulation 2015;132:A12156.